3. The Historical, Environmental and Socio-economic Context of Forests and Tree-based Systems for Food Security and Nutrition
© John A. Parrotta et al., CC BY http://dx.doi.org/10.11647/OBP.0085.03
Forests and tree-based systems are an important component of rural landscapes, sustaining livelihoods and contributing to the food security and nutritional needs of hundreds of millions of people worldwide. Historically, these systems developed under a wide variety of ecological conditions, and cultural and socio-economic contexts, as integrated approaches that combined management of forest and agricultural areas to provide primarily for the needs of producers and their local communities. Today they serve food and nutrition demands of growing global populations, both urban and rural. Population increase, globalisation, deforestation, land degradation, and ever-increasing demand and associated conflict for land (including forest) resources are placing pressure on these lands. Farmers have been encouraged to intensify food production on existing agricultural lands, by modifying some traditional practices (such as agroforestry) or abandoning others (such as shifting cultivation) that evolved over centuries to cope with biophysical constraints (e.g. limited soil fertility, climate variability) and changing socio-economic conditions. This chapter provides an overview of forests and tree-based systems and their role in enhancing food security and nutrition for rural communities and those served through the marketplace. The variability and viability of these management systems are considered within and across geographical regions and agro-ecological zones. Also discussed is the role of the social, cultural and economic contexts in which these systems exist, with a focus on three factors that affect the socio-economic organisation of forests and tree-based systems, namely: land and tree tenure and governance, human capital (including knowledge and labour) and financial capital (including credit). How these biophysical and socio-economic conditions and their complex interactions influence food security and nutrition outcomes, particularly for vulnerable segments of the population (i.e., the poor, women and children), are of particular concern.
Forests1 and trees outside of forests have ensured the food security and nutrition of human populations since time immemorial. Throughout the world, forests and associated ecosystems have been managed to enhance their production of a vast array of wild, semi-domesticated and domesticated foods, including fruits, nuts, tubers, leafy vegetables, mushrooms, honey, insects, game animals, fish and other wildlife (discussed in detail in Chapter 2). The development and spread of crop agriculture and animal husbandry over the past few centuries, and particularly since the early 20th century, has diminished dependence on forests for food security and nutrition in many societies, particularly those relying primarily on staple crops. Nonetheless forests and tree-based systems – which generally co-exist in the landscape with other land management practices – continue to play a very important role for food security and nutrition, often complementing other food production systems, particularly on lands unsuited to other forms of agriculture due to soil productivity constraints.
The earth’s diverse forest ecosystems and the human cultures associated with them through the course of history have produced a vast array of food systems connected to forests and trees. These forests and tree-based systems are based on the traditional wisdom, knowledge, practices and technologies of societies, developed and enriched through experimentation and adaptation to changing environmental conditions and societal needs over countless generations (Altieri, 2002; Berkes et al., 2000; Colfer et al., 2005; Galloway-McLean, 2010; Parrotta and Trosper, 2012). Traditional forest-related knowledge and farmer innovation have played a critical role in the development of highly diverse, productive and sustainable food production systems within and outside of forests (Anderson, 2006; Kuhnlein et al., 2009; Posey, 1999; Turner et al., 2011). Starting early in the 20th century, when anthropologists began documenting the ethnobotany and food production systems of indigenous and local communities worldwide, these forests and tree-based systems and the traditional knowledge upon which they are based have been “rediscovered” by a broader audience within the (formal) scientific community, principally among agricultural scientists and ecologists.
A number of inter-related factors continue to drive the general shift from forests and tree-based systems towards intensive agriculture (discussed in detail in Chapter 4). These include, among others, population growth, urbanisation, and the progressive movement from subsistence to market-driven economies and food production systems required to serve growing numbers of consumers globally. The resultant increased demand for staples and other food crops has led to expansion of mechanised agriculture and livestock production into forests and woodlands. This has frequently included introduction of crop and livestock species and production technologies developed under very different environmental and socio-cultural conditions. It should be noted, however that in some regions such as Amazonia, urbanisation has increased the demand for, and production of, foods from forests and tree-based systems (Padoch et al., 2008).
Deforestation continues unabated in many parts of the world, in large part the result of agricultural expansion and cattle ranching (particularly in Latin America) (FAO, 2010), driven notably by urbanisation and globalisation of agricultural trade (c.f. De Fries et al., 2010; Rudel et al., 2009). Further, an increasing proportion of the world’s remaining forests have been degraded both structurally and functionally. The drivers of forest degradation include unsustainable forest management for timber, fuelwood, wildlife and other non-timber forest products, overgrazing of livestock within forests, and uncontrolled human-induced fires, exacerbated in many regions by a number of factors, including climate change (Chazdon, 2014; Cochrane, 2003; ITTO, 2002; Thompson et al., 2012) and changing rural demographics (c.f. Uriarte et al., 2012).
These trends are not encouraging, particularly in light of extensive and ongoing land degradation, i.e., the long-term decline in ecosystem function and productivity caused by disturbances from which land cannot recover unaided. Land degradation currently affects hundreds of millions of hectares of agricultural lands and forests and woodlands, and an estimated 1.5 billion people who live in these landscapes (Zomer et al., 2009). Land degradation is the long-term result primarily of poor agricultural management (both historic and ongoing) associated with the expansion of extensive and intensive agricultural production practices into lands that are only marginally suitable for such activities. Without adequate organic or fossil fuel-derived fertilisers or other agricultural inputs (e.g. irrigation, pesticides, etc.) agricultural productivity typically declines in such areas, jeopardising food security for producers and those who depend on them.
In this chapter, we provide an overview of forests and tree-based systems and their role in enhancing food security and nutrition in rural communities. Our discussion includes not only management of forests, woodlands, agroforests and tree crops for direct food provisioning, but also the management of forested landscapes for the conditions they create that in turn affect other agricultural systems. The continuum of systems included in our analysis covers managed forests to optimise yields of wild foods and fodder, shifting cultivation, a broad spectrum of agroforestry practices, and single-species tree crop production (see Figure 3.1). We consider the variability and applicability of these management systems within and across geographical regions and biomes (agro-ecological zones). The social, cultural and economic contexts in which these systems exist and how they determine food security and nutrition outcomes are of particular concern. We therefore focus (in Section 3.4) on four factors that affect the socio-economic organisation of forests and tree-based systems, namely: land and tree tenure and governance; gender relations; human capital (including labour); and financial capital (including credit).
Fig. 3.1 The forest-tree-landscape continuum.
Photo 1 © Terry Sunderland, Photo 2 © Miguel Pinedo-Vasquez,
Photo 3 © Liang Luohui, Photo 4 © PJ Stephenson
3.2 Forests and Tree-based Systems: An Overview
3.2.1 Historical Overview and the Role of Traditional Knowledge
Most of the forest and tree-based systems found in the world today have deep historical roots, developed and enriched over generations through experimentation and adaptation to changing environmental conditions and societal needs. While the scientific community, development economists and policymakers have generally disregarded and under-valued local and indigenous knowledge, such knowledge and associated management practices continue to serve communities living in or near forests in meeting their food security, nutrition and other health needs (Altieri, 2004; Cairns, 2007; Cairns, 2015; Johns, 1996; Kuhnlein et al., 2009; Parrotta and Trosper, 2012).
Traditional knowledge includes such things as weather forecasting, the behaviour, ecological dynamics, and health values of countless forest food species. It has been used to develop techniques for modifying habitats (as discussed in Section 3.2.2), enhance soil fertility, manage water resources, in the breeding of agricultural crops, domesticated trees and animals, and management of habitats and species assemblages to increase their production of food, fodder, fuel, medicine and other purposes (c.f., Altieri, 2004; Feary et al., 2012; Lim et al., 2012; Oteng-Yeboah et al., 2012; Parrotta and Agnoletti, 2012; Pinedo-Vasquez et al., 2012; Ramakrishnan et al., 2012).
An often-cited example of the sucessful application of traditional knowledge on a massive scale is the re-greening of the Sahel in Burkina Faso, Mali and Niger (Reij, 2014) where hundreds of thousands of poor farmers have turned millions of acres of what had become semi-desert by the 1980s into more productive land. Traditional knowledge regarding shea nut (from the shea tree, Vitellaria paradoxa) harvesting and processing among women engaged in shea butter production in Ghana and Burkina Faso has led to local selection of trees for desired fruit and nut traits and culling of other trees for fuel or construction. This is enabling the expansion of intensively-managed shea parklands to meet growing export markets (Carney and Elias, 2014).
The local and indigenous knowledge that underpins traditional forest- and tree-based systems is eroding in most parts of the world (Collings, 2009; Maffi, 2005; Parrotta and Trosper, 2012) as a result of a number of pressures, notably shifts to a market-based economy, cultural homogenisation, and dramatic changes in governance arrangements related to forest lands and trees outside of forests in favour of state (or colonial) ownership and control (Garcia Latorre and Garcia Latorre, 2012; Jarosz, 1993; United Nations, 2009). Development and conservation policies that discourage the traditional forest management practices that have historically ensured food security within indigenous and local communities have inevitably led to the loss of the traditional knowledge underpinning these practices (Collings, 2009; Parrotta and Trosper, 2012).
There is, however, a growing recognition of the value of traditional knowledge and innovation underpinning the management of forests and tree-based systems by indigenous and local communities worldwide. Beyond its importance for food security and nutrition, the forested landscapes that traditional management practices have produced can be appreciated for their provision of ecosystem services (including carbon sequestration), as well as conservation of biological and cultural diversity (Cairns, 2015; De Foresta and Michon, 1997; Fox et al., 2000; Palm et al., 2005; Swift et al., 1996).
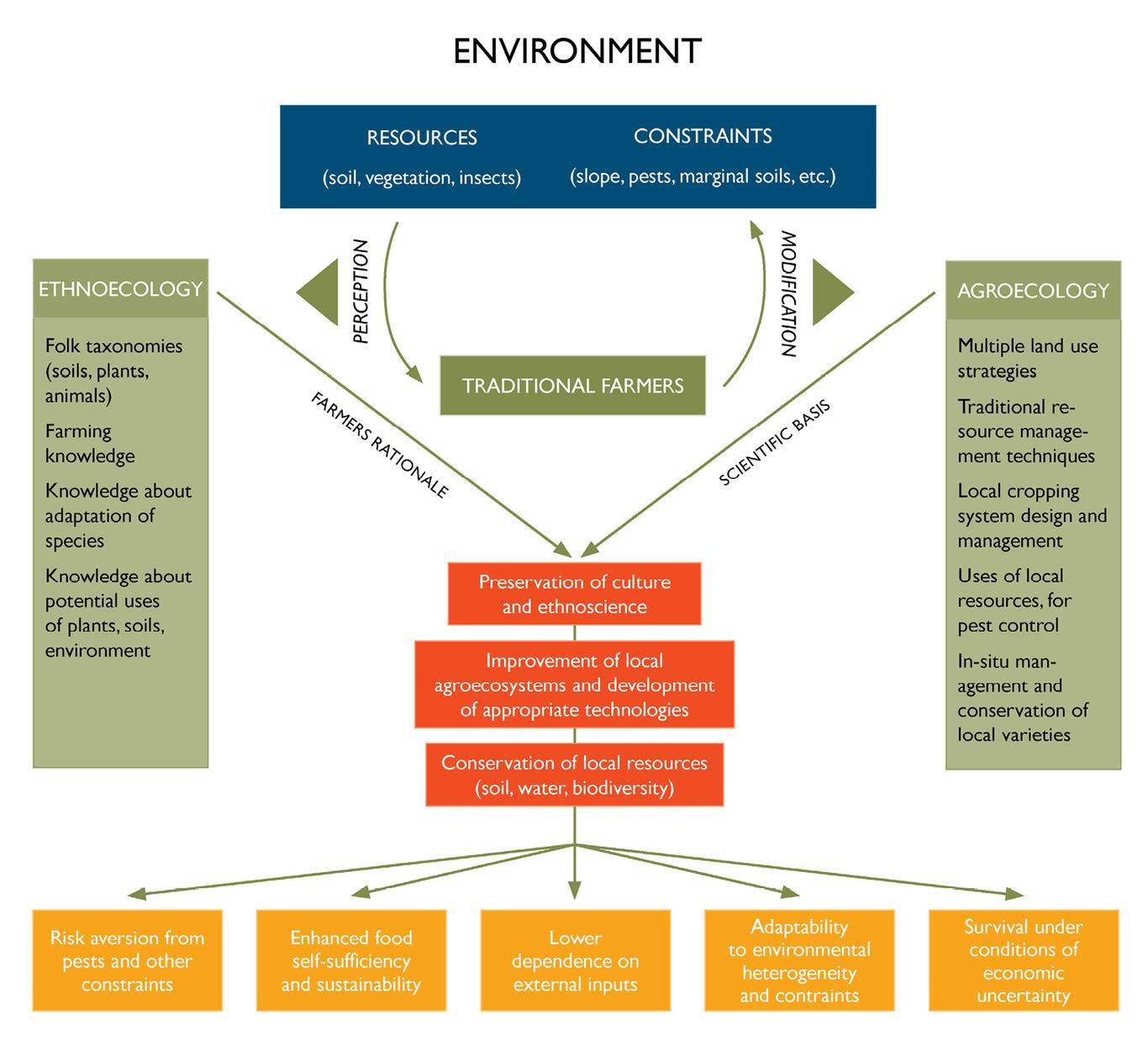
Only recently have the scientific community and decision-makers in dominant societies begun to appreciate the limitations of land use policies and the often unsustainable agricultural intensification practices that they have encouraged (c.f. Altieri, 2002; Sanchez, 1995). Part of this reassessment is a growing awareness of the value of forest-based food production systems and the traditional knowledge and wisdom that underpins them. Today, an increasing number of scientists in universities, research organisations and networks are involved in efforts to better understand and apply knowledge of forests and tree-based systems to help farmers and communities to maintain, further develop, and extend the use of these management practices to meet current and emerging challenges (such as land and forest degradation, climate change adaptation, and market changes). A useful framework for evaluating sustainability issues associated with these systems and the roles that agroecology, traditional knowledge and farmer innovation can all play in understanding and enhancing the resilience of forests and tree-based systems is presented in Figure 3.2 (Altieri, 2004).
Fig. 3.2 Agroecology and ethnoecology are complementary approaches for understanding and systematising the ecological rationale inherent in traditional agriculture and enhancing sustainability of forest and tree-based systems. Source: Altieri (2004)
3.2.2 Managed Forests, Woodlands and Parklands
People living in and near forests have, for millennia, been altering forests in many ways and on many levels. Although precise estimates are difficult to obtain, as many as 1.5 billion people are thought to be dependent on forests (Chao, 2012; Agrawal et al., 2013). Paleobotanical research in New Guinea by Hladik et al. (1993) has shown that people as early as the late Pleistocene (30,000-40,000 years ago) were manipulating the forest by trimming, thinning and ring-barking in order to increase the natural stands of taro, bananas and yams. Throughout the world, people have changed the diversity and density of edible plant and animal species, modified the structure of forest stands and populations of food trees, made gaps in forests to plant crops in temporary clearings, introduced new species, burned understories, transplanted seedlings, changed watercourses, and substantially altered the nutritional, economic and biodiversity value of many if not most, forests we see today (c.f. Boerboom and Wiersum, 1983; Sauer, 1969; Wiersum, 1997).
Fire is probably the most frequently cited and most effective management tool that past generations as well as today’s small farmers wield for changing and enriching forests and other areas with food and other useful plants. Fire is still widely used in shifting cultivation (or swidden) systems to temporarily increase soil fertility (through release of nutrients from standing vegetation), and in the management of both forests and grasslands around the world to enhance game production. Fire not only affects standing vegetation but also the soils upon which those forests stand and thus their potential productivity when cleared and planted to crops (Blate, 2005; Hammond et al., 2007; Hecht, 2009; McDaniel et al., 2005; Nepstad et al., 2001).
Many forms of traditional and contemporary forest management for food (including the creation of multi-storied agroforests, the planting of diverse forest gardens or the management of shifting cultivation fallows for food) have remained, with few exceptions, either invisible to researchers and planners or condemned by governments and conservationists (Hecht et al., 2014). Even the many contributions that woodlands make to agricultural production outside of forests have been largely overlooked (Foli et al., 2014).
There is little doubt that many of the forests that are now found throughout the tropics and elsewhere show the marks of management by people whether in the past or present (Balée, 2006). Often different types and patterns of forest manipulation have been superimposed in complex patterns whose histories and even purposes are not easily deciphered or understood. These patterns of forest disturbance, management, or manipulation continue to be developed and adapted to emerging needs and changing environmental and socio-economic conditions (Pinedo-Vasquez et al., 2012; Hecht et al., 2014). Rural communities living in and near forests around the globe and throughout history, and belonging to various communities, have not only enhanced the nutritional and economic value of their environments by increasing the supply of plant-based foods, they have also changed – and often increased – the availability of favoured animal species. Simple categories of hunting, gathering and agriculture, simply do not fit the realities of many of these livelihood strategies, while “forest management” does not adequately describe the multifaceted nature of these processes and practices. Some examples are outlined in Box 3.1.
The examples cited above give only a glimpse of how tropical forests have been and continue to be managed for food in complex and subtle ways that defy conventional categorisation. Even these few examples, however, challenge the ahistorical view held by many that old forests, particularly those of the tropics are “primordial” (Balée, 2006; Denevan, 1992) and question the facile dichotomisation of forests into “pristine” and “degraded”.
3.2.3 Shifting Cultivation Systems
Shifting cultivation, also known as swidden (or, more pejoratively, “slash-and-burn”), encompasses a highly diverse range of land use practices that human societies worldwide have used to manage forests for food over the past 10,000 years. Shifting cultivation is practised in a variety of landscapes, from steeply sloped hilly areas to flat lands and low-lying valleys, and in a variety of ecosystems ranging from tropical moist forests to dry tropical forests and savannahs, grasslands, and seasonal floodplains (Thrupp et al., 1997). Until the 19th and even into the 20th century, shifting cultivation was common in the temperate zones of the Mediterranean and Northern Europe as well as in the southwestern and northeastern pine woodlands of North America (Dove, 1983; Dove et al., 2013; Warner, 1991). Currently, shifting cultivation is practised in over 40 countries in tropical regions of Africa, South and Southeast Asia, and Latin America under a variety of environmental, social and political conditions (Mertz, 2009). It remains the dominant form of agriculture in many rural upland areas where it contributes to the creation of complex landscapes and livelihoods (Mertz et al., 2008; Raintree and Warner, 1986; Spencer, 1966).
Box 3.1 Contemporary examples of forest management systems employed to enhance food security and nutrition in Southeast Asia and Amazonia
The ”Forest Gardens” of West Kalimantan
On the island of Borneo there are significant forest stands that resemble “natural” forests but are in fact largely planted and are all heavily managed by farmers. A good example of such forests are the forest gardens that are commonly termed “tembawang” across the interior of the island. These complex forest gardens are largely found in what were once village sites and were originally formed by planting fruit trees and other trees around houses, by preserving useful species that came up spontaneously and by periodically weeding the areas selectively. When villages moved to other sites the gardens remained and grew, exhibiting an impressive tree diversity. For example in the village of Tae, an area of just one-fifth of a hectare was found to contain 224 trees belonging to 44 different species; 30 of which produce edible fruits, leaves or other edible products (Padoch and Peters, 1993; Padoch and Peluso, 1996). The most important fruits commonly found in tembawang include the especially prized durian (Durio zibethinus), as well as langsat (Lansium domesticum), jackfruit (Artocarpus heterophyllus), rambutan (Nephelium lappaceum), mangosteen (Garcinia mangostana), sugar palm (Arenga spp.) and the illipe nut (Shorea macrophylla) which produces an edible oil that also has industrial uses.
Managed forests of the Amazon estuary
The fruit of the açai palm (Euterpe oleracea) in the forests of the Amazon estuary has long been a staple of rural diets in Amazonian Brazil. It has recently also become an important source of cash, as consumption of the nutrient-rich açai fruit – once almost exclusively a local, rural food – has expanded to urban areas and into markets well beyond Amazonia. It is now highly prized and sold processed into a variety of products in North America, Europe and elsewhere (Brondizio, 2008; Brondizio et al., 2002; Padoch et al., 2008). The application of diverse management and planting practices and strategies is increasingly transforming the tidally-flooded forests of the estuary and beyond into açaí agroforests, locally called “açaizais” (Hiraoka, 1994; Brondizio, 2008). Açai agroforests include stands under different types and intensities of management, with varying population densities, structures, species diversity and composition. These practices range from selective weeding of existing açai-rich stands to further increase the production of the palm fruit, to enrichment planting and management of shifting cultivation fallows in the area. Often açai is not the only product that açai forest managers seek to promote, as açaizais contain other useful products including timbers, game and other fruits. Brondizio (2008) suggests that “ …while at the plot level one may observe a decline in tree species diversity in managed açaizais (avg 17 species) when compared to unmanaged floodplain forest (average 44 species), a broader landscape view (combining data from plots in different parts of the landscape) shows an increase of [native and exotic] tree species diversity (total 96 species).”
Building upon the management of others in the Amazon
Amazonian forests far from the estuary also abound in patches and plots that stand out from surrounding forests because of their richness in fruits and other foods. Many of these forest patches are almost certainly remnants of gardens, perhaps not unlike Borneo’s tembawang, that may have once been intensively managed but have since been largely abandoned. Other food-rich plots scattered throughout Amazonia include planted or protected vegetation along footpaths and rivers that are periodically manipulated by passersby, including indigenous groups that continue to seasonally trek following the changing availability of animals or fish, as well as other forest travellers or migrants (Alexiades, 2009; Anderson and Posey, 1989; Kerr and Posey, 1984; Rival, 2002). Many of these patches are further enriched and casually maintained by fruit harvesters, who often take the time to do some selective weeding, cut back intruding vines, or occasionally transplant new seedlings. In Brazil and Peru most of these forests are named after their most abundant and valuable tree species. In the Peruvian Amazon, zapotales (rich in the zapote fruit (Quararibea cordata)) are frequently found along paths used for centuries by indigenous and non-indigenous people. The exact origin of these stands is unknown, but many are believed to have originated centuries ago, and been maintained up to this day either intentionally or accidentally by people dispersing the seeds (while eating or processing food), protecting the seedlings and juveniles in the forests through selective weeding, and occasionally by transplanting seedlings from forests to the edges of pathways, agricultural fields or fallows. People not only value zapotes as a tasty fruit, but also as an attractor of game animals ranging from monkeys to tapirs.
While the importance of shifting cultivation for food security and nutrition in many tropical regions is indisputable, the numbers of people who depend on shifting cultivation and the land areas involved remain unclear. This is due to a general lack of useful demographic data, ethnographic studies, and explicit knowledge about the location and intensity of these practices, a failure of land cover/land use maps to identify these practices from the global to the sub-national scale (Mertz et al., 2009a; Padoch et al., 2007; Schmidt-Vogt et al., 2009). Earlier empirically-based assessments have yielded estimates of the numbers of people dependent on shifting cultivation ranging from 40 to more than 500 million worldwide (Russell, 1988; Goldammer, 1988; Kleinman et al., 1996; Sanchez et al., 2005). A more systematic study by Mertz et al. (2009a) provided conservative estimates of between 14 and 34 million people engaged in shifting cultivation in nine countries in Southeast Asia alone. Similarly, accurate estimates of land areas involved in shifting cultivation are also lacking, although it can be assumed that they include a significant proportion of the 850 million hectares of tropical secondary forests in Africa, Latin America and Asia (Mertz et al., 2008). There is a clear need for further research to provide more accurate estimates of shifting cultivator populations and land areas involved using a combination of remote sensing data, ethnographic studies and special information databases. Promising steps are being taken by scientists in this direction, for example by Hett et al. (2012) in their work in northern Laos.
These management systems usually begin with the formation of a gap in the forest, frequently a secondary forest. The forest gaps or clearings made by shifting cultivators may range from several hectares in size, especially in Southeast Asia when several households choose to farm contiguously, to only a few square metres. This phase of the cycle which usually, but not always, involves the use of fire, and creates a space to plant agricultural crops ranging from the dryland rice and vegetable combinations frequent in montane zones of Southeast Asia (Cairns, 2007; Conklin, 1957; Condominas, 1977; Padoch et al., 2007; Mertz et al., 2009b), to assemblages of cassava, banana, and a variety of tubers and herbs representative of Amazonian fields (Denevan et al., 1984; Denevan and Padoch, 1987; Padoch and de Jong, 1992). The agrobiodiversity of some of these systems is extremely high (Rerkasem et al., 2009). For example, the pioneering study of shifting cultivation fields in the Philippines by the Hanunoo people of Mindoro Island (Conklin, 1957) found over 280 types of food crops and 92 recognised rice varieties, with several dozen usually showing up in any particular field. Intensive cropping of annual species usually lasts for only a year or two after which management generally becomes less intensive, allowing for a more or less spontaneous or natural vegetation to gradually dominate the site.
In the past, the change in types or intensity of management was commonly characterised as “abandonment” of the field; more recently there has been considerable recognition that much of the “natural” or “forest” fallow can be and often is manipulated or managed by shifting cultivators for a variety of economic and food products (Cairns, 2007; Alcorn, 1981; Denevan and Padoch, 1987; Colfer et al., 1997; Colfer, 2008a; Padoch and de Jong, 1992). The “less intensive management” phase, or fallow, often relies heavily on the regrowth of forest vegetation for the provision of many of the environmental qualities necessary for efficient food production, including restoration of soil fertility and structure. The accumulation of biomass in the regrowing vegetation and the suppression of pests, diseases and weeds make agricultural production, especially in the tropics, a difficult and labour-demanding activity. Fallows or young regrowth also often feature many useful species that households collect and rely upon for food and the preparation of food. Thus shifting cultivation is increasingly seen and described as a complex and dynamic form of “swidden-fallow agroforestry” (Denevan and Padoch, 1987).
The complexity of alternating forest and field phases is further enhanced by other practices that result in the mixture of planted and spontaneous vegetation in swidden fields. When fields are first cleared, any useful tree species found in the plot are generally spared, left standing, and even protected from fire. These plants, frequently fruit trees, then become integral parts of the field together with planted crops and any spontaneous vegetation that survives weeding and further fires. “Selective weeding” is the norm; plants valuable for food or other purposes are again spared while those that are not valued are cut and removed. Especially in the later stages of the “fallow” phase, spontaneous or forest vegetation tends to predominate in shifting cultivators’ fields, the boundaries between forests and fields disappear, although the food value of these plots is often far higher than that of less “disturbed” forests (Rerkasem et al., 2009). Many areas of regrowth in these systems continue to be heavily managed for economic and other products, including such nutritionally valuable resources as bushmeat (Wadley and Colfer, 2004). “Garden hunting” is often carried out in shifting cultivation fields and fallows that can be rich in animals (Linares, 1976; Hiraoka, 1995) as they are attracted by the fruits that are frequently planted or spared. In summary, many shifting cultivation landscapes are largely forests that have been enriched with crops and a broad array of species by diverse management practices that are often applied iteratively and are difficult to classify or even see.
The dynamics of shifting cultivation have changed over time, and in some regions these changes have been rapid particularly since the mid-20th century. Many shifting cultivators have intensified their land use practices over time, including through the introduction of new crops and technologies that are not always well-suited to local agroecological conditions. While such changes can sometimes increase the cultivators’ immediate incomes, the agricultural results have often been adverse or unsustainable, especially if unsuitable land is overused or inappropriate inputs or crops are used. These changes have often resulted in instabilities in previously well-adapted shifting cultivation and resource use, jeopardising their ecological and in some cases economic sustainability (Raintree and Warner, 1986; Warner, 1991). For example, shortened cropping cycles or other management practices have in many situations contributed to soil fertility and productivity declines (Borggaard et al., 2003; Cairns and Garrity, 1999; Ramakrishnan, 1992). Destabilisation of traditional shifting cultivation systems is usually the result of a combination of socioeconomic and political changes, demographic pressures, and biophysical factors that force cultivators to change their practices (Table 3.1). Factors that commonly contribute to these changes include government restrictions of forest use, changes in land tenure systems, demographic pressures including large-scale migration and resettlements, and policies that promote cash crop production (Nair and Fernandes, 1984).
While such unstable conditions are not found in all shifting cultivation systems, they have reinforced negative perceptions of shifting cultivators and their practices (Fox et al., 2009; Mertz et al., 2009b). Arguments typically used to condemn shifting cultivation have included its low productivity, negative impacts on soils, hydrology and biodiversity conservation. However, broad generalisations regarding shifting cultivation are not helpful and obscure the fact that environmental impacts of shifting cultivation are diverse, and depend not only on farmers’ management practices, but the environmental, social, economic and political contexts in which they occur (c.f., Thrupp et al., 1997; Lambin et al., 2001). Efforts to ameliorate the perceived shortcomings or negative impacts of shifting cultivation can be counter-productive, particularly in relation to food security and nutrition. For example, recent studies on land use change in the Lao People’s Democratic Republic (also see Chapter 5), found that policies aimed at increasing forest cover, protecting wildlife, and promoting more intensive, commercial farming have had significant negative impacts on the well-being of rural community members and especially on their ability to adapt to change and respond to a variety of “shocks” that economic and environmental change may bring (Hurni et al., 2013; Castella et al., 2013).
Table 3.1 Causes of destabilisation and degradation in shifting
cultivation systems (adapted from Thrupp et al., 1997)
|
Outcomes of Destabilisation |
Proximate Causes |
Underlying Causes |
|
• Shortening or ceasing fallows • Over-exploitation of land/soils • Declining soil fertility • Decreasing yields • Increasing deforestation • Loss of biodiversity |
• Development of roads and • Expansion of monoculture • Scarcity of land and other resources available to cultivators • Changing demographic trends, e.g. migration and population growth • Lack of alternatives for production and income for rural people • Resettlement of new groups in frontier areas • Lack of access to stable markets for shifting cultivators |
• Inequitable political-economic • International/national economic policies, especially trade liberalisation, structural adjustment • Disrespect for, or neglect of, the rights of shifting cultivators • Lack of knowledge of environmental factors in agriculture • Lack of sustained economic development and employment for poor • Lack of political commitment for poverty alleviation • Inadequate attention to social needs in environmental policies |
A growing body of research indicates that in many areas where shifting cultivation is still practised, particularly where traditional knowledge regarding fallow management is well-developed and applied, these systems can be managed sustainably – without undermining soil fertility and jeopardising productivity – while conserving biodiversity and maintaining provision of an array of forest ecosystem services (c.f. Cairns, 2007; Cairns, 2015; Colfer et al., 2015; Cramb, 1993; Finegan and Nasi, 2004; Kleinman et al., 1996; Mertz et al., 2008; Palm et al., 2005; Parrotta and Trosper, 2012; Ramakrishnan, 1992; Swift et al., 1996). With respect to efforts to mitigate climate change through REDD+ programmes, it is important to note that while the secondary forest-dominated landscapes created through shifting cultivation do not store as much carbon as primary forests, their carbon sequestration potential is far greater than those dominated by alternative agricultural or single species tree crop management systems (c.f. Bruun et al., 2009; Chazdon, 2014; Martin et al., 2013). Such findings have important implications for REDD+ policies and programmes, particularly where they may exclude shifting cultivation areas (and their practitioners) from REDD+ funding consideration, or use REDD+ policies as a lever to eradicate shifting cultivation practices (Angelsen, 2008; Brown et al., 2011; Ziegler et al., 2012).
Finally, although shifting cultivation is a prominent feature of food production in forested areas in many tropical regions, the food values of forest mosaics that result from shifting cultivation systems have to date been little researched as they fall between conventional “farm” and “forest” categories. Shifting cultivation landscapes are often “illegible” to outsiders (Scott, 1999), are frequently devalued and labelled “degraded”. Yet what research there is suggests that these landscapes that harbour a great variety of plants and animals in fields and food-rich fallows and forests, and create multiple and diverse “edges”, have been the larders of human communities around the globe and throughout millennia (Andrade and Rubio-Torgler, 1994). As shifting cultivation systems disappear around the world (van Vliet et al., 2012; Padoch et al, 2008), being replaced by other forms of production that yield more food calories per area, it is important to understand what is being lost in micronutrient output, food diversity and resilience to shocks when these practices vanish.
Agroforestry encompasses a vast array of food production systems in which woody perennials are deliberately integrated in spatial mixtures or temporal sequences with crops and/or animals on the same land unit. These systems involve careful selection of species and management of trees and crops to optimise productivity and positive interactions among their components and minimise the need for chemical fertilisers and other inputs to maintain their productivity.
Like managed forests and shifting cultivation systems, most agroforestry practices are based on the traditional knowledge of people in local and indigenous communities. A staggering variety of agroforestry systems have been developed and modified by farmers in tropical, subtropical and temperate regions worldwide over centuries, or even millennia in some regions. The systematic study of agroforestry by the scientific community, which began only a few decades ago, has sought to understand the accumulated knowledge and wisdom of agroforestry practitioners using established theoretical bases from ecology and agroecology. This knowledge is being used to promote and in some cases modify these traditional systems in ways that will enhance their applicability, relevance and adaptability to changing environmental, economic and social conditions (Sanchez, 1995).
Overview of agroforestry systems and their variability
Agroforestry systems are typically classified on the basis of their structure, i.e., the nature and spatial and/or temporal arrangement of tree and non-tree components. Three broad classes are generally distinguished, based on the inclusion of agricultural crops and/or livestock in these systems: “agrisilvicultural systems” involving combinations of agricultural crops and trees or shrubs; “silvopastoral systems” that include combinations of trees and pasture for grazing livestock; and “agrosilvopastoral systems” combining crops, pastures and trees (Nair, 1993).
Agrisilvicultural systems include a very diverse array of agroforestry subsystems and practices, all of which involve the cultivation and management of trees and/or shrubs for food and/or non-food values (such as soil conservation or providing shelter for crops), generally in combination with agricultural crops. These subsystems and practices include for example, improved fallows, multilayer tree gardens and alley cropping. In some cases agrisilvicultural systems also combine the production of timber with agricultural crops, as is the case with “Taungya” which was originally used to promote teak plantations by the British colonial government in Burma in the late 19th century and which is widely practised today thoughout much of the tropics. Other agrisilvicultural systems include different plantation crop combinations, notably for fuelwood but also homegardens with fruit trees.
Silvopastoral systems include plantation crops with pastures and animals; trees on rangeland or pastures; and protein banks, involving concentrated production of protein-rich tree fodder outside of grazing areas.
Agrosilvopastoral systems include homegardens with domesticated animals; multipurpose woody hedgerows, involving fast-growing and coppicing fodder trees and shrubs in woody hedges for browse, mulch, green manure and soil improvement; apiculture with trees; aquaforestry where selected trees and shrubs line fish ponds, and multipurpose woodlots.
Within and across these broad categories, agroforestry systems vary in the functional characteristics of their components (especially of their tree and shrub components), including both productive functions (food, fodder, fuelwood, timber and other non-timber forest products) as well as protective functions (windbreaks and shelterbelts, soil conservation and fertility improvement, moisture conservation, and shade for crops, livestock and people). Considerable variation exists within all categories of agroforestry systems with respect to management intensity and the level of inputs used (such as labour, fertilisers and other agricultural inputs) which affect their adoption by farmers (Bannister and Nair, 2003; Franzel, 1999; Mercer, 2004; Scherr, 1995; see also discussion below in 3.4.4). They also differ in the predominant end uses of their products – ranging from subsistence (directly contributing to household food security and nutrition) as in the case of homegardens, to predominantly commercial, as in the case of cocoa, coffee, tea, rubber and oil palm agroforestry systems.
Regional and global patterns in agroforestry practice
Agroforestry systems serve a major role in food security and nutrition for their practitioners (and consumers of commercialised products) within a number of agroecological zones on all continents although the exact extent of these practices is difficult to quantify (notably because of a lack of standardised definitions and procedures for delineating the zone of influence of trees in mixed tree/crop systems (Nair et al., 2009)). Of particular importance to this book are those regions where food security is considered to be a more significant challenge. These include extensive areas where agroforestry systems also have a long history, i.e., the majority of tropical and sub-tropical humid, sub-humid, semi-arid and highland regions. The prevalence of different agroforestry systems in these regions, and their actual or potential contributions to enhanced food security and nutrition, are influenced by climate, natural vegetation and soils, and dominant land use systems, as well as a host of other socio-economic factors (Nair, 1993).
In humid and sub-humid tropical lowland regions, agroforestry is practised extensively in Southeast and South Asia, Central and West Africa, and Central and South America. In these regions, agroforestry can help to reduce deforestation and forest degradation, and overcome productivity constraints on conventional agriculture related to soil degradation caused by unsustainable forest management, poorly managed shifting cultivation (including reduction of fallow lengths), overgrazing, soil acidity, low soil fertility and high rates of soil erosion (Nair, 1993).
Tropical and sub-tropical highlands (over 1000m in elevation) with agroforestry potential include humid and sub-humid regions in the Himalayan region, parts of southern India and Southeast Asia, the highlands of east and central Africa, Central America and the Caribbean, and the Andes. Dominant land uses in these regions include shifting cultivation, arable farming, plantation agriculture and forestry, and ranching (in Central and South America). Agricultural productivity and food security in these regions may be constrained by soil erosion, shortening of fallows in shifting cultivation, overgrazing, deforestation and forest degradation, and fodder and fuelwood shortages (Nair, 1993).
Semiarid and arid regions where agroforestry systems are common include the cerrado of South America, savannah and sub-Saharan zones of Africa, drier regions of the Mediterranean, North Africa and the Near East, and parts of South Asia (Nair, 1993).
Parklands, one of the most extensive farming systems in the tropics and the dominant farming systems in semi-arid West Africa, cover the vast majority of cultivated area in Sahelian countries. This includes an estimated 90 percent (5.1 million ha) of all agricultural lands in Mali (Cissé 1995; Boffa, 1999) where scattered multipurpose trees such as baobab (Adansonia digitata L.), detar (Detarium microcarpum), néré (Parkia biglobosa), tamarind (Tamarindus indica), shea tree or karité (Vitellaria paradoxa) and ber (Ziziphus mauritiana) are managed on farmlands.
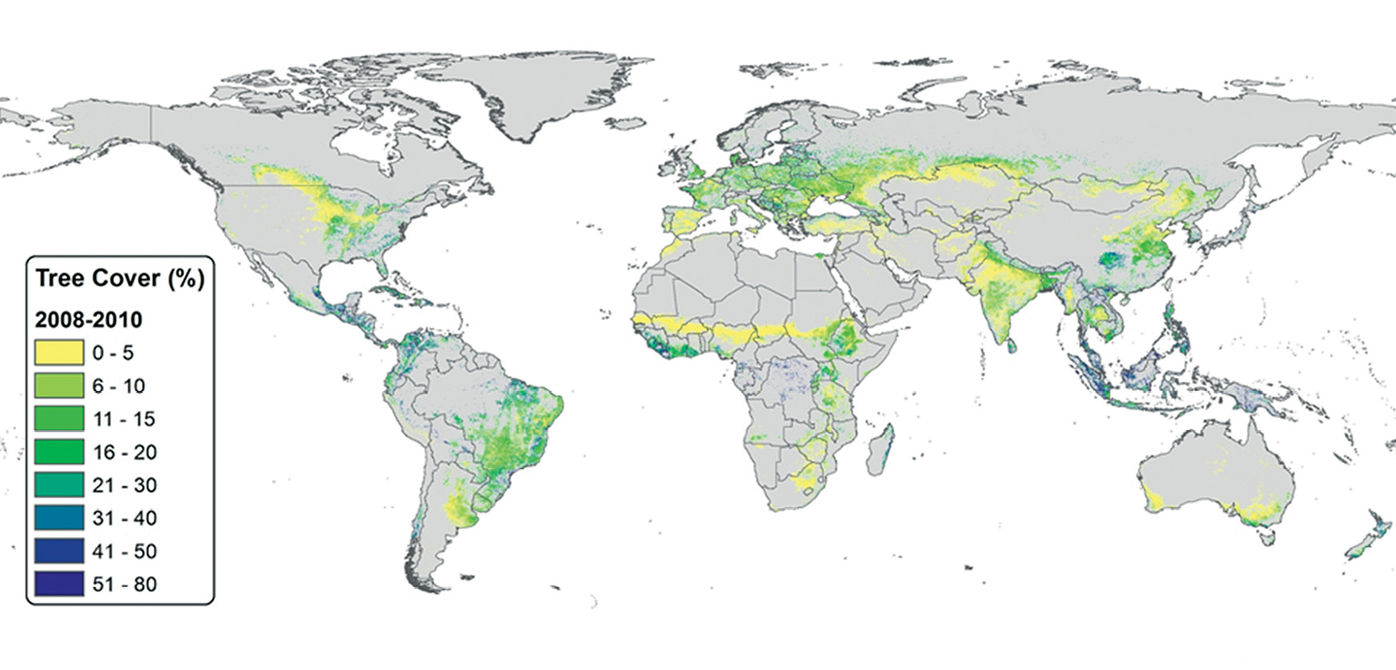
A recent geospatial analysis by Zomer et al. (2014) estimated the extent and recent changes in agroforestry practices at a global scale, based on remote sensing-derived global datasets on land use, tree cover and population. Agroforestry systems (defined in their study as agricultural lands with greater than 10 percent tree cover) were found to comprise 43 percent (over 1 billion ha) of all agricultural land globally (Figure 3.3). These lands include 320 million ha in South America, 190 million ha in sub-Saharan Africa, and 130 million ha in Southeast Asia. In Central America, 96 percent of agricultural lands were classified as agroforestry, as were over 80 percent of agricultural lands in Southeast Asia and South America. Globally, the amount of tree cover on agricultural land increased substantially between 2000 and 2010, with the area of >10 percent tree cover increasing from 40 to 43 percent (+82.8 million ha). The proportion of agricultural lands with varying levels of tree cover and proportions of people living in these landscapes in different regions of the world are presented in Table 3.2.
Fig. 3.3 Global estimates of tree cover (percent) on agricultural land
in the years 2008-2010 (averaged). Source: Zomer et al. (2014)
Zomer et al. (2009) found a strong relationship between aridity and tree cover in Southeast Asia, Central America and South America, although there are many exceptions to this rule (i.e., high tree cover found in more arid zones and low tree cover found in more humid zones) that must be explained by other factors, such as tenure, markets or other policies and institutions that affect incentives for tree planting and management, as well as context-specific historical trends (Zomer et al., 2014; Zomer et al., 2007; Zomer et al., 2009). Further, although patterns in the relationship between tree cover and human population densities in agricultural landscapes exist within aridity classes and continents, these correlations are neither consistently positive nor negative except in the very low or high range of tree cover, and there appears to be no general trade-off between human population density and tree cover in these landscapes. Additional work is needed to refine estimates of land cover (versus land use) in agricultural landscapes and the extent of agroforestry practice in its varied forms, both at the global level and at finer spatial scales, as well as their relationship with factors other than climate and population density.
Table 3.2 Percentage of land area and population living in agricultural areas with greater
than 10%, 20% and 30% tree cover in 2008-2010 (adapted from Zomer et al., 2014).
|
(% of all land area/persons in agricultural area) |
>10% tree cover |
>20% tree cover |
>30% tree cover |
|||
|
Region |
% land area |
% population |
% land area |
% population |
% land area |
% population |
|
North America |
42.4 |
66 |
26.3 |
46 |
15.5 |
30 |
|
Central America |
96.1 |
95 |
79.0 |
78 |
54.8 |
54 |
|
South America |
65.6 |
74 |
31.8 |
35 |
17.7 |
19 |
|
Europe |
45.0 |
46 |
20.4 |
19 |
11.6 |
10 |
|
North Africa/Western Asia |
11.0 |
13 |
5.5 |
4 |
3.3 |
2 |
|
sub-Saharan Africa |
30.5 |
39 |
15.0 |
16 |
8.4 |
7 |
|
Northern and Central Asia |
25.3 |
23 |
9.7 |
7 |
4.3 |
3 |
|
South Asia |
27.7 |
34 |
7.8 |
8 |
3.6 |
2 |
|
Southeast Asia |
79.6 |
73 |
62.9 |
46 |
49.9 |
30 |
|
East Asia |
47.5 |
57 |
22.1 |
21 |
11.8 |
8 |
|
Oceania |
33.3 |
80 |
23.8 |
67 |
17.0 |
52 |
|
Global average |
43.4 |
46 |
23.1 |
19 |
14.2 |
10 |
|
Change since 2000-2002 |
+3.7 |
+5 |
1.8 |
+2 |
+1.1 |
+2 |
3.2.5 Single-species Tree Crop Production Systems
Single-species tree crop production systems can be found in forest and agricultural landscapes in tropical, sub-tropical and temperate regions worldwide. They involve a wide variety of designs and management practices that have evolved over time in response to local, regional and global commoditization of domesticated forest species.
The domestication of forest tree species is rooted in antiquity. Genetic selection, vegetative propagation (including grafting) and cultivation of tree crops such as date palm (Phoenix dactilifera), olive (Olea europaea), sycamore fig (Ficus sycomorus), pomegranate (Punica granatum), apple (Malus x domestica), pear (Pyrus communis), apricot (P. armeniaca), almond (P. dulcis), sweet cherry (P. avium), peach (P. persica), mango (Mangifera indica) before avocado (Persea americana) all date back 4,000 to 6,000 years (Janick, 2005). In the case of the common fig (Ficus carica) its domestication may have begun at the time when wild grains such as rice, wheat and other staple crops were first cultivated in North Africa and Southwest Asia 11,000-12,000 years ago (Kislev et al., 2006).
Worldwide, many hundreds of tree species are cultivated today by farmers for household and local consumption, a lesser number for sale in urban markets, and still fewer for international markets. These cultivated species include beverage and confectionery crops (e.g. coffee, cocoa, tea), fruits, oils (e.g. oil palm, coconut), staples (e.g. bananas, plantains, breadfruit, peach palm and sago palm), spices (e.g. cinnamon, clove) and nuts. The diversity of forest species cultivated by farmers in tropical and subtropical regions is impressive; an indicative list presented by Smith et al. (1992) of domesticated tropical moist and wet forest trees for their edible fruits or nuts includes over 170 species. Production from these tree crop systems contributes significantly to the food security and nutrition of farmers – either directly for their nutritional value, or indirectly by providing income, as discussed in Chapter 2.
Tree crop systems are managed on large, medium or small scales either as single-species or multi-strata systems with other woody or herbaceous species. They may also be intercropped in agroforestry systems with annual or perennial crops in temporal or spatial sequences. For example, coffee production in Ethiopia mainly involves agroforestry-based systems, although there are both natural coffee forests and single-species plantations (Muleta, 2007). Similarly, cocoa is cultivated under the canopy of shade trees in traditional agroforests, although single-species plantations are also cultivated (Obiri et al., 2007). Weeding, fertiliser application, pest and disease control, and branch pruning are among the cultural practices used in tree crop systems for enhancement of yield (Table 3.3).
The introduction of new hybrids of some species with large international markets has led to a rapid expansion in acreage in producing countries. A number of major tree crops are listed in the FAO database, FAOSTATS, on agricultural commodities traded globally. These include: cocoa (Theobroma cacao), coffee (Coffea arabica, Coffea robusta), tea (Camellia sinensis), oil palm (Elaeis gineensis), coconut (Cocos nucifera), date palm (Phoenix dactylifera), mango (Mangifera indica), avocados (Persia americana), orange, tangerine, lemon, grapefruit (Citrus spp.), shea (Vitellaria paradoxa), guava (Psidium guajava), fig (Ficus carica), banana and plantain (Musa spp.), apple (Malus domestica), peach, plum, and apricot (Prunus spp.), olive (Olea europaea), cashew (Anacardium occidentale), walnut (Juglans spp.) and hazelnut (Corylus spp.). Information on a number of these tree crop species, their management and contributions to food security and nutrition, are summarised in Table 3.3 (see also Chapter 2).
Production of some tree crops with major global markets has been organised on a large scale with smallholder participation, making significant contributions to local and national economies (Watson, 1990). While smallholder farmers typically earn the least profit margin in tree crop commodity value chains, single-species tree crop systems do create employment and income opportunities locally and internationally as well as improved trade and foreign exchange balances for producing nations. For example, Ethiopia, the oldest exporter of coffee in the world, is the largest coffee producer and exporter in Africa. The cultivation, processing, trading, transportation and marketing of coffee provide employment for 15 million Ethiopians who depend on the industry for at least a significant part of their livelihood on a subsistence basis or as a sole source of income. The industry plays a fundamental role in both the cultural and socio-economic life of the nation (Muleta, 2007). In Uganda the coffee industry employs over 5 million people and the sector contributes 20-30 percent of the country’s foreign exchange earnings (Kiyingi and Gwali, 2013).
Climate change and its potentially devastating effects on crop production threaten the productivity of tree crop systems in many regions. For example, it is predicted that rising temperatures will dramatically reduce cocoa production between 2030 and 2050 in Côte d’Ivoire and Ghana, the world’s first and second cocoa producers accounting for 53 percent of the world’s cocoa output (CTA, 2012). This has necessitated a critical analysis of promising multi-purpose tree-based systems that have the potential for ensuring sustainable income and food security while mitigating climate change effects. Shade-grown cocoa and coffee are also being advocated in response to certification schemes and also the increasing demand for “specialty” products (Afari-Sefa et al., 2010; WOCAT, 2007). Generally, growing tree crops under the shade of upper canopy forest trees is considered to be more ecologically and economically sustainable than open-grown systems (WOCAT, 2007). However, the value of such systems for biodiversity conservation is very much context-specific, and has been questioned in the case of shade coffee (Tejada-Cruz et al., 2010).

Theobroma cacao (cocoa) pods.
Photo © sarahemcc, Wikimedia
Box 3.2 Shade-grown cocoa
Although it has been argued that the perennial nature of tree crop systems makes them inherently more sustainable and less environmentally damaging in comparison with annual food crop systems (Watson, 1990), their biodiversity impacts, particularly for the production of cocoa and coffee, have increased with the expansion of plantations in many producing countries. In the case of cocoa, the total area under cultivation worldwide increased by 3 million ha (4.4 million to 7.4 ha) in the last 50 years (Clough et al., 2010), contributing to the ongoing transformation of many lowland tropical forest landscapes in Latin America, Africa and Southeast Asia that began centuries ago (Schroth and Harvey, 2007). Expansion of cocoa farms accounts for much of the deforestation in lowland West Africa (Gockowski and Sonwa, 2011) where intact tropical forests have been converted for this purpose. This transformation has been expedited by the development and introduction of highly productive cocoa hybrid varieties that require little or no forest tree shade. However, since open-grown cocoa requires increased investments in agro-chemical inputs to support optimum productivity, it has a shorter productive period with deleterious effects on soil fertility and plantation health (Ruf and Schroth, 2004). In contrast, cocoa traditionally grown under filtered shade of forest trees often results in a multi-strata agroforestry system that is considered to be one of the best examples of permanent agriculture that preserves a forest environment and biodiversity (Ruf and Schroth, 2004; Rice and Greenberg, 2000). Under optimal soil conditions and rainfall regimes, shade grown cocoa may produce good yields for 60-100 years whereas optimum production may last for 20 or less years without shade (Ruf and Schroth, 2004; Obiri et al., 2007; Obiri et al., 2011).
Table 3.3 Geographical distribution, management and nutritional values of selected tree crops with international markets.
|
Common (and scientific) name & centre of origin |
Major producing countries |
Establishment and |
Principle food uses and nutritional value |
References |
|
Sweet orange Most likely Southeast Asia |
Cultivated worldwide in tropical, subtropical and Mediterranean climates: Largest producer is Brazil, followed by USA, India, Mexico, China, Spain, Italy, Egypt, Iran, Indonesia, Turkey, Pakistan and South Africa. |
Grown in agroforestry systems with food crops and in monocrop plantations; propagated from seeds/seedlings or vegetatively from grafted seedlings. Weed control, insect pest and disease control, fertiliser application, irrigation and branch pruning required to sustain productivity. |
Fruit is eaten fresh, or processed for its juice or fragrant peel for marmalade. Orange juice is a rich source of vitamin C; the edible peel has significant contents of vitamin C, dietary fibre, total polyphenols, carotenoids, limonene and dietary minerals, such as potassium and magnesium. |
FAOSTAT Statistical Database: http://faostat.fao.org/ |
|
Apple Central Asia in southern Kazakhstan, Kyrgyzstan, Tajikistan, and Xinjiang, China. |
Cultivated worldwide in temperate and some subtropical regions. Largest producer is China, followed by USA, Turkey, Iran, Poland, Italy, France, India, Russia, Chile, Argentina and Brazil. |
Grown in orchards and agroforestry systems. Generally propagated by grafting, although wild apples grow readily from seed. Apple trees highly susceptible to fungal and bacterial diseases and insect pests. Intensive programme of chemical sprays important to maintain high fruit quality, tree health, and high yields in commercial plantations. |
Fruit often eaten fresh but also cooked in prepared foods (especially desserts) and drinks. Used for juice, vinegar and other beverages and confectionery. Fruit contains significant dietary fibre and modest vitamin C content, with otherwise a generally low content of essential nutrients compared to other fruits. Apple peels contain various phytochemicals with unknown nutritional value, including quercetin, epicatechin, and procyanidin B2. |
FAOSTAT Statistical Database: http://faostat.fao.org/; Lauri et al. (2006); USDA Nutrient Database: http://ndb.nal.usda.gov/ndb/search/list |
|
Mango Tropical South and Southeast Asia |
Cultivated throughout the tropics and subtropics. Largest producer is India, followed by China, Thailand, Indonesia, Pakistan, Mexico, Brazil, Philippines, Egypt, Kenya. |
Grown in smallholder agroforestry systems and in large scale monocrop plantations; propagated from seeds, seedlings and grafted seedling. Weed control, insect pest and disease control, fertiliser application, irrigation and branch pruning required to sustain productivity. |
Fruits are eaten fresh or used to prepare juices, smoothies, sherbets or other desserts. Also used (dried or fresh) in cooking, preparation of chutneys and preserves. Both green and ripe mango fruits are rich in carbohydrates, minerals and vitamin C. Fruits and sometimes leaves used as livestock fodder. |
FAO (1982); FAOSTAT Statistical Database: http://faostat.fao.org/; Mukherjee (1972). |
|
Avocado Mexico and Central America |
Cultivated worldwide in tropics, subtropical and Mediterranean climates. Largest producer is Mexico, followed by Indonesia, Chile, USA, Dominican Republic, Colombia, Brazil, Peru, China and Kenya. |
Grown in agroforestry systems with food crops and in orchards; propagated from seeds/seedlings or asexually from grafted seedlings. Weed control, insect pest and disease control, fertiliser application, irrigation and branch pruning required to sustain productivity. |
The fruit is eaten fresh and for preparation of various recipes; it is a major ingredient in vegetarian diets. A typical serving of avocado (100 g) is a very good source of several B vitamins and vitamin K, and a good source of vitamin C, vitamin E and potassium. Avocados also contain phytosterols and carotenoids, such as lutein and zeaxanthin, and diverse fats, mostly oleic acid but also palmitic acid and linoleic acid, among others. |
Chen et al. (2008); Dreher and Davenport (2013); FAOSTAT Statistical Database: NutritionData.com. 2013. |
|
Common (and scientific) name & centre of origin |
Major producing countries |
Establishment and |
Principle food uses and nutritional value |
References |
|
Common fig Middle East and western Asia |
Cultivated in many temperate and subtropical countries worldwide, particularly in the Middle East and areas with a Mediterranean climate. Major producers include: Turkey, Egypt, Iran, Morocco, Algeria, Syria, USA, Greece, Spain, Afghanistan, Brazil, Tunisia and Italy. |
Propagated from seeds, but more commonly by vegetative methods, i.e., cuttings, air-layering or grafting. |
Figs are consumed fresh or dried and are often processed as a paste for pastries or canned. The fruit can be fermented and distilled into alcohol. Dried figs are a rich source (> 20% of the daily value) of dietary fibre and the essential mineral, manganese, while vitamin K and numerous other minerals are in moderate content. Figs contain diverse phytochemicals, including polyphenols such as gallic acid, chlorogenic acid, syringic acid, (+)-catechin, (−)-epicatechin and rutin. |
FAOSTAT Statistical Database: http://faostat.fao.org/; Janick (2005); USDA Nutrient Database: http://ndb.nal.usda.gov/ndb/search/list; |
|
Cocoa Southeastern Mexico to Amazon Basin |
Cultivated in humid tropics. Top 10 producing countries (in 2005): Cote d’Ivoire, Ghana, Indonesia, Nigeria, Brazil, Cameroon, Ecuador, Colombia, Mexico, Papua New Guinea. |
Grown both in large agroindustrial plantations and by small producers, the bulk of production coming from millions of small producers; Planted under forest shade or in monocrop plantations; propagated from seeds/seedlings. Pruning, ferti liser application, pest, disease and weed control, pod harvesting and bean processing are main cultural practices for managing cocoa plantations. |
Seeds or beans contain 40-50% fat as cocoa butter used for chocolate, cocoa mass and powder; pulp used for juice, smoothies, jelly and nata; fermented pulp distilled into alcoholic beverages. |
CacaoNet. (2012); FAOSTAT Statistical Database: http://faostat.fao.org/; |
|
Coconut Asia-Pacific |
Coastal regions throughout humid tropics and subtropics. Major producers include: Indonesia, Philippines, and India, followed by Brazil, Sri Lanka, Vietnam, Papua New Guinea, Mexico, Thailand, Malaysia and Tanzania. |
Cultivated in a variety of agroforestry systems and in monocrop plantations; propagated from seeds (nuts) and seedlings. |
Fruit (nut) contains water suspended in the endosperm with an outer hard shell (mesocarp) and fibrous husk(exocarp).Water from immature fruits consumed as a refreshing beverage rich in vitamins and trace minerals; Endosperm when mature contains 35-40% oil, 10% carbohydrate and 3% protein; oil extracted from dried endosperm (copra) used as a cooking oil, in margarine, cocoa butter, beverages and numerous non-food products; dried endosperm used in confectionery, cooking, and may be ground into flour for baking. |
FAOSTAT Statistical Database: http://faostat.fao.org/; Opeke (1982); Parrotta (1993) |
Table 3.3, cont. Geographical distribution, management and nutritional values of selected tree crops with international markets.
|
Common (and scientific) name & centre of origin |
Major producing countries |
Establishment and |
Principle food uses |
References |
|
Shea Guinea and Sudan savannah zone from Senegal to Sudan, and to western Ethiopia and Uganda. |
Managed in natural stands near human settlements, as well as planted throughout its African range. Major producers include Nigeria, Mali, Burkina Faso, Ghana, Côte d’Ivoire, Benin and Togo. |
Throughout its range it is managed in natural stands and in agroforestry systems (parklands), either involving livestock and/or staple crop production. Control of bush fire, insects and parasites and drought are major management activities. |
Shea butter extracted from the seeds widely used in cosmetics as a moisturiser, salve or lotion. It is one of the most important sources of vegetable oil in rural areas of the savannah zone of West Africa, used in food preparation in many African countries, and occasionally (mixed with other oils) in the chocolate industry as a substitute for cocoa butter. Shea butter is composed of five principal fats mostly stearic, oleic but also palmitic, linoleic, and arachidic; it is a rich source of vitamins A and E, and contains phenolic compounds known to have antioxidant properties. |
Hall et al. (1996); Masters et al. (n.d.); PROTA database: http://database.prota.org/PROTAhtml/Vitellaria paradoxa_En.htm |
|
Cashew Northeastern Brazil |
Cultivated in many tropical countries worldwide. Major producers include: India, Côte d’Ivoire, Brazil, Indonesia, Vietnam, Nigeria, Benin, Guinea-Bissau, Mozambique and Philippines. |
Grown in smallholder agroforestry systems and in large scale monocrop plantations from seeds, seedlings and grafted seedling. Weeding, mulching of young plants, fertiliser application and pruning of branches enhance growth and yield. |
Nut (kernel) eaten as a snack food or used in cooking. They are a rich source of protein, carbohydrate and fat and contains minerals such as Ca, P, Na, K, Mg, Fe, Cu, Zn and Mn. Cashew kernel lipids are rich in unsaturated fats, mainly oleic acid. It is also a good source of antioxidants. The spongy, juicy, pear shaped stalk (cashew apple) contains sugars, tannins, phenols, amino acids, ascorbic acid, riboflavin, minerals and fibre. It is used to prepare juices or distilled into a liqueur (feni);also used to prepare pickle and other food products. |
FAOSTAT Statistical Database: http://faostat.fao.org/; Johnson (1973); Ohler (1979); Saroj and Rupa (2014)
|
|
Walnut (Juglans regia) Central Asia, including Uzbekistan, Kyrgyzstan, Tajikistan, Turkmenistan and southern Kazakhstan. |
Largest producer is China, followed by Iran, USA, Turkey, Mexico, Ukraine, India, Chile, France and Romania. |
Commonly propagated from seeds. Insect pest and disease control, fertiliser application, branch pruning required to sustain productivity. |
Walnuts are eaten raw, toasted, pickled or cooked in various recipes; also processed for oil. 100 grams of walnuts contain 15.2 grams of protein, 65.2 grams of fat, and 6.7 grams of dietary fibre. They are rich in vitamins, particularly thiamine (B1), B6, folate (B9), and in trace metals, particularly manganese, but also magnesium, phosphorus, iron, and zinc. Unlike most nuts that are high in monounsaturated fatty acids. |
FAOSTAT Statistical Database: http://faostat.fao.org/; Molnar et al. (2011); USDA Nutrient Database: http://ndb.nal.usda.gov/ndb/search/list |
|
Common (and scientific) name & centre of origin |
Major producing countries |
Establishment and |
Principle food uses |
References |
|
Oil palm Tropical West and Southwest Africa, between Angola and the Gambia. |
Cultivated in many countries in the humid tropics outside of its African range since the mid-20th century when large-scale plantations were established in Malaysia. At present (2014-2015), Indonesia is the major producer, followed by Malaysia and Nigeria. Smaller producer countries include Thailand, Colombia, Benin, Cameroon, Kenya and Ghana. Largest importers of palm oil include India, the European Union and China. |
Cultivated in mixed cropping with food crops in smallholder systems and increasingly in large scale monoculture plantations. Propagated by seeds. Weed control, insect pest and disease control, fertiliser application, irrigation and branch pruning required to sustain productivity. |
An edible oil derived from the mesocarp (reddish pulp) of the fruit kernels is used for household cooking (especially in tropical Africa and Southeast Asia) and industrial food and non-food applications worldwide (e.g. margarine, cosmetics, soaps, toothpaste, waxes, lubricants and ink). From a nutritional and health perspective, palm oil has an especially high concentration of saturated fat, specifically of palmitic acid, as well as the monounsaturated oleic acid. While palm oil is an important source of calories and a food staple in poor communities, its overall health impacts, particularly in relation to cardiovascular disease, are controversial. Much of the palm oil that is consumed as food is to some degree oxidised rather than in the fresh state, and this oxidation appears to be responsible for the health risk associated with consuming palm oil. |
Edem (2002); USDA Foreign Agricultural Service: http://apps.fas.usda.gov/psdonline/; USDA Nutrient Database: http://ndb.nal.usda.gov/ndb/search/list |
|
Common fig Middle East and western Asia |
Cultivated in many temperate and subtropical countries worldwide, particularly in the Middle East and areas with a Mediterranean climate. Major producers include: Turkey, Egypt, Iran, Morocco, Algeria, Syria, USA, Greece, Spain, Afghanistan, Brazil, Tunisia and Italy. |
Propagated from seeds, but more commonly by vegetative methods, i.e., cuttings, air-layering or grafting. |
Figs are consumed fresh or dried and are often processed as a paste for pastries or canned. The fruit can be fermented and distilled into alcohol. Dried figs are a rich source (> 20% of the Daily Value) of dietary fibre and the essential mineral, manganese, while vitamin K and numerous other minerals are in moderate content. Figs contain diverse phytochemicals, including polyphenols such as gallic acid, chlorogenic acid, syringic acid, (+)-catechin, (−)-epicatechin and rutin. |
FAOSTAT Statistical Database: http://faostat.fao.org/; Janick (2005); USDA Nutrient Database: http://ndb.nal.usda.gov/ndb/search/list; |
|
Cocoa Southeastern Mexico to |
Cultivated in humid tropics. Top 10 producing countries (in 2005): Cote d’Ivoire, Ghana, Indonesia, Nigeria, Brazil, Cameroon, Ecuador, Colombia, Mexico, Papua New Guinea. |
Grown both in large agroindustrial plantations and by small producers, the bulk of production coming from millions of small producers; Planted under forest shade or in monocrop plantations; propagated from seeds/seedlings. Pruning, fertiliser application, pest, disease and weed control, pod harvesting and bean processing are main cultural practices for managing cocoa plantations. |
Seeds or beans contain 40-50% fat as cocoa butter used for chocolate, cocoa mass and powder; pulp used for juice, smoothies, jelly and nata; fermented pulp distilled into alcoholic beverages. |
CacaoNet. (2012); FAOSTAT Statistical Database: http://faostat.fao.org/; |
3.3 The Influence of Forest Landscape Configuration, Management and Use on Food Security and Nutrition
Forests and associated food production systems do not exist in isolation. They are part of broader economic, political, cultural and ecological landscapes. Such landscapes usually comprise diverse patches of different land use types, which may include forest and non-forest, different food production systems, and numerous other land uses. The following discussion considers the ways in which different land use-patches interact with each other in space and time to influence the productivity and sustainability of forests and tree-based systems.
3.3.1 Interactions between Landscape Components
Positive contributions of forests to agricultural productivity
Forests provide an array of direct and indirect contributions to agriculture at different scales (MA, 2005). At the broad scale, forests contribute to the recycling of nutrients, suppression of agricultural pests, detoxification of noxious chemicals, control of hydrological processes and genetic resources for future adaptation to climate change (Foley et al., 2005; MA, 2005; Plantegenest et al., 2007). In a study carried out in 56 countries in Africa, Asia and Central/South America it was found that a ten percent increase in deforestation would result in a 4-28 percent increase in flood frequency (Bradshaw et al., 2007), with large impact on rural and agrarian populations (FAO and CIFOR, 2005; Jonkman, 2005). Forests also contribute to climate change mitigation, having the capacity to absorb a significant fraction of global carbon emissions which could have positive impacts on food production (FAO, 2012).
At the local scale, forests and trees outside forests are essential for ecosystem services such as pollination (Ricketts, 2004; Ricketts et al., 2008), pest regulation and regulation of the microclimate (Kort, 1988), as discussed in Chapter 2. They can also preserve genetic diversity of domesticated and wild food species and enhance soil fertility and agricultural productivity (Tscharntke et al., 2005a; Bianchi et al., 2006; Ricketts et al., 2008; Boyles et al., 2011). For example, 75 percent of the most important crop species benefit from pollination services (Klein et al., 2007) accounting for 153 billion Euros annually (Gallai et al., 2009). In many African countries farmer-managed forest regeneration programmes are estimated to have doubled the agricultural yields over nearly five million hectares with significant potential for the future (World Bank, 2013). Green foliage collected from forests can also represent an important resource for compost to enhance productivity of field crops, such as areca nut plantations in India (Sinu et al., 2012).
As discussed in Chapter 2 and earlier in this chapter, forests are also a direct source of food, fuel, fodder and medicines, benefiting not only people living within forested landscapes (c.f. Colfer, 2008a; Kuhnlein et al., 2009), but those living elsewhere, including urban areas. For example, it is estimated that about 2.4 billion people, or 40 percent of the population of low- and middle-income countries, rely on woodfuel for cooking, with some 746 million people boiling their water with wood (FAO, 2014).
The provision of such forest benefits can be dependent on the spatial configuration of the landscape and proximity to forests. For example, Ickowitz et al. (2014) found that after controlling for confounding factors (such as distance to market and road density) children’s dietary diversity increased with tree cover across 21 African states. Wild harvested meat also provides a significant source of food in many regions, including for example in Central Africa where a critical portion of protein and fat often comes from this source (Nasi et al., 2008). Forests can also contribute to nutrition by providing sources of income that can be spent to buy food in markets.
Negative effects of forests on agricultural productivity
Forests can also have negative impacts on nearby agricultural production, for example by harbouring agricultural pests and diseases that reduce agricultural yield, and others that more directly harm human health. New insect pests can be introduced into an area through the transportation of wood or nursery stock associated with forestry and horticultural activities (Cock, 2003). Forest wildlife species and arthropods (insects, ticks, etc.) can spread disease pathogens and parasites to livestock and humans, such as malaria, encephalitis, rabies, Ebola, SARS, and several others (Bengis et al., 2002; Belotto et al., 2005; Colfer, 2008b; Olson et al., 2010; Tomalak et al., 2011; Wilcox and Ellis, 2006). In light of the recent West African Ebola crisis, it has been argued that these risks create an opportunity to conserve forest animal species by emphasising the dangers involved in consuming wild meat (Williams, 2014). However, this argument has been rejected by others, who emphasise the complex relationship between people,forests and hunting practices that produce the risk of disease transmission (Pooley et al., 2015).
Forests are a critical habitat for wildlife species but can also be a source of human-wildlife conflict, particularly where agroforestry buffers between forests and farms provide suitable habitat for wild species (Naughton-Treves et al., 1998). When agricultural fields, agroforestry systems or homegardens are raided by wild animals, crop damage can result in significant economic losses on farms and during post-harvest stages of food production, and in some cases total crop devastation (Ntiamoa-Baidu, 1997; Hockings and McLennan, 2012). Around Kibale National Park (Uganda) – a large forested reserve harbouring crop raiding species such as baboons and chimpanzees – average financial losses for farmers in a six month period were estimated at USD 74 with more severe crop damage closer to the park boundary (Mackenzie and Ahabyona, 2012). In the struggle to protect crops, both humans and wildlife can be put in danger, undermining conservation efforts due to increased human-wildlife conflict and increasing farm labour costs (Hill, 2000; Pérez and Pacheco, 2006). In India, elephants kill over 400 people and destroy crops valued at two to three million USD every year (Bist, 2006; Rangarajan et al., 2010).
Impacts of other land use patches on forests
Forests can be impacted positively or negatively by other nearby or distant land uses in ways that affect their own role as food production systems, as habitat for biodiversity, or their structure and function more generally. Forests located near farming and urban areas may be more exposed to air, water and other types of pollution. Forests are vulnerable to emissions of reactive pollutants such as SO2, NOx, HNO3 and NH3 as well as elevated levels of ozone and excessive mineral salts (Fowler et al., 1999; Likens et al., 1996). These potentially phytotoxic pollutants, largely studied in the northern hemisphere, are damaging to forest health although it is difficult to identify specific pollutant effects given the high level of interactivity between pollutants, and between pollutants and climate change (Bytnerowicz et al., 2007; Paoletti et al., 2010). Atmospheric pollutants can also severely damage forests through acid rain (Likens et al., 1996).
Proximity to human settlements and roads can increase the likelihood of invasive species being introduced to, and perhaps damaging, forest environments (Bradley and Mustard, 2006; Bartuszevige et al., 2006). In most cases the introduction of non-native species may have little impact since they often fail to survive in a new habitat. However, those that do become established and thrive can cause severe and widespread economic and ecological losses, such as a reduction in forest and agricultural productivity, species population declines and even extinctions (Holmes et al., 2009). For example, in Canada the Asian longhorned beetle (Anoplophora glabripennis) threatens the hardwood and maple syrup industries, while the impacts of yellow star thistle (Centaurea solstialis) on cattle production have cost Californian ranchers and the state an estimated USD 17 million (Eagle et al., 2007). In French Polynesia and other Pacific islands, Miconia calvescens (an introduced tropical American tree), has shaded out native plant species in some areas and, due to its shallow rooting habit, increased erosion and frequency of landslides (Meyer and Malet, 1997; Environment Canada, 2004; Moore, 2005).
Scale and fragmentation issues
Many of the interactions described above are influenced by the scale and spatial configuration of different land use patches. The process of forest fragmentation, occurring when formerly forested lands are converted permanently to pastures, agricultural fields, or human-inhabited developed areas, can result in changes in ecosystem functions that alter the supply and distribution of ecosystem services vital for agriculture (Tscharntke et al., 2012). Reduced connectivity of forest patches affects the ability of pollinators, pest predators (Tscharntke et al., 2005b; Kremen, 2005), water and nutrients (Brauman et al., 2007; Power, 2010) to move across a landscape. However, there is growing evidence that in agricultural landscapes forest fragments continue to provide ecosystem services, including pollination and pest control services (Ricketts, 2004; Ricketts et al., 2008; Holzschuh et al., 2010), water regulation and purification services (Foley et al., 2005). Forest fragments in agricultural landscapes can also change dispersal patterns for fungi and soil organisms that affect decomposition (Plantegenest et al., 2007). In some cases, managing landscape configuration to enhance forest fragment connectivity may be a more effective tool for optimising agricultural landscapes for multiple ecosystem services rather than simply limiting further forest loss (Mitchell et al., 2014). It is however important that sufficiently large forest patches and connectivity are maintained, as high levels of forest loss can result in abrupt landscape-scale loss of native forest specialist species in the long term (Pardini et al., 2010).
In many parts of the world, traditional agricultural landscape management approaches have been developed to more closely link agricultural and forest (or woodland) management and ensure continuity in the provision of ecosystem services from forests. For example, Japan’s traditional socio-ecological production landscapes, known as satoyama (“sato” =home village; “yama” =wooded hills and mountains), comprise integral social and ecological networks of villages and their surrounding agricultural lands, open forestlands and forests, in which forests are managed for multiple values, including biodiversity conservation and the ecosystem services that forests and woodlands provide to agriculture (Indrawan et al., 2014). Similar landscape management systems are found throughout Asia and elsewhere in forms that are adjusted to regional biophysical conditions (e.g. Agnoletti, 2006; Bélair et al., 2010; Johann et al., 2012; Kumar and Takeuchi, 2009; Ramakrishnan et al., 2012; Youn et al., 2012).
3.3.2 The Influence of Landscape Use and Management of Forests and Tree-based Systems on Nutrition
Many factors influence the actual or potential contributions of forests and tree-based systems to food security and nutrition of producers, their families and other consumers. These include the productivity of these management systems, the resilience of these systems to withstand shocks (weather and other events), the choice of food species cultivated and managed, and the extent to which the food products are utilised for household or local consumption, or marketed to earn income which may then be used to purchase other foods. The variety of forest and tree management practices that typically co-exist within rural landscapes may contribute to the broader food system in varying degrees, since a substantial portion of people’s diet is often traded or purchased (Powell et al., 2015).
Two main types of studies can be used to evaluate how different landscape, forest and tree management approaches may impact nutrition. The first type involves studies that compare the diets of one or more ethnic groups at different stages of transition from one livelihood strategy to another, with the different livelihood strategies having different land use patterns. A selection of such studies and their main results are summarised in Table 3.4.
Table 3.4 Studies examining differences in diet between groups during livelihood and land use transitions.
|
Transition/Location |
Findings related to diet |
Study |
|
Shifting cultivation to plough-farming in the Philippines |
Two Tiruray communities at opposite poles of this transition were studied. Hunting, fishing, and gathering of wild resources have virtually disappeared. The traditional communities had lower average intake of energy, protein, fat, calcium, iron, vitamin A and higher average intake of thiamine and riboflavin (B vitamins) compared to those in sedentary agriculture. |
|
|
Comparison of diets of tribes with settled/paddy-based agriculture, to those with shifting cultivation and those with hunting and gathering, in India |
A comparison of tribes from northeast India shows that those that engaged in the most hunting (Padams) had highest percent energy from protein, highest iron, calcium and vitamin A intake. The tribe with least animal source foods (Noktoe) had second highest vitamin A intake, likely due to greater dependence on wild and cultivated vegetables. The tribes practising mixed shifting and paddy cultivation (Padam, Minyong and Galongs) had better diets than those without paddy cultivation (Nokte). In central and western India, a hunter-gatherer forest dwelling tribe (Marias) had lowest calcium, iron and vitamin A intake. Forest dwelling subsistence agriculture tribe (Baiga) had highest iron, vitamin A, compared to settled rice-based agricultural tribe (Gonds), despite much higher energy intake by Gonds. |
|
|
Hunter-gatherers in transition to settled agro-pastoralism; San of /ai/ai, in Botswana |
Traditional (hunting and gathering): Percentage of caloric intake from: vegetables (85), meat (12), milk (1), maize (2). Mixed (diet of wild and domestic food): Percentage of caloric intake from: vegetables (65), meat (11), milk (17), maize (7). Settled (agro-pastoralism): Percentage of caloric intake from: vegetables (10), meat (10), milk (29), maize (43), sugar (9). Settled communities have much lower contribution to diet from vegetables and meat and much greater intake of milk, maize meal and sugar. |
|
|
Comparing hunter-gatherers to neighbouring agricultural communities in Cameroon |
Yassa: Agriculture and fish-based subsistence. Average daily per capita intake: 34g of vegetables; 199g fish; 24g meat. Mvae: Subsistence based on agriculture and hunting (in forest and on coast).Average daily per capita intake: 100g vegetables; 62g fish; 129g meat. Bakola: hunter-gatherer based subsistence. Average daily per capita intake: 54g vegetables; 22g fish; 216g meat. Much higher intake of meat and high animal source food intake in hunter-gatherer group, higher vegetable consumption in agricultural community. |
|
|
Hunter-gatherer to sedentary urban/agriculture in Borneo |
Remote/traditional communities had more diverse diets with more meat, better nutritional status and physical fitness and greater contribution of forest resources to diet compared to sedentary agricultural or urban communities. |
|
|
Hunter-gatherer to market-oriented rice cultivation in Borneo |
People in resettled area with better access to markets, where people’s livelihood strategies focus on market-oriented rice production had poorer diets compared to those in a remote area (possibly due to lower use of wild foods and less time for production of non-staples) |
|
|
Agricultural community in forested landscape mosaic, transition after introduction of payments for ecosystem services (PES) in Mexico |
Community perceived loss of food security, and greater dependence on purchased food. They perceived lower maize yields due to shorter fallows (less agricultural land/no new land available), lower meat consumption (no more hunting, all meat now has to be purchased and the money from PES cannot fully compensate for loss of hunting). |

Village near Corbett National Park, India.
Photo © PJ Stephenson
Other studies that have compared the capacity of different forests and tree-based systems to produce nutritionally-important foods such as fruits and vegetables and animal sources of foods (usually done by modelling) offer insights as to their relative contribution to diet and nutrition. Differences in the diets of traditional hunter-gatherer communities and neighbouring agricultural ones in India seem to be very context specific (sometime better, sometimes worse). In many places more traditional subsistence groups had more meat in their diets, based on studies from India (Gupta, 1980), Cameroon (Koppert et al., 1993), Borneo (Colfer, 2008a; Dounias et al., 2007) and Botswana (Hausman and Wilmsen, 1985). Comparing primary forests with secondary or heavily modified forest systems, the latter provide a greater number and quantity of useful plant species (but not always animal species) than primary forests, based on studies from the Brazilian (Parry et al., 2009), Bolivian (Toledo and Salick, 2006) and Peruvian Amazon (Gavin, 2004) and from Panama (Smith, 2005). Considering shifting cultivation, the abandonment of this practice may be associated with less use of wild foods including wild meat and vegetables (and uptake of micronutrients such as iron and vitamin A), but the few existing studies have not demonstrated that shifting cultivation is associated with better dietary intake, based on studies in the Philippines (Schlegel and Guthrie, 1973) and India (Gupta, 1980). Complex agroforests have been found more likely to provide enough fruits and nutrients per unit of land than less diverse agroforestry systems, based on results of farm modelling studies from Central America and West Java) (Cerda et al., 2014; Marten and Abdoellah, 1988). Regarding home gardens, four separate reviews of the impacts of agricultural interventions on nutrition outcomes all concluded that there is convincing evidence for the positive impact of home garden interventions on nutrition, especially access to fruits and vegetables and intake of vitamin A (Berti et al., 2004; Girard et al., 2012; Masset et al., 2012; Powell et al., 2015; Tontisirin et al., 2002).
More research is needed into the detailed contribution of different forms of forest and tree management systems to nutrition.
3.4 The Socio-economic Organisation of
Forests and Tree-based Systems
The viability of production system options available to farmers, including forests and tree-based systems, is influenced by an array of biophysical and socio-economic factors. Understanding both the opportunities and constraints on the retention or adoption of these production options is of prime importance to all concerned with enhancing the food security and nutrition of farmers and rural communities as well as the urban and increasingly globalised populations whose food they produce.
Challenges faced by families and communities that rely on forests and tree-based systems for their food security and nutrition include heterogeneous and unpredictable environmental conditions (e.g. unpredictable weather exacerbated by climate change, fragile and/or marginal soils), forest degradation, deforestation and associated biodiversity losses. Production systems are also embedded in underlying “invisible” social, economic and political structures, and are influenced by social and cultural norms, values, beliefs, customs and traditions. Such factors determine social and gender relations and their interaction within production systems, and shape the cultural identities of different ethnic and social groups and communities and indigenous peoples, and their food and livelihood preferences and choices. Social, economic and political structures also embody power relations which determine access to land, trees and other productive resources, and participation by different stakeholders in forest and natural resource governance mechanisms and the resulting outcomes in terms of resource appropriation or sharing and conflict resolution.
The socio-economic organisation in the four production systems identified earlier in this chapter is highly diverse and complex, with considerable variations between and within continents and countries. Even a single landscape often comprises peoples or social groups of different ethnic or religious affiliation, class, caste, political ideology or agricultural profession (pastoralists, sedentary farmers, foresters, plantation managers, hunters and gatherers) who may have overlapping, complementary or quite distinct production systems.
This section concentrates on the three factors directly affecting the socio-economic organisation of production: land and tree tenure, gender relations, human capital and financial capital (including credit), with a focus at the community and household level. These factors and their interrelationships are constantly evolving in response to external changes that include: shocks (such as drought, disease, food price hikes), longer-term climate change trends, public action (policies, laws, administrative procedures), infrastructrure development, innovations and new technologies, improved extension services, changes in governance frameworks and institutions, popular demand voiced through protest and social movements, and new opportunities brought about by changes in markets for land, labour, agricultural and tree products, and forest sub-soil resources (such as minerals, fossil fuels). While the drivers of these changes are discussed in Chapter 4, the implications for the socio-economic organisation of production in forests and tree-based systems are addressed in this section, with particular focus on the livelihoods, food security and nutrition of the poor.
3.4.2 Land, Tree and Related Natural Resource Tenure
The four forest- and tree-based systems described earlier in this chapter (Section 3.2) are governed by a web of highly complex land tenure systems in which rights to land, trees and other natural resources such as water are commonly categorised as: private, communal, open access and state (Box 3.3). The related tenure rights can be defined through formal or statutory legal arrangements (de jure), which predominate in private or state land, or by customary practices (de facto) which are prevalent in communal and open access regimes.
Box 3.3 Land tenure categories
Representing the relationship, whether legally or customarily defined, among people, as individuals or groups, with respect to land (including land-related natural resources such as water and trees), land tenure is commonly categorised as:
Private: the assignment of rights to a private party who may be an individual, a married couple, a group of people, or a corporate body such as a commercial entity or non-profit organisation. For example, within a community, individual families may have exclusive rights to agricultural parcels and certain trees. Other members of the community can be excluded from using these resources without the consent of those who hold the rights.
Communal: a right of commons may exist within a community where each member has a right to use independently the holdings of the community. For example, members of a community may have the right to graze cattle on a common pasture.
Open access: specific rights are not assigned to anyone and no-one can be excluded.
State: property rights are assigned to some authority in the public sector. For example, in some countries, forest lands may fall under the mandate of the state, whether at a central or decentralised level of government.
Source: FAO, 2002a.
Note: The rights to subsoil resources such as minerals, natural gas and oil are almost always reserved for the state (RRI, 2012).
Forests and tree-based systems are characterised by different land right regimes (defined in Table 3.5), though there are marked context-specific variations in practice. Shifting cultivation is practised generally on land that is not privately owned while agroforestry is commonly practised on private land in South Asia, parts of North Africa, and Europe and on communal land in sub-Saharan Africa. Plantations and smaller tree crop stands grown by corporations/large farmers and smallholders respectively are usually on private land which provides the tenure security needed to protect costly, long-term investments. However, in countries where communal tenure is fairly secure, smallholder tree crops are also found on communal land (for example, cocoa trees in Ghana (Quisumbing et al., 2003), or oil palm on collectively-held customary land in Indonesia (Li, 2014)). Corporations quite commonly lease state land for tree plantations, for example, in Indonesia for oil palm (Li, 2014) and in many countries in Southeast Asia, Africa and Latin America for industrial timber concessions (c.f. Hatcher and Bailey, 2010). Finally, all four types of tenure can apply to managed forests, with the actual distribution by tenure varying by region and country.
Table 3.5. Generalised overview of types of tenure rights associated with
forests and tree-based systems.
|
Forest/Tree-based system |
Rights |
|||
|
Private |
Communal |
Open Access |
State |
|
|
Managed forest |
✔ |
✔ |
✔ |
✔ |
|
Shifting cultivation |
✔ |
✔ |
✔ |
|
|
Agroforestry |
✔ |
✔ |
✔ |
|
|
Single-species tree crop systems |
✔ |
✔ |
✔ |
|
Bundles of rights, incentives and food security
In practice, different tenure regimes can co-exist in the same landscape, and even within some tenure regimes two or more individuals or groups can have different rights to a specific area of land or related natural resources (such as trees), either simultaneously or in different seasons. Thus it is useful to think of “bundles of rights” that can be held by different holders of the rights (FAO, 2002a; Bomuhangi et al., 2011). A frequently-used classification, developed by Schlager and Ostrom (1992), distinguishes: access, withdrawal, management, exclusion and alienation rights. Access rights enable entry to the land, such as the right to walk in a forest. Withdrawal rights include the right to take something from the land, such as forest foods, firewood, timber. While in many countries communities have withdrawal rights for subsistence or small scale commercial activities, in some cases such as Thailand, legislation does not recognise customary rights of forest communities, rather criminalising extraction of forest products and land occupation (RRI, 2012). Management rights cover the right to use or change the land, such as to plant trees or crops or to graze animals, or to make improvements to the land, such as better water management. In many countries, traditional management systems developed by local communities and indigenous people to regulate access and withdrawal rights by community members have been replaced by government-authorised systems, subject to certain conditions. These can bring benefits, for example, in reducing deforestation and increasing community access to fuelwood and fodder and control over NTFPs, but they can also weaken a community’s capacity to function flexibly and effectively to meet community needs for food and other livelihood requirements (Larson et al., 2010; RRI, 2012; Barry and Meinzen-Dick, 2014). Exclusion rights prevent others from using the land or resource, while alienation rights enable the transfer of land to others, by sale, lease or bequest.
Table 3.6 illustrates the complexity of these bundles of rights for the four forest- and tree-based systems. While not compatible with systems of shifting cultivation, private tenure permits all five rights (i.e. “full ownership”) in the other three systems. Communal right regimes operate in all four systems, and are particularly extensive in Latin America and Africa. They are usually managed by (informal) community mechanisms (sometimes government-authorised under specific conditions) and enjoy some exclusion rights. Importantly, they do not have alienation rights. Open access regimes are confined to shifting cultivation and, in a few countries, some managed forests, where users only have access and withdrawal rights. Finally, in most countries the state owns the major share of managed forests and tree plantations, commonly delegating management rights to state bodies and/or formal community organisations under strict conditions, or leasing land for tree plantations to corporate bodies with all rights except alienation.
Table 3.6 Bundles of rights typically associated with different forest- and tree-based systems.
Source: Adapted by authors from FAO, 2002b and Schlager and Ostrom, 1992. (The tenure categories are taken from FAO, 2002b, given in Box 3.3, and also used in Table 3.5).
|
Forest/Tree based Systems and Tenure |
Rights |
||||
|
Access |
Withdrawal |
Management |
Exclusion |
Alienation |
|
|
Managed forest |
|||||
|
Private |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Communal |
✔ |
✔ |
CG |
CG |
X |
|
Open Access |
✔ |
✔ |
X |
X |
X |
|
State |
✔ |
✔ |
✔ |
SB / CG (CO) |
✔ |
|
Shifting cultivation |
|||||
|
Communal |
✔ |
✔ |
CG |
CG |
X |
|
Open Access |
✔ |
✔ |
X |
X |
X |
|
State |
✔ |
✔ |
✔ |
CG (CO) |
✔ |
|
Agroforestry |
|||||
|
Private |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Communal |
✔ |
✔ |
CG |
CG |
X |
|
State |
✔ |
✔ |
✔ |
SB (CO) |
✔ |
|
Single-species Tree Crop systems |
|||||
|
Private |
✔ |
✔ |
✔ |
✔ |
✔ |
|
Communal |
✔ |
✔ |
CG |
CG |
X |
|
State |
SB / CB |
SB / CB |
SB / CB |
SB / CB |
SB |
(CG) Traditional Community Groups; (CB) Corporate Bodies; (SB) State Body; (CO) Community Organisation with formal/legal rights and obligations. X = Not permitted
More recently, the Schlager and Ostrom (1992) classification has been expanded (RRI, 2012; Stevens et al., 2014) to include the dimensions of duration and extinguishability.
Duration considers whether the rights are held in perpetuity or for a limited time period. Permanent rights are vital to safeguard the sovereignty and autonomy of indigenous peoples (RRI, 2012) and because “indigenous people’s right to food is inseparable from their right to land, territories and resources, culture and self-determination” (Damman et al., 2013). Often, in customary systems the duration of rights is determined by evidence of continuous use (e.g. in Meghalaya, India (Kumar and Nongkynrih, 2005); and in Gambia (Dey, 1981)). Long-term rights provide security and incentives to invest and maintain sustainable forest and tree management practices (RRI, 2012). In Viet Nam, for example, long term (50 years or more) use rights to forest lands have been secured through Land Use Certificates, with a total of 1.8 million certificates having been issued by December 2010 (FAO, 2014).
The right of extinguishability ensures “due process and compensation” when governments exercise their universal right of “eminent domain” to expropriate lands for the “public good”. While private land owners as well as communities and indigenous peoples with de jure use rights to state or communal forest land generally have legal entitlements to due process and compensation, communities with de facto rights are vulnerable to losing their land and their livelihoods (RRI, 2012). For example, herders in Mongolia protested at government issuance of gold mining rights to national and foreign companies, as they lost pastures and forests and their water was polluted by the mines (New Zealand Nature Institute, 2006). Logging concessions as well as illegal logging on indigenous peoples’ land in Indonesia and Peru, have displaced thousands of people from forests on which they depend for their food and livelihoods (United Nations, 2009). Even with official de jure rights, in many instances weak government protection may make it difficult for communities to assert their rights. For example, Peru and Colombia have ratified various international conventions and covenants regarding indigenous peoples and the right to adequate food for all, and have demarcated and titled a large part of indigenous and community land, yet they have authorised hydrocarbon and mining companies to operate on this land, without consultation or consent by the indigenous peoples and communities concerned.
Multiple rights to a specific parcel of land or to specific natural resources on it can be held simultaneously or successsively by several people or groups (Bruce, 1999; Fuys and Dohrn, 2010). These complex rights mean that even a single landscape that might contain forests, agroforestry with trees, crops, pastures and animals, and lakes/rivers, would be subject to a web of different property rights regimes or, as conceptualised by Bruce (1999), “tenure niches”. For food security and livelihoods, it is important to recognise that these “bundles of rights” can be further broken down, with different individuals, families, kinship and other groups (cross-cut by gender, class and agricultural specialisation) accessing different “rights” to the same resources. The exercise of these rights can be complementary, for example, where some people (especially men) may have ownership or usufruct rights to trees, and others (especially women) to certain products from these trees such as fruit and small branches for fuel (Rocheleau and Edmunds, 1997). In Zimbabwe for example, in communal tenure systems among the Baganda, only men use fig trees (Ficus natalensis) to produce bark cloth, hang beehives and create boundaries while only women use figs for soil improvement and as shade for other crops. In northern Thailand upland residents have rights to collect bamboo on individually-owned lowland farms (Fuys and Dohrn, 2010).
Rights to trees may be different from rights to the land on which they grow, particularly in the case of customary tenure systems (Howard and Nabanoga, 2007). However, even under private tenure, they may be different, for example, in Morocco the state owns argan trees even if they are grown on private land (Biermayr-Jenzano et al., 2014). Under customary tenure, an individual’s rights to trees may depend on his/her rights to the land on which they are grown, while planting trees can also establish rights to land. However, bundles of rights to trees and their products can also be held by different individuals (with or without the land ownership or use rights), simultaneously or at different times, for different purposes (Fortmann and Bruce, 1988). These rights are often nested and layered in space as well as among rights holders, creating differential entitlements to benefits that are also related to the broader social structures (Howard and Nabanoga, 2007), and the social and religious/spiritual norms, values and practices of the concerned communities.
The exercise of multiple rights can cause conflicts despite the existence of mediation mechanisms (Bruce, 1999). For example, in the state-owned argan forest areas in southwestern Morocco, tensions are rife between nomadic camel and goat herders with grazing rights and local residents with rights to exploit the argan fruit (Biermayr-Jenzano et al., 2014). In Senegal, disputes between Wolof farmers and Peul herders over the use of branches from the baobab trees for fodder undermined the Peuls’ food security and livelihoods. These disputes were exacerbated by a government decree protecting the baobab tree (Rose, 1996).
As Schlager and Ostrom observe (1992) “Different bundles of property rights, whether they are de facto or de jure, affect the incentives individuals face, the types of actions they take, and the outcomes they achieve”. These rights are ultimately critical for ensuring food security and nutrition.
3.4.3 Gender, Rights to Land and Trees, and Food Security
Reviewing country-level statistics and a large number of field studies, Lastarria-Cornhiel et al. (2014) conclude that most land tenure systems are gender-biased, allocating primary rights to land to male members of the community and family. Gender differences in ownership or use rights to trees are particularly complex and vary by culture. In many countries, trees on state, community or open access land belong to the state. Women in matrilineal systems often have stronger rights, though sometimes these are controlled by their brothers or maternal uncles. Gender differences in the way land is accessed also contribute to differences in tenure security. In sub-Saharan Africa, men often acquire use and management rights to land through inheritance or allocation by their clan or lineage, while women more commonly acquire temporary use rights (and occasionally permanent rights) through marriage and to a considerably lesser extent through fathers and brothers (Rocheleau and Edmunds, 1997; Howard and Nabanoga, 2007; Kiptot and Franzel, 2012; Meinzen-Dick et al., 2014; Lastarria-Cornhiel et al., 2014). In such customary systems, women frequently lose their land use rights if their marriages are dissolved (through separation, divorce or death of their spouse), particularly if they do not have sons. In Latin America, women are more likely to acquire land through inheritance (so their rights are not affected if their marriages dissolve) and men through purchases in land markets (Doss et al., 2008). Paradoxically, the emergence of land rental markets in customary systems, particularly in sub-Saharan Africa, can facilitate women’s access to land as male owners are more ready to rent to women because they are prohibited from acquiring permanent land rights (Giovarelli, 2006; Lastarria-Cornhiel et al., 2014).
Rural men and women often acquire different types of assets (Meinzen-Dick et al., 2014). Men are more likely to own large livestock such as cattle and buffaloes and women small livestock such as poultry and goats (Kristjanson et al., 2014). In rural Philippines women tend to have higher educational levels (and thus better access to non-farm work) while their brothers are more likely to inherit family land (Quisumbing et al., 2004). In Asia, women are more likely to own jewellery, and men are more likely to own land and assets such as farm equipment and vehicles (Agarwal, 1994b; Antonpoulos and Floro, 2005, cited in Meinzen-Dick et al., 2014).
Where the state owns trees, the use rights are either vested in the community, which exercises management responsibilities or in the male leaders of the lineage or households (Rocheleau and Edmunds, 1997). Often the effectiveness of women’s rights depends on their voice in local institutions that are commonly male-dominated (Agarwal, 2010; Lastarria-Cornhiel et al., 2014). In the case of community-owned land and state land managed by communities, women often have secondary rights legitimised through their relationship to men. Howard and Nabanoga (2007) found highly complex gender-differentiated rights to trees and their products among the Baganda in Uganda that varied according to their location in homesteads, croplands, common lands or state forests. While only men owned trees on private land, women’s customary rights to plant resources in gendered spaces on common or state land were as strong as men’s. Rocheleau and Edmunds’ (1997) review of studies in Africa also found that women’s rights are substantial, particularly in customary systems where they have rights to fuelwood, medicinal plants and wild foods in the “bush” or forests, in “in-between” spaces not valued by men, such as bush along roadsides, fences, and boundaries between men’s trees and crops, as well as home gardens near their houses, and also to certain tree products (e.g. fruit, fuelwood, leaves, fodder) growing on men’s land. Agarwal (1994b) found that in Sri Lanka women sometimes received coconut trees as dowry and their brothers would periodically send them a share of the harvest.
However, these cases cannot be generalised, even in customary systems. For example, in Ghana, women have been able to acquire their own trees, through acquisition of private land through the market and sale of cash crops such as cocoa (Berry, 1989, 1993 cited in Rocheleau and Edmond, 1997; Lastarria-Cornhiel, 1997) or as gifts of cocoa trees from their husbands in compensation for their labour on the men’s cocoa trees (Quisumbing et al., 2003). In the Colombian Pacific region, Afro-Colombians have highly complex tenure systems that permit both men and women to own trees that they have planted or inherited, and their products such as fruit and tree snails (Asher, 2009).

Forest and agriculture mosaic landscape, Cat Ba, Vietnam. Photo © Terry Sunderland
The nature and security of women’s rights to land, trees and their products are of central importance to ensuring household food security. Gender differences in the types and relative sizes of productive assets and control of income are critical for food security as a large body of evidence shows that women are more likely to spend their income (from their own production or wage labour) on food, healthcare and education of their children (Haddad et al., 1997; Agarwal, 1997; Njuki et al., 2011; FAO, 2011; Kennedy and Peters, 1992; Duflo and Udry, 2004; Meinzen-Dick et al., 2014).
The interrelationships between women’s rights to trees and their products and household food security and nutrition raise two major issues. The first is the need for women’s security of tenure. This is clearly demonstrated by Fortmann et al. (1997), who found in their study of two Zimbabwe villages in the communal areas that women were much less likely than men to plant fruit and other trees within the homestead or on household woodlots because the trees and their produce belonged to their husbands (as household head), and they lost their use rights to the produce if he died or they divorced (even if they still lived nearby). However, both men and women were equally likely to plant trees on community woodlots where the duration of their rights to the trees was secure as long as they remained village residents. Furthermore, while richer men planted considerably more trees than poor men, indicating a greater ability to engage in commercial production, this was not the case for richer women who planted a few trees for subsistence and had less risky ways of earning, such as producing annual crops for sale, beer brewing and handicraft sales.
The second issue is the complementarity between men’s and women’s access to different products from the same trees, sometimes in different seasons, and from different tenure systems. For example, in Uganda, jackfruits located in different areas are used differently by men and women. Women reported 60 percent of uses in homegardens, which were mainly for subsistence especially during periods of food shortage (they use leaves for fodder and medicine) while men reported over 80 percent of uses on croplands that were for sale and subsistence, as well as fuel. Jackfruits on common land and in state forests were only used for subsistence fuel (Howard and Nabanoga, 2007).
Land ownership or use rights may not be sufficient to exercise control over the use, management and the products of trees on their land (Agarwal, 1994a; Rocheleau and Edmunds, 1997; Deere et al., 2013). Even where women have land ownership rights, research in the Gender Asset Gap Project in Ecuador, Ghana and the state of Karnataka in India found that land did not automatically translate into decision-making on what to grow, how much of the crop to sell, and over the use of the income generated from crop sales (Deere et al., 2013).
3.4.4 Human Capital, Control and Decision-making in Forests and Tree-based Systems
Rights to forests and trees and their products are embedded in the broader social systems that also determine access to human and financial capital, decision-making processes and control of the products or income from their sale, thus affecting the way in which these property rights are used. Since social systems are not static, these rights can be negotiated or changed over time (Meinzen-Dick et al., 1997; Rocheleau and Edmunds, 1997).
In many customary and open access tenure systems, the notion that individuals own their labour power and the products of their labour is widespread. Rights to forest land and trees are commonly established by the act of clearing primary forest. For example, the Lauje in Sulawesi, Indonesia, considered that the person who invested labour in clearing land or planting trees owned the land and the trees, and could alienate these through gift, sale or exchange (Li, 1998). Similarly, in sub-Saharan Africa, rights to land are derived from the labour expended to clear or cultivate the land. Land is commonly held under lineage-based systems, in which a male lineage member is entitled to land to support his family, and can use this as long as it is being cultivated. His heirs would normally be given the land that was cultivated at the time of his death (Platteau, 1992). Women are sometimes prevented by men from clearing land, for example, in The Gambia, as this would make the land “women’s property” and their husbands or other male relatives would have no control over it if their husbands died or they divorced (Dey, 1981).
In open access and communal forest systems (including local and indigenous communities’ formal or informal use of state land), the availability of human capital (commonly proxied as labour and education (Meinzen-Dick et al., 2014), though also covering traditional knowledge and skills and health that are less easily quantified) is one of the main factors affecting the ability of an individual, household or community to clear, maintain, and use forests and tree products. While labour is a key factor, specialised knowledge and skills that are often gender- and age-specific are also critical. For example, women often specialise in forest medicinal plants and fuelwood, and men in hunting wild animals for food, while either may have rich knowledge of other foods and fodder, depending on their cultures.
Often very poor families with few resources except their labour are highly dependent on forest products for their food security and livelihoods (Jodha,1986; Fisher, 2004; Adhikari, 2005; Narain et al., 2008). However, the literature indicates that while resource dependence (defined by Narain et al., 2008, as the share of resource income in overall income) tends to decline with overall income, the relationships are complex and there is no consistent trend. For example, Fisher (2004) and Narain et al. (2008) found that forest income declined with the household head’s level of education in Malawi and Madhya Pradesh (India); similarly, Adhikari (2005) found that in Nepal, forest income declined with the household’s average level of education. Both Adhikari and Narain et al. found that forest income increased with household livestock holdings as such households required more fodder. The results were also affected by the availability and type of labour, and by education/skills.
More remote villages may have higher dependence on forest resources as they have fewer opportunities for off-village labour, and are likely to have higher costs for purchasing resources and food (Narain et al., 2008). Duchelle et al. (2014) found that in the more remote communities in Pando (Bolivia) forest income made up 64 percent of total household income compared with only 12 percent in the region of Acre in Brazil, just across the border, which is better connected to markets and towns, and off-farm work opportunities.
Agroforestry systems (on private or communal land), woodlots and small tree stands are becoming an increasingly important smallholder livelihood strategy in many countries for a variety of reasons (see Section 3.2.4) of which a critical one is labour. Trees demand less labour than most field crops and are attractive where labour is scarce, expensive or difficult to manage. Households with sufficient income from non-farm sources, which therefore may not need to cultivate their land intensively, may also plant trees to provide food and other products, or to retain surplus land as an alternative to renting out or selling the land (Arnold and Dewees, 1998).
Shortages of labour (especially male labour) as well as land are leading to shorter fallows and longer cultivation periods in many shifting cultivation systems (Hunt, 1984; AIPP and IWGIA, 2014). Land shortages, for example, in the uplands of Southeast Asia, are the result of increasing population densities from endogenous growth and in-migration by large numbers of lowlanders, as well as loss of access to land taken over by the governments (Cairns and Garrity, 1999). Analyses of studies from across Southeast Asia have shown that increasing returns to labour is usually much more important than increasing yields per unit of land area (Cairns and Garrity, 1999).
The intrahousehold division of labour and control of the product, by gender and age, is highly complex across and within forests and tree-based systems, regions, countries and cultures. In many cases women provide substantial labour and management of particular forest/tree products but men control the disposal or marketing of these products and the distribution/use of the benefits (World Bank et al,. 2009; Rocheleau and Edmunds, 1997). Case studies in seven Asian countries showed that indigenous women perform about 70 percent of the work in shifting cultivation. Men identify suitable land and do the hard physical work in land preparation. Women also help in clearing the land, selecting seeds and weeding, while both men and women harvest and conduct the rituals during the cultivation cycle together (AIPP and IWGIA, 2014). In some parts of Africa, women are involved in small retailing of forest products and men in wholesale trade (Kiptot and Franzel, 2012). This may affect incentives to increase production and sustainable resource management, with negative implications for improving food security and livelihoods. Based on her field work in Africa, Whitehead (1985) distinguishes between sex-sequential labour processes on a single product and sex-segregated labour processes on similar or different products. She considered women’s claim on the product of their labour to be weaker in the first case, as their contributions were submerged in the conjugal role. In contrast, in Southeast Asia, Li (1998) found that the key issue was not the division of labour itself but the extent to which labour investment is directly connected to the creation of the property.
Women are often disadvantaged in access to and control of agricultural labour (Dey Abbas, 1997; FAO, 2011; Hill and Vigneri, 2014). Kumar and Quisumbing (2012) found that in Ethiopia, female-headed households tended to be smaller than male-headed households, and have a larger proportion of female members which disadvantaged them as many agricultural operations are male-intensive. This is particularly the case for ploughing, a task which cultural norms proscribe for women. Similar constraints were reported for Botswana (Fortmann, 1983; Peters, 1986) and Zambia (Feldstein and Poats, 1990). In many sub-Saharan African countries, women are also obliged by custom to provide labour, food and sometimes cash crops for male-controlled households. These obligations often take precedence over women’s rights to work on their personal fields, trees or other income-generating activities (Dey Abbas, 1997; van Koppen, 1990; Hill and Vigneri, 2014). Women also have heavy domestic demands on their labour, which limits the time they can spare for their agricultural work (Quisumbing and Pandofelli, 2009).
Interestingly, despite women’s labour and cash/credit constraints, female-managed cocoa farms in Ghana were as productive as male-managed farms (Hill and Vigneri, 2014). Women were able to compensate by using labour exchange groups and relying more on labour-intensive production methods rather than the use of purchased modern inputs. This balancing of labour and non-labour inputs confirms the review of evidence in FAO (2011) that women are as productive as men, if they have the same level of inputs.
3.4.5 Financial Capital and Credit: Using and Investing in Forests and Trees
Financial capital includes savings/debt (including in banks, credit unions, cooperatives, informal savings clubs or tontines), gold/jewellery income, credit, insurance, state transfers and remittances (Carloni, 2005; IFPRI, 2013). Savings are often in the form of livestock assets, for example, as is the case in Acre (Brazil) (Duchelle et al., 2014).
It is frequently argued that poor households (especially those headed by women) are more dependent on forest resources for food and income than richer households although the evidence is mixed (Adhikari, 2005). A growing body of evidence suggests that the role of capital and/or credit is critical in enabling households or individuals to exploit forest resources. For example, a study by Adhikari (2005) in Nepal found that households with land and livestock assets gained more from community forests because they were able to make greater use of intermediate forest products such as leaf litter, fodder and grass products. Female-headed households benefitted less than male-headed households, as they had fewer livestock and had minimal involvement as office bearers in the forest user groups. These findings are consistent with those of Velded (2000) who found that the benefits from common grazing land among the Fulani in Mali were exclusively related to capital, technology and skill levels, and those of Narain et al. (2008) in relation to complementarity of asset ownership in Jhabua (India).
For the majority of smallholders in local or indigenous communities, forest income is often insufficient to support investment in forest and tree resources. A number of countries have introduced small grants and microcredit schemes for smallholders, sometimes through the mechanisms of producer cooperatives or, particularly in Latin America, by facilitating relations beween banks and small forestry producers (FAO, 2014). In Viet Nam, through its 2007 Decision 147 on the promotion of forests for productive purposes, the government encouraged households to engage in the plan to establish 250,000 ha of new plantations per year till 2015 by providing low credit rates for smallholders (FAO, 2014).
These schemes seem to neglect earlier evidence (Arnold and Dewees, 1998) which showed that tree planting only requires low inputs of capital and that subsidies can lead to adoption of inappropriate tree species or lead to distortions in land use. Arnold and Dewees (1998) also refer to widespread evidence that seedling distribution, fertiliser and cash subsidies tend to be captured by larger farmers, who are not food insecure.
The adaptation of shifting cultivation systems to “dual economies” among many indigenous communities in Asia reflects also the importance of improved market access as well as greater opportunities to access credit or wage labour to invest earnings in farming and improve food security and livelihoods (AIPP and IWGIA, 2014). The report by AIPP and IWGIA (2014) provides examples of resulting innovative combinations of shifting cultivation with agroforestry (e.g. fruit and cashew orchards in Cambodia, rubber gardens in Indonesia), growing high value cash crops in shifting cultivation fields (e.g. vegetables, herbs, ginger, turmeric in India and Bangladesh), establishing separate, permanent fields for cash crops (e.g. tobacco, maize, flowers, pineapple, vegetables in Thailand, India, Bangladesh) and improving fallow management by planting specific trees in India.
Numerous studies cite evidence that women generally have less access to capital than men. They are often prevented by social norms or their heavy domestic and caring work from engaging in paid work outside the home or community (where wages are generally lower than in more distant, urban, jobs) and have less capacity to establish or buy tree gardens (Li, 1998). Women’s lack of financial capital is often cited as a reason for their greater dependence on common property resources, as in Ethiopia (Howard and Smith, 2006).

Women selling mangoes in a roadside market in Guinea.
Photo © Terry Sunderland
Forests and tree-based systems have historically played a major role in supporting livelihoods as well as meeting the food security and nutritional needs of people worldwide. These systems, including natural forests that are managed to optimise yields of wild foods and fodder, shifting cultivation, a wide variety of agroforestry systems and single-species tree crops, are still dominant components of rural landscapes in many parts of the world, and remain critical to food security and nutrition of hundreds of millions of people worldwide.
They offer a number of advantages over permanent (crop) agriculture given their adaptability to a broader range of environmental conditions (e.g. soils, topography and climate) and changing socio-economic conditions and the diversity of food products derived from them.
Most forests and tree-based systems we see in the world today – particularly managed forests, shifting cultivation and agroforestry systems – are underpinned by the accumulated traditional knowledge of local and indigenous communities. This knowledge has been crucial to the development and modification of these systems over generations under diverse and variable environmental conditions and to meet changing socio-economic needs.
Only rarely and relatively recently have agricultural and forest scientists, extension agents and development organisations begun to understand the importance and relevance of many of these systems, and begun to work with farmers to combine the best of traditional and formal scientific knowledge to enhance their productivity and direct (food security and nutrition) and indirect (income) benefits to their practitioners.
Despite their widespread use, particularly in regions of the world where food security and nutrition are of particular concern, the data needed for decision-makers to make informed choices is quite limited, especially at the global and national level. Further research is needed on: the actual extent of most of these systems, the numbers of people who rely on one or more such systems to meet their household food and/or income needs, and the relative value of different forests and tree-based systems on the diets and health of those who manage them. Such information is of great importance to policymakers, planners and development agencies seeking to improve the lives of food-insecure populations.
Differences in diets and nutrition associated with different subsistence strategies/different forms of land use (e.g. managed forests, shifting agriculture, agroforests, and single-species tree crop systems) are not widely documented. Studies comparing hunter-gatherers and low-population-density forest communities to more sedentary and urbanised groups have generally shown that the former consumed more meat but their diets were not necessarily better. The few existing studies suggest that the impact of transitions from one form of subsistence and land use to another is context-specific and influenced by social, cultural and economic factors.
A number of studies have shown a link between tree cover and dietary diversity and consumption of nutritious foods. Although we do not yet understand the pathways of this relationship, it suggests that maintenance of tree cover around rural homes and communities may lead to more nutritious diets.
Forests and tree-based systems are part of broader economic, political, cultural and ecological landscapes that typically include a mosaic of different food production systems and other land uses. How these different land use patches interact with each other in space and time can profoundly influence the productivity and sustainability of forests and tree-based systems as well as their food security and nutrition outcomes.
Tenure regimes in all four forest and tree-based systems are highly complex, and rights to trees may be different from rights to the land on which they are grown. Different bundles of rights are nested and overlap in these different systems, varying by geographical, social, cultural, economic and political factors, and affecting the access of different population groups to the trees and their products for food, income and other livelihood needs.
Most tenure systems are gender-biased, allocating primary rights to men. Since women represent 43 percent of the global agricultural labour force, and there is evidence of feminisation of agriculture in numerous developing countries, women’s weak and often insecure rights of access to land, forests and trees is undermining their engagement in innovation in forests and agroforestry systems with huge costs for the food security and nutrition of their families.
Rights to land, forests and trees in customary systems are commonly based on labour expended in clearing land or planting trees. Richer households with more assets (including livestock) are able to claim or make greater use of forest common property resources. However, poorer households often have a higher dependence, as a proportion of their total income, on forest resources for food security and livelihoods.
Tree planting and management requires low inputs of capital, mainly for labour, fertilisers and pesticides, and subsidies can lead to adoption of inappropriate trees or lead to distortions in land use. Such subsidies are often captured by larger farmers, who are not food insecure. Thus policies and incentives that improve demand and market prospects for trees rather than subsidising the establishment phase are more effective in promoting food security and improved livelihoods for the poor.
Adhikari, B., 2005. Poverty, property rights and collective action: Understanding the distributive aspects of common property resource management. Environment and Development Economics 10: 7-31. http://dx.doi.org/10.1017/s1355770x04001755
Afari-Sefa,V., Gockowski, J., Agyeman, N.F and Dziwornu, A.K., 2010. Economic Cost-benefit Analysis of Certified Sustainable Cocoa Production in Ghana. Poster presented at the Joint 3rd African Association of Agricultural Economists (AAAE) and 48th Agricultural Economists Association of South Africa (AEASA) Conference, Cape Town, South Africa, September 19-23, 2010. http://ageconsearch.umn.edu/bitstream/97085/2/33. Cost benefit of cocoa in Ghana.pdf
Agarwal, B., 1994a. Gender and command over property: A critical gap in economic analysis and policy in South Asia. World Development 22: 1455-1478. http://dx.doi.org/10.1016/0305-750x(94)90031-0
Agarwal, B., 1994b. A Field of One’s Own: Gender and Land Rights in South Asia. Cambridge: Cambridge University Press.
Agarwal, B., 1997. “Bargaining” and gender relations: Within and beyond the household. Feminist Economics 3(1): 1-51. http://dx.doi.org/10.1080/135457097338799
Agarwal, B., 2010. Gender and Green Governance: The Political Economy of Women’s Presence Within and Beyond Community Forestry. Oxford: Oxford University Press.
Agnoletti, M. (ed.), 2006. The Conservation of Cultural Landscapes. Wallingford, UK: CAB International.
Agrawal, A., Cashore, B., Hardin, R., Shepherd, G., Benson, C. and Miller, D., 2013. Economic Contributions of Forests. Background Paper to UNFF tenth Session, Istanbul, 8-19 April 2013. http://www.un.org/esa/forests/pdf/session_documents/unff10/EcoContrForests.pdf
AIPP and IWGIA, 2014. Shifting Cultivation, Livelihood and Food Security. New and Old Challenges for Indigenous Peoples in Asia. Chiang Mai: Asia Indigenous Peoples’ Pact (AIPP) and the International Work Group for Indigenous Affairs (IWGIA). http://www.iwgia.org/iwgia_files_publications_files/0694_AIPPShifting_cultivation_livelihoodfood_security.pdf
Alcorn, J.B., 1981. Huastec noncrop resource management: Implications for prehistoric rain forest management. Human Ecology 9(4): 395-417. http://dx.doi.org/10.1007/bf01418729
Alexiades, M.N., 2009. Mobility and Migration in Indigenous Amazonia: Contemporary Ethnoecological Perspectives. Oxford: Berghahn.
Altieri, M.A., 2002. Agroecology: The science of natural resource management for poor farmers in marginal environments. Agriculture, Ecosystems and Environment 93: 1-24. http://dx.doi.org/10.1016/s0167-8809(02)00085-3
Altieri, M.A., 2004. Linking ecologists and traditional farmers in the search for sustainable agriculture. Frontiers in Ecology and the Environment 2(1): 35-42. http://dx.doi.org/10.2307/3868293
Anderson, A.B. and Posey, D.A., 1989. Management of a tropical scrub savanna by the Gorotire Kayapo. Advances in Economic Botany 7: 159-173.
Anderson, K., 2006. Tending the Wild: Native American Knowledge and the Management of California’s Natural Resources. Berkeley, CA, USA: University of California Press. http://permaculteur.free.fr/ecoanarchisme/tending_the_wild.pdf
Andrade, G.I. and Rubio-Torgler, H., 1994. Sustainable use of the tropical rain forest: Evidence from the avifauna in a shifting-cultivation habitat mosaic in the Colombian Amazon. Conservation Biology 8(2): 545-554. http://dx.doi.org/10.1007/978-1-4612-4018-1_27
Angelsen, A., 2008. Moving Ahead with REDD: Issues, Options and Implications. Bogor, Indonesia: Center for International Forestry Research. http://dx.doi.org/10.17528/cifor/002601
Arnold, M. and Dewees, P. 1998. Rethinking Approaches to Tree Management by Farmers. Overseas Development Institute (ODI) Natural Resource Perspectives, No. 26. http://www.odi.org/sites/odi.org.uk/files/odi-assets/publications-opinion-files/2414.pdf
Asher, K., 2009. Black and Green: Afro-Colombians, Development, and Nature in the Pacific Lowlands. Durham, NC: Duke University Press.
Balée, W., 2006. The research program of historical ecology. Annual Review of Anthropology 35: 75-98. http://dx.doi.org/10.1146/annurev.anthro.35.081705.123231
Bannister, M.E. and Nair, P.K.R., 2003. Agroforestry adoption in Haiti: The importance of household and farm characteristics. Agroforest Systems 57: 149-157. http://dx.doi.org/10.1023/a:1023973623247
Barros, H.R., Ferreira, T.A. and Genovese, M.I., 2012. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chemistry 134(4): 1892-8. PMID 23442635. http://dx.doi.org/10.1016/j.foodchem.2012.03.090
Barry, D. and Meinzen-Dick, R., 2014. The invisible map: Community tenure rights. In: The Social Lives of Forests: Past, Present and Future of Woodland Resurgence, edited by S. Hecht, K. Morrison and C. Padoch. Chicago and London: University of Chicago Press. http://dx.doi.org/10.7208/chicago/9780226024134.001.0001
Bartuszevige, A.M., Gorchov, D.L. and Raab, L., 2006. The relative importance of landscape and community features in the invasion of an exotic shrub in a fragmented landscape. Ecography 29: 213-222. http://dx.doi.org/10.1111/j.2006.0906-7590.04359.x
Bélair, C., Ichikawa, K., Wong, B.Y.L. and Mulongoy K.J. (eds.), 2010. Sustainable Use of Biological Diversity in Socio-ecological Production Landscapes, Technical Series No. 52. Montreal: Secretariat of the Convention on Biological Diversity. https://www.cbd.int/doc/publications/cbd-ts-52-en.pdf
Belotto, A., Leanes, L.F., Schneider, M.C., Tamayo, H. and Correa, E., 2005. Overview of rabies in the Americas. Virus Research 111: 5-12. http://dx.doi.org/10.1016/j.virusres.2005.03.006
Bengis, R.G., Kock, R.A. and Fischer, J., 2002. Infectious animal diseases: The wildlife/livestock interface. Revue Scientifique et Technique (International Office of Epizootics) 21: 53-65. PMID: 11974630
Berkes, F., Colding, J. and Folke, C., 2000. Rediscovery of traditional ecological knowledge as adaptive management. Ecological Applications 10: 1251-1262. http://dx.doi.org/10.1890/1051-0761(2000)010[1251:roteka]2.0.co;2
Berti, P.R., Krasevec, J. and Fitzgerald, S., 2004. A review of the effectiveness of agriculture interventions in improving nutrition outcomes. Public Health Nutrition 7(5): 599-609. http://dx.doi.org/10.1079/phn2003595
Bianchi, F.J.J., Booij, C.J. and Tscharntke, T., 2006. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proceedings of the Royal Society B: Biological Sciences 273: 1715-1727. http://dx.doi.org/10.1098/rspb.2006.3530
Biermayr-Jenzano, P., Kassam S.N. and Aw-Hassan, A., 2014. Understanding Gender and Poverty Dimensions of High Value Agricultural Commodity Chains in the Souss-Masaa-Draa Region of South-western Morocco. ICARDA working paper, Mimeo. Amman, Jordan.
Bist, S.S., 2006. Elephant conservation in India—an overview. Gajah 25: 27-35. http://www.asesg.org/PDFfiles/Gajah/25-27-Bist.pdf
Blate, G.M., 2005. Modest trade-offs between timber management and fire susceptibility of a Bolivian semi-deciduous forest. Ecological Applications 15: 1649-1663. http://dx.doi.org/10.1890/04-0385
Boerboom, J.H.A. and Wiersum, K.F., 1983. Human impact on tropical moist forest. In: Man’s Impact on Vegetation, edited by W. Holzner, M.J.A. Werger and I. Ikusima. The Hague: W. Junk.
Boffa, J.-M., 1999. Agroforestry Parklands in Sub-saharan Africa. FAO Conserv. Guide 34. Rome: Food and Agriculture Organization of the United Nations. http://www.fao.org/docrep/005/x3940e/x3940e00.htm
Bomuhangi, A., Doss, C. and Meinzen-Dick, R., 2011. Who owns the land? Perspectives from rural Ugandans and implications for land acquisitions. IFPRI Discussion Paper 01136. Washington DC: IFPRI. http://dx.doi.org/10.1080/13545701.2013.855320
Borggaard, O.K., Gafur, A. and Petersen, L., 2003. Sustainability appraisal of shifting cultivation in the Chittagong Hill Tracts of Bangladesh. Ambio 32(2): 118-123. http://dx.doi.org/10.1579/0044-7447-32.2.118
Boyer, J. and Liu, R.H., 2004. Apple phytochemicals and their health benefits. Nutrition Journal 3(1): 5. http://dx.doi.org/10.1186/1475-2891-3-5
Boyles, J.G., Cryan, P.M., McCracken, G.F. and Kunz, T.H., 2011. Economic importance of bats in agriculture. Science 332: 41-42. http://dx.doi.org/10.1126/science.1201366
Bradley, B.A. and Mustard, J.F., 2006. Characterizing the landscape dynamics of an invasive plant and risk of invasion using remote sensing. Ecological Applications 16: 1132-1147. http://dx.doi.org/10.1890/1051-0761(2006)016[1132:ctldoa]2.0.co;2
Bradshaw, C.J.A., Sodhi, N.S., Peh, K.S.-H. and Brook, B.W., 2007. Global evidence that deforestation amplifies flood risk and severity in the developing world. Global Change Biology 13: 2379-2395. http://dx.doi.org/10.1111/j.1365-2486.2007.01446.x
Brauman, K.A., Daily, G.C., Duarte, T.K. and Mooney, H.A., 2007. The nature and value of ecosystem services: An overview highlighting hydrologic services. Annual Review of Environment and Resources 32: 1-32. http://dx.doi.org/10.1146/annurev.energy.32.031306.102758
Brondizio, E.S., 2008. The Amazonian Caboclo and the Açaí Palm: Forest Farmers in the Global Market. New York: New York Botanical Garden Press.
Brondizio, E.S., Safar, C.A.M. and Siqueira, A.D., 2002. The urban market of açaí fruit (Euterpe oleracea Mart.) and rural land use change: Ethnographic insights into the role of price and land tenure constraining agricultural choices in the Amazon estuary. Urban Ecosystems 6(1-2): 67-97. http://dx.doi.org/10.1023/a:1025966613562
Brown, H.C.P., Smit, B., Sonwa, D.J., Somorin, O.A. and Nkem, J., 2011. Institutional perceptions of opportunities and challenges of REDD+ in the Congo Basin. Journal of Environment & Development 20(4): 381-404. http://dx.doi.org/10.1177/1070496511426480
Bruce, J., 1999. Legal bases for the management of forest resources as common property. Forests, Trees and People Community Forestry Note 14. Rome: FAO. http://www.fao.org/docrep/012/x2581e/x2581e00.pdf
Bruun, T.B., de Neergaard, A., Lawrence, D. and Ziegler, A., 2009. Environmental consequences of the demise in swidden agriculture in Southeast Asia: Carbon storage and soil quality. Human Ecology 37: 375-388. http://dx.doi.org/10.1007/s10745-009-9257-y
Bytnerowicz, A., Omasa, K. and Paoletti, E., 2007. Integrated effects of air pollution and climate change on forests: A northern hemisphere perspective. Environmental Pollution 147: 438-445. http://dx.doi.org/10.1016/j.envpol.2006.08.028
CacaoNet, 2012. A Global Strategy for the Conservation and Use of Cacao Genetic Resources, as the Foundation for a Sustainable Cocoa Economy (B. Laliberté, compiler). Montpellier, France: Bioversity International. http://www.bioversityinternational.org/uploads/tx_news/A_global_strategy_for_the_conservation_and_use_of_cacao_genetic_resources__as_the_foundation_for_a_sustainable_cocoa_economy_1588.pdf
Cairns, M.F. (ed.), 2007. Voices from the Forest: Integrating Indigenous Knowledge into Sustainable upland Farming. Washington, DC: Resources for the Future.
Cairns, M.F. (ed.), 2015. Shifting Cultivation and Environmental Change: Indigenous People, Agriculture and Forest Conservation. London: Earthscan Publications (Routledge).
Cairns, M. and Garrity, D.P., 1999. Improving shifting cultivation in Southeast Asia by building on indigenous fallow management strategies. Agroforestry Systems 47: 37-48. http://dx.doi.org/10.1023/a:1006248104991
Carloni, A. 2005. Rapid Guide for Missions. Analysing Local Institutions and Livelihoods. Institutions for Rural Development 1. Rome: FAO. http://www.fao.org/3/a-a0273e.pdf
Carney, J and Elias, M., 2014. Gendered knowledge and the African shea-nut tree. In: The Social Lives of Forests: Past, Present and Future of Woodland Resurgence, edited by S. Hecht, K. Morrison and C. Padoch. Chicago and London: University of Chicago Press. http://dx.doi.org/10.7208/chicago/9780226024134.001.0001
Castella, J.-C., Lestrelin, G., Hett, C., Bourgoin, J., Fitriana, Y.R., Heinimann, A. and Pfund, J.-L., 2013. Effects of landscape segregation on livelihood vulnerability: Moving from extensive shifting cultivation to rotational agriculture and natural forests in Northern Laos. Human Ecology 41.1 (Feb. 2013): 63-76. http://dx.doi.org/10.1007/s10745-012-9538-8
Cerda, R., Deheuvels, O., Calvache, D., Niehaus, L., Saenz, Y., Kent, J., Vilchez, S., Villota, A., Martinez, C. and Somarriba, E., 2014. Contribution of cocoa agroforestry systems to family income and domestic consumption: Looking toward intensification. Agroforestry Systems 88(6): 1-25. http://dx.doi.org/10.1007/s10457-014-9691-8
Chao, S., 2012. Forest Peoples: Numbers Across the World. Moreton-in-Marsh, UK: Forest Peoples Programme. http://www.forestpeoples.org/sites/fpp/files/publication/2012/05/forest-peoples-
numbers-across-world-final_0.pdf
Chazdon, R.L. 2014. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation. Chicago: University of Chicago Press.
Chen, H., Morrell, P.L., Ashworth, V.E.T.M., De La Cruz, M. and Clegg, M.T., 2008. Tracing the geographic origins of major avocado cultivars. Journal of Heredity 100(1): 56-65. PMID 18779226. http://dx.doi.org/10.1093/jhered/esn068
Cissé, M.I., 1995. Les parcs agroforestiers au Mali. Etat des connaissances et perspectives pour leur amélioration. AFRENA Rep. 93. Nairobi: ICRAF.
Clough, Y., Abrahamczyk, S., Adams, M.-O., Anshary, A., Ariyanti, N., et al., 2010. Biodiversity patterns and trophic interactions in human-dominated tropical landscapes in Sulawesi (Indonesia): Plants, arthropods and vertebrates. In: Tropical Rainforests and Agroforests Under Global Change, edited by T. Tscharntke, C. Leuschner, E. Veldkamp, H. Faust, E. Guhardja and A. Bidin. Environmental Science and Engineering Series. Berlin: Springer Verlag. http://dx.doi.org/10.1007/978-3-642-00493-3
Cochrane, M.A., 2003. Fire science for rainforests. Nature 421: 913-919. http://dx.doi.org/10.1038/nature01437
Cock, M.J.W., 2003. Biosecurity and Forests: An Introduction—with Particular Emphasis on Forest Pests. FAO Forest Health and Biosecurity Working Paper FBS/2E. Rome: FAO. ftp://ftp.fao.org/docrep/fao/006/J1467E/J1467E.pdf
Colfer, C.J.P., 2008a. The Longhouse of the Tarsier: Changing Landscapes, Gender and Well Being in Borneo. Phillips, Maine: Borneo Research Council, in cooperation with CIFOR and UNESCO.
Colfer, C.J.P., 2008b. Human Health and Forests: A Global Overview of Issues, Practice and Policy. London: Earthscan.
Colfer, C.J.P., Colchester, M., Joshi, L., Puri, R.K., Nygren, A., Lopez, C., 2005. Traditional knowledge and human well-being in the 21st century. In: Forests in the Global Balance—Changing Paradigms, IUFRO World Series No. 17, edited by G. Mery, R. Alfaro, M. Kanninen. and M. Lovobikov. 2005. Helsinki: International Union of Forest Research Organizations. http://www.iufro.org/science/special/wfse/forests-global-balance
Colfer, C.J.P., Minarchek, R.D., Cairns, M., Aier, A., Doolittle, A., Mashman, V., Odame, H.H., Roberts, M., Robinson, K. and Van Esterik, O., 2015. Gender analysis and indigenous fallow management. In: Shifting Cultivation and Environmental Change: Indigenous People, Agriculture and Forest Conservation, edited by M. Cairns. London: Earthscan.
Colfer, C.J.P., Peluso, N.L. and Chin, S.C., 1997. Beyond Slash and Burn: Building on Indigenous Management of Borneo’s Tropical Rain Forests. Bronx, NY: New York Botanical Garden.
Collings, N., 2009. Environment. In: The State of the World’s Indigenous Peoples. United Nations. Department of Economic and Social Affairs, Division for Social Policy and Development, Secretariat of the Permanent Forum on Indigenous Issues Report No. ST/ESA/328. http://www.un.org/esa/socdev/unpfii/documents/SOWIP/en/SOWIP_web.pdf
Condominas, G., 1977. We Have Eaten the Forest: The Story of a Montagnard Village in the Central Highlands of Vietnam. New York: Hill and Wang.
Conklin, H. C., 1957. Hanunoo Agriculture: A Report on an Integral System of Shifting Cultivation in the Philippines. Rome: FAO.
Cramb, R.A., 1993. Shifting cultivation and sustainable agriculture in East Malaysia: A longitudinal case study. Agricultural Systems 42: 209-226. http://dx.doi.org/10.1016/0308-521x(93)90055-7
CTA, 2012. Climate change: Concerns for cocoa. SPORE No. 159: 9.
Damman, S., Kuhnlein, H.V. and Erasmus, B., 2013. Human rights implications of Indigenous Peoples’ food systems and policy recommendations. In: Indigenous Peoples’ Food Systems and Well-being. Interventions and Policies for Healthy Communities, edited by H.V. Kuhnlein, B. Erasmus, D. Spigelski and B. Burlingame. Rome: FAO and CINE (Centre for Indigenous Peoples’ Nutrition and Environment). http://www.fao.org/3/a-i3144e.pdf
De Foresta, H. and Michon, G., 1997. The agroforest alternative to Imperata grasslands: When smallholder agriculture and forestry reach sustainability. In: Agroforestry Innovations for Imperata Grassland Rehabilitation, edited by D.P. Garrity. Dordrecht, Netherlands: Kluwer Academic Publishers and Nairobi: International Centre for Research in Agroforestry. http://dx.doi.org/10.1007/bf00142877
DeFries, R.S., Rudel. T., Uriarte, M. and Hansen, M., 2010. Deforestation driven by urban population growth and agricultural trade in the twenty-first century. Nature Geoscience 3: 178-181. http://dx.doi.org/10.1038/ngeo756
Deere, C.D., Boakye-Yiadom, L., Doss, C., Oduro, A.D., Swaminathan, H., Twyman, J. and Suchitra, J.Y. 2013. Women’s Land Ownership and Participation in Agricultural Decision-making: Evidence from Ecuador, Ghana and Karnataka, India. The Gender Asset Gap Project Research Brief Series No. 2. Bangalore: Indian Institute of Management. http://genderassetgap.org/sites/default/files/ResearchBrief2.pdf
Denevan, W.M., 1992. The pristine myth: The landscape of the Americas in 1492. Annals of the Association of American Geographers 82(3): 369-385. http://dx.doi.org/10.1111/j.1467-8306.1992.tb01965.x
Denevan, W.M. and Padoch, C., 1987. Swidden-fallow Agroforestry in the Peruvian Amazon. New York: New York Botanical Garden.
Denevan, W.M., Treacy, J.M., Alcorn, J.B., Padoch, C., Denslow, J. and Paitan, S.F., 1984. Indigenous agroforestry in the Peruvian Amazon—Bora Indian management of swidden fallows. Interciencia 9: 346-357. http://pdf.usaid.gov/pdf_docs/PNAAT727.pdf
Dey, J. 1981. Gambian women: Unequal partners in rice development projects? Journal of Development Studies 17(3): 109-122. http://dx.doi.org/10.1080/00220388108421801
Dey Abbas, J., 1997. Gender asymmetries in intrahousehold resource allocation in Sub-Saharan Africa: Some policy implications for land and labor productivity. In: Intrahousehold Resource Allocation in Developing Countries: Methods, Models and Policy, edited by L. Haddad, J. Hoddinott and H. Alderman. Baltimore: John Hopkins University Press, for IFPRI. https://www.pep-net.org/sites/pep-net.org/files/typo3doc/pdf/intrahhres1.pdf
Doss, C., Grown, C. and Deere, C.D., 2008. Gender and Asset Ownership: A Guide to Collecting Individual Level Data. Policy research working paper 4704. Washington DC: World Bank. http://dx.doi.org/10.1596/1813-9450-4704
Dounias, E., Selzner, A., Koizumi, M. and Levang, P. 2007. From sago to rice, from forest to town: The consequences of sedentarization for the nutritional ecology of Punan former hunter-gatherers of Borneo. Food and Nutrition Bulletin 28(2, suppl.): 294S-302S(9). http://www.ncbi.nlm.nih.gov/pubmed/17658075
Dove, M., 1983., Theories of swidden agriculture, and the political economy of ignorance. Agroforestry Systems 1: 85-99. http://dx.doi.org/10.1007/bf00596351
Dove, M.R., Smith, D.S., Campos, M.T., Mathews, A. S., Rademacher, A., Rhee, S. and Yoder, L.M., 2013. Globalisation and the construction of Western and non-Western knowledge. In: Local Science vs Global Science: Approaches to Indigenous Knowledge in International Development, edited by P. Sillitoe. Oxford and New York: Berghan Books.
Dreher, M.L. and Davenport, A.J., 2013. Hass avocado composition and potential health effects. Critical Reviews in Food Science and Nutritio 53 (7): 738-50. PMID 23638933. http://dx.doi.org/10.1080/10408398.2011.556759
Duchelle, A., Almeyda Zambrano, A.M., Wunder, S., Börner, J. and Kainer, K. 2014. Smallholder Specialization Strategies along the Forest Transition Curve in Southwestern Amazonia. World Development. http://dx.doi.org/10.1016/j.worlddev.2014.03.001
Duflo, E. and Udry, C., 2004. Intrahousehold Resource Allocation in Côte d’Ivoire: Social Norms, Separate Accounts and Consumption Choices. NBER working paper No. 10498. Cambridge MA: National Bureau of Economic Research. http://dx.doi.org/10.3386/w10498
Eagle, A.J., Eiswerth, M.E., Johnson, W.S., Schoenig, S.E. and Cornelis van Kooten, G., 2007. Costs and losses imposed on California ranchers by yellow starthistle. Rangeland Ecology & Management 60: 369-377. http://dx.doi.org/10.2111/1551-5028(2007)60[369:calioc]2.0.co;2
Edem, D.O., 2002. Palm oil: Biochemical, physiological, nutritional, hematological and toxicological aspects: A review. Plant Foods for Human Nutrition 57(3): 319-341. http://dx.doi.org/10.1023/a:1021828132707
Environment Canada, 2004. An Invasive Alien Species Strategy for Canada. Ottawa: Environment Canada. http://publications.gc.ca/collections/collection_2014/ec/CW66-394-2004-eng.pdf
FAO, 1982. Fruit-bearing Forest Trees: Technical Notes. FAO Forestry Paper 34. Rome: Food and Agriculture Organization of the United Nations. http://www.fao.org/docrep/015/t0006e/t0006e00.pdf
FAO, 2002a. Land Tenure and Rural Development. FAO Land Tenure Studies 3. Rome: FAO. ftp://ftp.fao.org/docrep/fao/005/y4307E/y4307E00.pdf
FAO, 2002b. Gender and Access to Land. FAO Land Tenure Studies 4. Rome: FAO. ftp://ftp.fao.org/docrep/fao/005/y4308e/y4308e00.pdf
FAO, 2010. Global Forest Resources Assessment 2010: Main Report. FAO Forestry Paper 163. Rome: Food and Agriculture Organization of the United Nations. http://www.fao.org/docrep/013/i1757e/i1757e.pdf
FAO, 2011. State of Food and Agriculture. Women in Agriculture: Closing the Gender Gap for Development. Rome: FAO. http://www.fao.org/docrep/013/i2050e/i2050e.pdf
FAO, 2012. Roles of Forests in Climate Change. http://www.fao.org/forestry/climatechange/53459/en/
FAO, 2014. State of the World‘s Forests. Enhancing the Socioeconomic Benefits from Forests. Rome: FAO. http://www.fao.org/3/a-i3710e.pdf
FAO and CIFOR, 2005. Forests and Floods: Drowning in Fiction or Thriving on Facts? Bangkok: Centre for International Forestry Research, Bogor and Food and Agriculture Organisation of the United Nations. http://www.fao.org/docrep/008/ae929e/ae929e00.htm
FAOSTAT Statistical Database, 2010. Rome: Food and Agriculture Organization of the United Nations. http://faostat.fao.org/
Feary, S.A., Eastburn, D., Sam, N. and Kennedy, J., 2012. Western Pacific. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_11
Feldstein, H.S. and Poats, S.V., 1990. Working Together. Gender Analysis in Agriculture. West Hartford, CT: Kumarian.
Figueira, A., Janick, J. and BeMiller, J.N., 1993. New products from Theobroma cacao: Seed pulp and pod gum. In: New Crops, edited by J. Janick and J.E. Simon. New York: Wiley.
Finegan, B. and Nasi, R., 2004. The biodiversity and conservation potential of swidden agricultural landscapes. In: Agroforestry and Biological Conservation in Tropical Landscapes, edited by G. Schroth, G.A.B. da Fonseca, C.A. Harvey, C. Gascon, H.L. Vasconcelos and A.-M.N. Izac, Washington, DC: Island Press. http://library.uniteddiversity.coop/Permaculture/Agroforestry/Agroforestry_and_Biodiversity_Conservation_in_Tropical_Landscapes.pdf
Fisher, M., 2004. Household welfare and forest dependence in southern Malawi. Environment and Development Economics 9(2): 135-154. http://dx.doi.org/10.1017/s1355770x03001219
Foley, J.A., DeFries, R., Asner, G.P., Barford, C., Bonan, G., Carpenter, S.R., Chapin, F.S., Coe, M.T., Daily, G.C., Gibbs, H.K., Helkowski, J.H., Holloway, T., Howard, E.A., Kucharik, C.J., Monfreda, C., Patz, J.A., Prentice, I.C., Ramankutty, N. and Snyder, P.K., 2005. Global Consequences of Land Use. Science 309: 570-574. http://dx.doi.org/10.1126/science.1111772
Foli, S., Reed, J., Clendenning, J., Petrokofsky, G., Padoch, C. and Sunderland, T., 2014. To what extent does the presence of forests and trees contribute to food production in humid and dry forest landscapes?: A systematic review protocol. Environmental Evidence 3: 15. http://www.environmentalevidencejournal.org/content/3/1/15 http://dx.doi.org/10.17528/cifor/005476
Fortmann, L., 1983. Who plows? The effect of economic status on women’s participation in agriculture in agriculture in Botswana. Mimeo.
Fortmann, L., Antinori, C. and Nabanne, N., 1997. Fruits of their labors: Gender, property rights, and tree planting in two Zimbabwe villages. Rural Sociology 62(3): 295-314. http://dx.doi.org/10.1111/j.1549-0831.1997.tb00653.x
Fortmann, L. and Bruce, J. W. (eds.), 1988. Whose Trees? Proprietary Dimensions of Forestry. Boulder and London: Westview Press.
Fowler, D., Cape, J.N., Coyle, M., Flechard, C., Kuylenstierna, J., Hicks, K., Derwent, D., Johnson, D. and Stevenson, D., 1999. The global exposure of forests to air pollutants. In: Forest Growth Responses to the Pollution Climate of the 21st Century, edited by L.J. Sheppard and J.N. Cape. Dordrecht, Netherlands: Springer. http://dx.doi.org/10.1007/978-94-017-1578-2_1
Fox, J., Truong, D.M., Rambo, A.T., Tuyen, N.P., Cuc, L.T. and Leisz, S., 2000. Shifting cultivation: A new old paradigm for managing tropical forests. BioScience 50(6): 521-528. http://dx.doi.org/10.1641/0006-3568(2000)050[0521:scanop]2.0.co;2
Fox, J., Fujita, Y., Ngidang, D., Peluso, N., Potter, L., Sakuntaladewi, N., Sturgeon, J. and Thomas, D., 2009. Policies, Political-Economy, and Swidden in Southeast Asia. Human Ecology 37(3): 305-322. http://dx.doi.org/10.1007/s10745-009-9240-7
Franzel, S., 1999. Socioeconomic factors affecting the adoption potential of improved tree fallows in Africa. Agroforestry Systems 47: 305-321. http://dx.doi.org/10.1023/a:1006292119954
Fuys, A. and Dohrn, S. 2010. Common property regimes: Taking a closer look at resource access. In: Beyond the Biophysical. Knowledge, Culture and Power in Agriculture and Natural Resource Management, edited by L. German, J. Ramisch and R. Verma. Dordrecht, Heidelberg, London, New York: Springer. http://dx.doi.org/10.1007/978-90-481-8826-0_9
Gallai, N., Salles, J.-M., Settele, J. and Vaissiere, B.E., 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological Economics 68: 810-821. http://dx.doi.org/10.1016/j.ecolecon.2008.06.014
Galloway-McLean, K., 2010. Advance Guard: Climate Change Impacts, Adaptation, Mitigation and Indigenous Peoples—A Compendium of Case Studies. Darwin, Australia: United Nations University-Traditional Knowledge Initiative. http://www.unutki.org/news.php?doc_id=101&news_id=92
García Latorre, J. and García Latorre, J., 2012. Globalization, local communities, and traditional forest-related knowledge. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_12
Gavin, M.C., 2004. Changes in forest use value through ecological succession and their implications for land management in the Peruvian Amazon. Conservation Biology 18(6): 1562-70. http://dx.doi.org/10.1111/j.1523-1739.2004.00241.x
Giovarelli, R., 2006. Overcoming gender biases in established and transitional property rights systems. In: Land Law Reform: Achieving Development Policy Objectives. Law, Justice, and Development Series, edited by J.W. Bruce, R. Giovarelli, L. Rolfes, D. Bledsoe and R. Mitchell. Washington, DC: World Bank Publications. http://dx.doi.org/10.1596/978-0-8213-6468-0
Girard, A.W., Self, J.L., McAuliffe, C. and Olude, O., 2012. The effects of household food production strategies on the health and nutrition outcomes of women and young children: A systematic review. Paediatric and Perinatal Epidemiology 26: 205-22. http://dx.doi.org/10.1111/j.1365-3016.2012.01282.x
Gockowski, J. and Sonwa, D., 2011. Cocoa intensification scenarios and their predicted impact on CO2 emissions, biodiversity conservation, and rural livelihoods in the Guinea rain forest of West Africa. Environmental Management 48: 307-321. http://dx.doi.org/10.1007/s00267-010-9602-3
Goldammer, J.G., 1988. Rural land-use and wildland fires in the tropics. Agroforestry Systems 6: 235-252. http://dx.doi.org/10.1007/bf02220124
Gupta, P.NS., 1980. Food consumption and nutrition of regional tribes of India. Ecology of Food and Nutrition 9(2): 93-108. http://dx.doi.org/10.1080/03670244.1980.9990587
Haddad, L. Hoddinott, J. and Alderman, H. (eds.), 1997. Intrahousehold Resource Allocation in Developing Countries: Methods, Models and Policy. Baltimore: John Hopkins University Press, for IFPRI. http://dx.doi.org/10.2307/1244597
Hall, J.B., Aebischer, D.P., Tomlinson, H.F., Osei-Amaning, E. and Hindle, J.R., 1996. Vitellaria Paradoxa: A Monograph. Bangor, UK: University of Wales.
Hammond D.S., ter Steege, H. and van der Borg, K., 2007. Upland soil charcoal in the wet tropical forests of central Guyana. Biotropica 39: 153-160. http://dx.doi.org/10.1111/j.1744-7429.2006.00257.x
Hatcher, J. and Bailey, L. 2010. Tropical Forest Tenure Assessment: Trends, Challenges and Opportunities. Washington DC and Yokohama: RRI and ITTO.
Hausman, A.J. and Wilmsen, E.N., 1985. Economic change and secular trends in the growth of San children. Human Biology 57(4): 563-571.
Hecht, S.B., 2009. Kayapó savanna management: Fire, soils, and forest islands in a threatened biome. In: Amazonian Dark Earths: Wim Sombroek’s Vision, edited by W.I. Woods, W.G. Teixeira, J. Lehmann, C. Steiner, A.M.G.A. WinklerPrins and L. Rebellato. Heidelberg: Springer. http://dx.doi.org/10.1007/978-1-4020-9031-8_7
Hecht, S.B., Morrison, K.D. and Padoch, C. (eds.), 2014. The Social Lives of Forests: Past, Present, and Future of Woodland Resurgence. Chicago: University of Chicago Press. http://dx.doi.org/10.5860/choice.52-0834
Hett, C., Castella, J.-C., Heinimann, A., Messerli, P. and Pfund, J.-L., 2012. A landscape mosaics approach for characterizing swidden systems from a REDD+ perspective. Applied Geography 32: 608-618. http://dx.doi.org/10.1016/j.apgeog.2011.07.011
Hill, C.M., 2000. Conflict of interest between people and baboons: Crop raiding in Uganda. International Journal of Primatology 21: 299-315. http://sanrem.cals.vt.edu/1048/Conflict of Interest Between People and Baboons.pdf
Hill, R.V. and Vigneri, M. 2014. Mainstreaming gender sensitivity in cash crop market supply Chains. In: Gender in Agriculture. Closing the Knowledge Gap, edited by A. Quisumbing, R. Meinzen-Dick, T. Raney, A. Croppenstedt, J. Behrman and A. Peterman. Rome and Dordrecht: FAO and Springer. http://dx.doi.org/10.1007/978-94-017-8616-4_13
Hiraoka, M., 1994. Mudanças nos padrões econômicos de uma população ribeirinha do estuário do Amazonas. In: Povos das Águas: Realidade e perspectivas na Amazônia, edited by L. Furtado, A.F. Mello and W. Leitão. Belém, Para, Brazil: MPEG/Universidade Federal do Pará.
Hiraoka, M., 1995. Aquatic and land fauna management among the floodplain riberenos of the Peruvian Amazon. In: The fragile tropics of Latin America: Sustainable Management of Changing Environments, edited by T. Nishizawa and J.I. Uitto. Tokyo: United Nations University.
Hladik, C.M., Linares, O.F., Hladik, A., Pagezy, H. and Semple, A., 1993. Tropical forests, people and food: An overview. In: Tropical Forests, People and Food. Biocultural Interactions and Applications to Development, Man and Biosphere Series No. 13, edited by C.M. Hladik, A. Hladik, O.F. Linares, H. Pagezy, A. Semple and M. Hadley. Paris: UNESCO and New York: Parthenon.
Hockings, K.J. and McLennan, M.R., 2012. From forest to farm: Systematic review of cultivar feeding by chimpanzees—management implications for wildlife in anthropogenic landscapes. PLoS ONE 7, e33391. http://dx.doi.org/10.1371/journal.pone.0033391
Holmes, T.P., Aukema, J.E., Von Holle, B., Liebhold, A. and Sills, E., 2009. Economic impacts of invasive species in forests. Annals of the New York Academy of Sciences 1162: 18-38. http://dx.doi.org/10.1111/j.1749-6632.2009.04446.x
Holzschuh, A., Steffan-Dewenter, I. and Tscharntke, T., 2010. How do landscape composition and configuration, organic farming and fallow strips affect the diversity of bees, wasps and their parasitoids? Journal of Animal Ecology 79: 491-500. http://dx.doi.org/10.1111/j.1365-2656.2009.01642.x
Howard, P.L. and Smith, E., 2006. Leaving Two Thirds out of Development: Female Headed Households and Common Property Resources in the Highlands of Tigray, Ethiopia. Livelihood Support Programme (LSP) Working Paper 40. Rome: FAO. http://www.fao.org/3/a-ah624e.pdf
Howard, P. L. and Nabanoga, G., 2007. Are there customary rights to plants? An inquiry among the Baganda (Uganda), with special attention to gender. World Development 35(9): 1542-1563. http://dx.doi.org/10.1016/j.worlddev.2006.05.021
Hunt, D., 1984. The Labour Aspects of Shifting Cultivation in African Agriculture. Rome: FAO.
Hurni, K., Hett, C., Heinimann, A., Messerli, P. and Wiesmann, U., 2013. Dynamics of shifting cultivation landscapes in northern Lao PDR between 2000 and 2009 based on an analysis of MODIS time series and Landsat images. Human Ecology 41(1): 21-36. http://dx.doi.org/10.1007/s10745-012-9551-y
Ibarra, J.T., Barreau, A., Del Campo, C., Camacho, C.I., Martin, G.J. and McCandless S.R., 2011. When formal and market-based conservation mechanisms disrupt food sovereignty: Impacts of community conservation and payments for environmental services on an indigenous community of Oaxaca, Mexico. International Forestry Review 13(3): 318-337. http://dx.doi.org/10.1505/146554811798293935
Ickowitz, A., Powell, B., Salim, M.A. and Sunderland, T.C.H., 2014. Dietary quality and tree cover in Africa. Global Environmental Change 24: 287-294. http://dx.doi.org/10.1016/j.gloenvcha.2013.12.001
Indrawan, M., Yabe, M., Nomura, H. and Harrison, R., 2014. Deconstructing satoyama—The socio-ecological landscape in Japan. Ecological Engineering 64: 77-84. http://dx.doi.org/10.1016/j.ecoleng.2013.12.038
IFPRI, 2013. Reducing the Gender Asset Gap through Agricultural Development. A Technical Resource Guide. Washington DC: IFPRI. http://dx.doi.org/10.1007/978-94-017-8616-4_5
ITTO, 2002. ITTO Guidelines for the Restoration, Management and Rehabilitation of Degraded and Secondary Tropical Forests. ITTO Policy Development Series No. 13. Yokohama, Japan: International Tropical Timber Organization. http://www.cifor.org/library/1175/itto-guidelines-for-the-restoration-management-and-rehabilitation-of-degraded-and-secondary-tropical-forests/
Janick, J., 2005. The origins of fruits, fruit growing, and fruit breeding. Plant Breeding Review 25: 255-320. http://dx.doi.org/10.1002/9780470650301.ch8
Jarosz, L., 1993. Defining and explaining tropical deforestation: Shifting cultivation and population growth in colonial Madagascar (1896-1940). Economic Geography 69(4): 366-379. http://dx.doi.org/10.2307/143595
Jodha, N.S., 1986. Common Property Resources and Rural Poor in Dry Regions of India. Economic and Political Weekly 21: 1169-81. http://www.jstor.org/stable/4375858
Johann, E., Agnoletti M., Bölöni, J., Erol, S.Y., Holl, K., Kusmin, J., García Latorre, J., García Latorre, J., Molnár, Z., Rochel, X., Rotherham, I.D., Saratsi, E., Smith, M., Tarang, L., van Benthem, M. and van Laar, J., 2012. Europe. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. https://dx.doi.org/10.1007/978-94-007-2144-9_6
Johns, T., 1996. The Origins of Human Diet and Medicine: Chemical Ecology. Tucson AZ, USA: University of Arizona Press.
Johnson, D., 1973. The botany, origin, and spread of the cashew Anacardium occidentale L. Journal of Plantation Crops 1(1-2): 1-7.
Jonkman, S.N., 2005. Global perspectives on loss of human life caused by floods. Natural Hazards 34(2): 151-175. http://dx.doi.org/10.1007/s11069-004-8891-3
Kennedy, E. and Peters, P. 1992. Household food security and child nutrition: The interaction of income and gender of household head. World Development 20(8): 1077-1085. http://dx.doi.org/10.1016/0305-750x(92)90001-c
Kerr, W.E. and Posey D.A., 1984. Notas sobre a agricultura dos índios Kayapó. Interciência 9(6): 392-400.
Kiptot, E. and Franzel, S., 2012. Gender and agroforestry in Africa: A review of women’s participation. Agroforestry Systems 84: 35-58. http://dx.doi.org/10.1007/s10457-011-9419-y
Kislev, M.E., Hartmann, A. and Bar-Yosef, O., 2006. Early domesticated fig in the Jordan Valley. Science 312(5778): 1372-1374 (2 June 2006). http://dx.doi.org/10.1126/science.1125910
Kiyingi, I. and Gwali, S., 2013. Productivity and profitability of robusta coffee agroforestry systems in central Uganda. Uganda Journal of Agricultural Sciences 13(1): 85-93. http://www.researchgate.net/publication/236901244_Productivity_and_profitability_of_Robusta_coffee_agroforestry_systems_in_central_Uganda
Klein, A.-M., Vaissière, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C. and Tscharntke, T., 2007. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society B: Biological Sciences 274: 303-313. http://dx.doi.org/10.1098/rspb.2006.3721
Kleinman, P.J.A., Pimentel, D. and Bryant, R.B., 1996. Assessing ecological sustainability of slash-and-burn agriculture through soil fertility indicators. Agronomic Journal 88: 122-127. http://dx.doi.org/10.2134/agronj1996.00021962008800020002x
Kort, J., 1988. Benefits of windbreaks to field and forage crops. Agriculture, Ecosystems and Environment 22-23: 165-190. http://dx.doi.org/10.1016/b978-0-444-43019-9.50018-3
Koppert, G.J.A., Dounias, E., Froment, A. and Pasquet, P., 1993. Food consumption in three forest populations of the southern coastal areas of Cameroon: Yassa—Mvae—Bakola. In: Tropical Forests, People and Food. Biocultural Interactions and Applications to Development. Man and Biosphere Series No. 13. Paris: UNESCO/The Parthenon Publishing Group. http://www.cifor.org/publications/pdf_files/research/forests_health/17.pdf
Kremen, C., 2005. Managing ecosystem services: What do we need to know about their ecology? Ecology Letters 8: 468-479. http://dx.doi.org/10.1111/j.1461-0248.2005.00751.x
Kristjanson, P., Waters-Buyer, A., Johnson, N., Tipilda, A., Njuki, J., Baltenweck, I., Grace, D., MacMillan, S., 2014. Livestock and women’s livelihoods. In: Gender in Agriculture. Closing the Knowledge Gap, edited by A. Quisumbing, R. Meinzen-Dick, T. Raney, A. Croppenstedt, J. Behrman and A. Peterman. Rome and Dordrecht: FAO and Springer. http://dx.doi.org/10.1007/978-94-017-8616-4_9
Kuhnlein, H.V., Erasmus, B. and Spigelski, D., 2009. Indigenous Peoples’ Food Systems. Rome: Food and Agriculture Organization of the United Nations. ftp://ftp.fao.org/docrep/fao/012/i0370e/i0370e00.pdf
Kumar, B.M. and Takeuchi, K., 2009. Agroforestry in the Western Ghats of peninsular India and the satoyama landscapes of Japan: A comparison of two sustainable land use systems. Sustainability Science 4(2): 215-232. http://dx.doi.org/10.1007/s11625-009-0086-0
Kumar, C. and Nongkynrih, K. 2005. Customary tenurial forest practices and the poor in Khasi—Jaintia Society of Meghalaya. Case study submitted for the joint study Rural Common Property in a Perspective of Development and Modernization. Delhi: CIFOR and North Eastern Hill University.
Kumar, N. and Quisumbing, A., 2012. Policy Reform Toward Gender Equality in Ethiopia: Little by Little the Egg Begins to Walk. IFPRI Discussion Paper 1126. Washington DC: IFPRI. http://dx.doi.org/10.2139/ssrn.2184985
Landscan, 2010. Landscan Global Population Database. http://www.eastview.com/online/landscan
Lambin, E.F., Turner, B.L., Geist, H.J., Agbola, S.B., Angelsen, A., Bruce, J.W., Coomes, O.T., Dirzo, R., Fischer, G., Folke, C., George, P.S., Homewood, K., Imbernon, J., Leemans, R., Li, X., Moran, E.F., Mortimore, M., Ramakrishnan, P.S., Richards, J.F, Skånes, H., Steffen, W., Stone, G.D., Svedin, U., Veldkamp, T.A., Vogel, C. and Xu, J., 2001. The causes of land-use and land-cover change: Moving beyond the myths. Global Environmental Change 11: 261-269. http://dx.doi.org/10.1016/s0959-3780(01)00007-3
Larson, A.M., Barry, D. and Dahal G.R., 2010. New rights for forest-based communities? Understanding processes of forest tenure reform. International Forestry Review 12(1): 78-96. http://dx.doi.org/10.1505/ifor.12.1.78
Lastarria-Cornhiel, S., 1997. Impact of privatization on gender and property rights in Africa. World Development 25(8): 1317-1333. http://dx.doi.org/10.1016/s0305-750x(97)00030-2
Lastarria-Cornhiel, S., Behrman, J., Meinzen-Dick, R. and Quisumbing, A., 2014. Gender equity ann land: Toward secure and effective access for rural women. In: Gender in Agriculture. Closing the Knowledge Gap, edited by A. Quisumbing, R. Meinzen-Dick, T. Raney, A. Croppenstedt, J. Behrman and A. Peterman. Rome and Dordrecht: FAO and Springer. http://dx.doi.org/10.1007/978-94-017-8616-4_6
Lauri, P.-É., Maguylo, K. and Trottier, C., 2006. Architecture and size relations: An essay on the apple (Malus x domestica, Rosaceae) tree. American Journal of Botany 93(93): 357-368. http://dx.doi.org/10.3732/ajb.93.3.357
Li, T.M., 1998. Working separately but eating together: Personhood, property and power in conjugal relations. American Ethnologist 25(4): 675-694. http://dx.doi.org/10.1525/ae.1998.25.4.675
Li, T.M., 2014. Social Impacts of Oil Palm in Indonesia. A Gendered Perspective from West Kalimantan. Toronto: University of Toronto, for CIFOR. http://dx.doi.org/10.17528/cifor/005579
Likens, G.E., Driscoll, C.T. and Buso, D.C., 1996. Long-term effects of acid rain: Response and recovery of a forest ecosystem. Science 272: 244-246. http://dx.doi.org/10.1126/science.272.5259.244
Lim, H.F., Liang, L., Camacho, L.D., Combalicer, E.A. and Singh, S.K.K., 2012. Southeast Asia. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_10
Linares, O.F., 1976. ”Garden hunting” in the American tropics. Human Ecology 4(4): 331-349. http://dx.doi.org/10.1007/bf01557917
MA (Millennium Ecosystem Assessment), 2005. Ecosystems and Human Well-being: Synthesis. Washington DC: Island Press. http://www.millenniumassessment.org/documents/document.356.aspx.pdf
Mackenzie, C.A. and Ahabyona, P., 2012. Elephants in the garden: Financial and social costs of crop raiding. Ecological Economics 75: 72-82. http://dx.doi.org/10.1016/j.ecolecon.2011.12.018
Maffi L., 2005. Linguistic, cultural, and biological diversity. Annual Review of Anthropology 29: 599-617. http://dx.doi.org/10.1146/annurev.anthro.34.081804.120437
Marten, G.G. and Abdoellah, O.S., 1988. Crop diversity and nutrition in west java. Ecology of Food and Nutrition 21(1): 17-43. http://dx.doi.org/10.1080/03670244.1988.9991016
Martin, P.A., Newton, A.C. and Bullock, J.M., 2013. Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proceedings of the Royal Society B 280: 20132236. http://dx.doi.org/10.1098/rspb.2013.2236
Masset, E., Haddad, L., Cornelius, A. and Isaza-Castro, J., 2012. Effectiveness of agricultural interventions that aim to improve nutritional status of children: Systematic review. BMJ (British Medical Journal) 344: d8222. http://dx.doi.org/10.1136/bmj.d8222
Masters, E.T., Yidana, J.A. and Lovett, P.N. [n.d.]. Reinforcing Sound Management through Trade: Shea Tree Products in Africa. Rome: Food and Agriculture Organization. http://www.fao.org/docrep/008/y5918e/y5918e11.htm
McDaniel, J., Kennard, D. and Fuentes, A., 2005. Smokey the tapir: Traditional fire knowledge and fire prevention campaigns in lowland Bolivia. Society and Natural Resources 18: 921-931. http://dx.doi.org/10.1080/08941920500248921
Meinzen-Dick, R., Brown, L., Feldstein, H. and Quisumbing, A., 1997. Gender, property rights, and natural resources. World Development 25(8): 1303-1315. http://dx.doi.org/10.1016/s0305-750x(97)00027-2
Meinzen-Dick, R., Johnson, N., Quisumbing, A., Njuki, J., Behrman, J., Rubin, D., Peterman, A. and Waithanji, E., 2014. The gender asset gap and its implications for agricultiral and rural development. In: Gender in Agriculture. Closing the Knowledge Gap, edited by A. Quisumbing, R. Meinzen-Dick, T. Raney, A. Croppenstedt, J. Behrman, and A. Peterman. Rome and Dordrecht: FAO and Springer. http://dx.doi.org/10.1007/978-94-017-8616-4_5
Mercer, D.L., 2004. Adoption of agroforestry innovations in the tropics: A review. Agroforestry Systems 61-62(1-3): 311-328. http://dx.doi.org/10.1007/978-94-017-2424-1_22
Mertz, O., 2009. Trends in shifting cultivation and the REDD mechanism. Current Opinion in Environmental Sustainability 1(2): 156-160. http://dx.doi.org/10.1016/j.cosust.2009.10.002
Mertz, O., Leisz, S., Heinimann, A., Rerkasem, K., Thiha, Dressler,W., Cu, P.V., Vu, K.C., Schmidt-Vogt, D., Colfer, C.J.P., Epprecht, M., Padoch, C. and Potter, L., 2009a. Who counts? The demography of swidden cultivators. Human Ecology 37: 281-289. http://dx.doi.org/10.1007/s10745-009-9249-y
Mertz, O., Padoch, C., Fox, J., Cramb, R.A., Leisz, S.J., Lam, N.T. and Vien, T.D., 2009b. Swidden change in Southeast Asia: Understanding causes and consequences. Human Ecology 37(3): 259-264. http://dx.doi.org/10.1007/s10745-009-9245-2
Mertz, O., Wadley, R.L., Nielsen, U., Bruun, T.B., Colfer, C.J.P., de Neergaard, A., Jepsen, M.R., Martinussen, T., Zhao, Q., Noweg, G.T. and Magid, J., 2008. A fresh look at shifting cultivation: Fallow length an uncertain indicator of productivity. Agricultural Systems 96: 75-84. http://dx.doi.org/10.1016/j.agsy.2007.06.002
Meyer, J.-Y. and Malet, J.-P., 1997. Study and management of the alien invasive tree Miconia calvescens DC. (Melastomataceae) in the Islands of Raiatea and Tahaa (Society Islands, French Polynesia): 1992-1996. Report, Cooperative National Park Resources Studies Unit. Honolulu: University of Hawaii at Manoa, Department of Botany. http://hdl.handle.net/10125/7368
Mitchell, M.G.E., Bennett, E.M. and Gonzalez, A., 2014. Forest fragments modulate the provision of multiple ecosystem services. Journal of Applied Ecology 51: 909-918. http://dx.doi.org/10.1111/1365-2664.12241
Molnar, T.J., Zaurov, D.E., Capik, J.M., Eisenman, S.W., Ford, T., Nikolyi, L.V. and Funk, C.R, 2011. Persian Walnuts (Juglans regia L.) in Central Asia. In: Northern Nut Growers Association (NNGA) 101st Annual Report. http://www.ippfbe.org
Moore, B.A., 2005. Alien Invasive Species: Impacts on Forests and Forestry. Rome: Food and Agriculture Organisation of the United Nations. http://www.fao.org/docrep/008/j6854e/j6854e00.htm
Mukherjee, S.K., 1972. Origin of mango (Mangifera indica). Economic Botany 26(3): 260-264. http://dx.doi.org/10.1007/bf02861039
Muleta, D., 2007. Microbial Inputs in Coffee (Coffea arabica L.) Production Systems, Southwestern Ethiopia: Implications for Promotion of Biofertilizers and Biocontrol Agents. PhD thesis, Swedish University of Agricultural Sciences/Uppsala. http://pub.epsilon.slu.se/1657/1/Am_Thesis_template_LC_PUBL..pdf
Nair, P.K.N., 1993. An Introduction to Agroforestry. Dordrecht, Netherlands: Kluwer Academic Publishers. http://dx.doi.org/10.1007/978-94-011-1608-4
Nair, P.K.R. and Fernandes, E.,1984. Agroforestry as an Alternative to Shifting Cultivation. FAO Soils Bulletin No. 53: 169-182.
Nair, P.K.R., Kumar, B.M. and Nair, V.D., 2009. Agroforestry as a strategy for carbon sequestration. Journal of Plant Nutrition and Soil Science 172(1): 10-23. http://dx.doi.org/10.1002/jpln.200800030
Narain, U., Gupta, S. and van ‘t Veld, K., 2008. Poverty and the environment: Exploring the relationship between household incomes, private assets and natural assets. Land Economics 84(1): 148-167. http://dx.doi.org/10.3368/le.84.1.148
Nasi, R., Brown, D., Wilkie, D., Bennett, E., Tutin, C., Van Tol, G. and Christophersen, T., 2008. Conservation and Use of Wildlife-based Resources: The Bushmeat Crisis. In: CBD Technical Series No. 33, Montreal: Secretariat of the Convention on Biological Diversity. https://www.cbd.int/doc/publications/cbd-ts-33-en.pdf
Naughton-Treves, L., Treves, A., Chapman, C. and Wrangham, R., 1998. Temporal patterns of crop-raiding by primates: Linking food availability in croplands and adjacent forest. Journal of Applied Ecology 35: 596-606. http://dx.doi.org/10.1046/j.1365-2664.1998.3540596.x
Nepstad, D., Carvalho, G., Barros, A.C., Alencar, A., Capobianco, J.P., Bishop, J., Moutinho, P., Lefebvre, P., Silva, U.L. and Prins, E., 2001. Road paving, fire regime feedbacks, and the future of Amazon forests. Forest Ecology and Management 154: 395-407. http://dx.doi.org/10.1016/s0378-1127(01)00511-4
New Zealand Nature Institute, 2006. Rural Livelihoods and Access to Forest Resources in Mongolia. LSP Working Paper 32. Rome: FAO, Livelihood Support Programme (LSP). http://www.fao.org/3/a-ah253e.pdf
Njuki, J, Kaaria, S., Chamunorwa, A. and Chiuri, W., 2011. Linking smallholder farmers to markets, gender, and intrahousehold dynamics: Does the choice of commodity matter? Eur European Journal of Development Research 23(3): 426-443. http://dx.doi.org/10.1057/ejdr.2011.8
Ntiamoa-Baidu, Y., 1997. Wildlife and Food Security in Africa. Rome: FAO. http://www.fao.org/docrep/w7540e/w7540e00.htm
Obiri, D.B., Bright, G.A., McDonald, M.A., Anglaaere, L.C.N. and Cobbina, J., 2007. Financial analysis of shaded cocoa in Ghana. Agroforestry Systems 71(2): 139-149. http://dx.doi.org/10.1007/s10457-007-9058-5
Obiri, D.B., Depinto, A. and Tetteh, F., 2011. Cost-benefit Analysis of Agricultural Climate Change Mitigation Options: The Case of Shaded Cocoa in Ghana. Research report prepared for IFPRI, Washington. 56pp.
Ohler, J.G., 1979. Cashew processing. Tropical Abstracts 21(9): 1792-2007.
Olson, S.H., Gangnon, R., Silveira, G.A. and Patz, J.A., 2010. Deforestation and malaria in Mâncio Lima County, Brazil. Emerging Infectious Diseases 16(7): 1108-1115. http://dx.doi.org/10.3201/eid1607.091785
Opeke, L.K., 1982. Tropical Tree Crops. Chichester, UK: John Wiley & Sons.
Oteng-Yeboah, A., Mutta, D., Byarugaba, D. and Mala, W.A., 2012. Africa. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_2
Padoch, C., Coffey, K., Mertz, O., Leisz, S., Fox, J., Wadley, R.L., 2007. The demise of swidden in Southeast Asia? Local realities and regional ambiguities. Geografisk Tidsskrift-Danish Journal of Geography 107: 29-41. http://dx.doi.org/10.1080/00167223.2007.10801373
Padoch, C., Brondizio, E., Costa, S., Pinedo-Vasquez, M., Sears, R.R. and Siqueira, A., 2008. Urban forest and rural cities: Multi-sited households, consumption patterns, and forest resources in Amazonia. Ecology and Society 13(2): 2. http://www.ecologyandsociety.org/vol13/iss2/art2/
Padoch, C. and de Jong, W., 1992. Diversity, variation, and change in ribereño agriculture. In: Conservation of Neotropical Forests: Working from Traditional Resource Use, edited by K. Redford and C. Padoch. Columbia University Press: New York.
Padoch, C. and Peluso, N., 1996. Borneo in Transition: People, Forests, Conservation and Development. Kuala Lumpur: Oxford University Press.
Padoch, C. and Peters, C., 1993. Managed forest gardens in West Kalimantan, Indonesia. In: Perspectives on Biodiversity: Case Studies of Genetic Resource Conservation and Development, edited by C.S. Potter, J.I. Cohen and D. Janczewski. Washington, DC: AAAS.
Palm, C.A., Sanchez, P.A., Ericksen, P.J. and Vosti, S.A. (Eds.), 2005. Slash-and-burn Agriculture: The Search for Alternatives. New York: Columbia University Press.
Paoletti, E., Schaub, M., Matyssek, R., Wieser, G., Augustaitis, A., Bastrup-Birk, A.M., Bytnerowiczg, A., Günthardt-Goergb, M.S., Müller-Starckc, G. and Serengilh, A., 2010. Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environmental Pollution 158: 1986-1989. http://dx.doi.org/10.1016/j.envpol.2009.11.023
Pardini, R., Bueno, A.A., Gardner, T.A., Prado, P.I. and Metzger, J.P., 2010. Beyond the fragmentation threshold hypothesis: Regime shifts in biodiversity across fragmented landscapes. PLoS ONE 5: e13666. http://dx.doi.org/10.1371/journal.pone.0013666
Parrotta, J.A., 1993. Cocos nucifera L. Coconut, palma de coco. Res. Note SO-ITF-SM-57. New Orleans, LA: USDA Forest Service, Southern Forest Experiment Station. http://www.fs.fed.us/global/iitf/pubs/sm_iitf057 (7).pdf
Parrotta, J.A. and Agnoletti, M., 2012. Traditional forest-related knowledge and climate change. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_13
Parrotta, J.A. and Trosper, R.L. (eds.), 2012. Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity. Dordrecht, Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9
Parry, L., Barlow, J. and Peres, C.A., 2009. Hunting for sustainability in tropical secondary forests. Conservation Biology 23(5): 1270-1280. http://dx.doi.org/10.1111/j.1523-1739.2009.01224.x
Pérez, E. and Pacheco, L.F., 2006. Damage by large mammals to subsistence crops within a protected area in a montane forest of Bolivia. Crop Protection 25: 933-939. http://dx.doi.org/10.1016/j.cropro.2005.12.005
Perfecto, I., Vandermeer, J., Mas, A. and Soto Pinto, L., 2005. Biodiversity, yield, and shade coffee certification. Ecological Economics 54: 435-446. http://dx.doi.org/10.1016/j.ecolecon.2004.10.009
Peters, P.E. 1986. Household management in Botswana: Cattle, crops and wage labor. In: Understanding Africa’s Rural Households and Farming Systems, edited by J.L. Moock. Boulder and London:Westview.
Pinedo-Vasquez, M., Hecht, S. and Padoch, C., 2012. Amazonia. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_4
Plantegenest, M., Le May, C. and Fabre, F., 2007. Landscape epidemiology of plant diseases. Journal of the Royal Society Interface 4: 963-972. http://dx.doi.org/10.1098/rsif.2007.1114
Platteau, J-P. 1992. Land Reform and Structural Adjustment in Sub-Saharan Africa: Controversies and Guidelines. FAO Economic and Social Development Paper 107. Rome: FAO.
Pooley, S., Fa, J.E. and Nasi, R., 2015. No conservation silver lining to Ebola. Conservation Biology 29(3): 965-967. http://dx.doi.org/10.1111/cobi.12454
Posey, D.A. (ed.), 1999. Cultural and Spiritual Values of Biodiversity. London: Intermediate Technology Publications, UNEP. http://dx.doi.org/10.3362/9781780445434
Powell, B., Thilsted Haraksingh, S., Ickowitz, A., Termote, C., Sunderland, T. and Herforth, A., 2015. Improving diets with wild and cultivated biodiversity from across the landscape. Food Security, in press. http://dx.doi.org/10.1007/s12571-015-0466-5
Power, A.G., 2010. Ecosystem services and agriculture: Tradeoffs and synergies. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 2959-2971. http://dx.doi.org/10.1098/rstb.2010.0143
Quisumbing, A.R., Payongayong, E., Aidoo, J.B. and Otsuka, K., 2003. Women’s land rights in the transition to individualized ownership: Implications for the management of tree resources in western Ghana. In : Household Decisions, Gender, and Development. A Synthesis of Recent Research, edited by A. Quisumbing. Washington DC: IFPRI.
Quisumbing, A.R., Estudillo, J.P. and Otsuka, K., 2004. Land and Schooling: Transferring Wealth Across Generations. Baltimore: John Hopkins University Press, for IFPRI.
Quisumbing, A.R. and Pandofelli, L,. 2009. Promising Approaches to Address the Needs of Poor Female Farmers: Resources, Constraints, and Interventions. IFPRI Discussion Paper 00882. Washington DC: IFPRI. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.227.3028&rep=rep1&type=pdf
Raintree, J. and Warner, K., 1986. Agroforestry pathways for the intensification of shifting cultivation. Agroforestry Systems 4(1): 39-54. http://dx.doi.org/10.1007/bf01834701
Ramakrishnan, P.S., 1992. Shifting Agriculture and Sustainable Development: An Interdisciplinary Study from North-eastern India. Man and Biosphere Book Series No. 10. Paris: UNESCO and Caernforth, Lancaster, UK: Parthenon Publishing.
Ramakrishnan, P.S., Rao, K.S., Chandrashekara, U.M., Chhetri, N., Gupta, H.K., Patnaik, S., Saxena, K.G. and Sharma, E., 2012. South Asia. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_9
Rangarajan, M., Desai, A., Sukumar, R., Easa, P.S., Menon, V., Vincent, S., Ganguly, S., Talukdar, B.K., Singh, B., Mudappa, D., Chowdhary, S. and Prasad, A.N., 2010. Securing the Future for Elephants in India. The Report of the Elephant Task Force. New Delhi: Ministry of Environment and Forests. http://www.moef.nic.in/downloads/public-information/ETF_REPORT_FINAL.pdf
Reij, C., 2014. Re-greening the Sahel: Linking adaptation to climate change, poverty reduction, and sustainable development in drylands. In: The Social Lives of Forests: Past, Present and Future of Woodland Resurgence, edited by S. Hecht, K. Morrison and C. Padoch. Chicago and London: University of Chicago Press.
Rerkasem, K., Lawrence, C., Padoch, D. Schmidt-Voght, D., Zeigler, A.D. and Bruun, T.B., 2009. Consequences of swidden transitions for crop and fallow biodiversity in Southeast Asia. Human Ecology 37: 347-360. http://dx.doi.org/10.1007/s10745-009-9250-5
Rice, R.A. and Greenberg, R., 2000. Cacao cultivation and the conservation of biological diversity. AMBIO: A Journal of the Human Environment 29(3): 81-87, 167-173. http://dx.doi.org/10.1639/0044-7447(2000)029[0167:ccatco]2.0.co;2
Ricketts, T.H., 2004. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conservation Biology 18: 1262-1271. http://dx.doi.org/10.1111/j.1523-1739.2004.00227.x
Ricketts, T.H., Regetz, J., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Bogdanski, A., Gemmill-Herren, B., Greenleaf, S.S., Klein, A.M., Mayfield, M.M., Morandin, L.A., Ochieng, A. and Viana B.F., 2008. Landscape effects on crop pollination services: Are there general patterns? Ecology Letters 11: 499-515. http://dx.doi.org/10.1111/j.1461-0248.2008.01157.x
Rival, L.M., 2002. Trekking Through History: The Huaorani of Amazonian Ecuador. New York: Columbia University Press.
Rocheleau, D. and Edmunds, D., 1997. Women, men and trees: Gender, power and property in forest and agrarian landscapes. World Development 25(8): 1351-1371. http://dx.doi.org/10.1016/s0305-750x(97)00036-3
RRI, 2012. What Rights? A Comparative Analysis of Developing Countries’ National Legislation on Community and Indigenous Peoples’ Forest Tenure Rights. Washington DC: Rights and Resources Initiative. http://www.rightsandresources.org/
Rose, L., 1996. Disputes in Common Property Regimes. Land Tenure Center Paper 154. Madison: Land Tenure Center, University of Wisconsin-Madison.
Rudel, T.K., DeFries, R., Asner, G.P. and Laurance, W.F., 2009. Changing Drivers of Deforestation and New Opportunities for Conservation. Conservation Biology 23(6): 1396-1405. http://dx.doi.org/10.1111/j.1523-1739.2009.01332.x
Ruf, F. and Schroth, G., 2004. Chocolate forests and monocultures: A historical review of cocoa growing and its conflicting role in tropical deforestation and forest conservation. In: Agroforestry and Biodiversity Conservation in Tropical Landscapes, edited by G. Schroth, G.A.B. Da Fonseca, C.A. Harvey, C. Gascon, H.L. Lasconcelos and A.N. Izac. Washington, DC: Island Press. http://library.uniteddiversity.coop/Permaculture/Agroforestry/Agroforestry_and_Biodiversity_Conservation_in_Tropical_Landscapes.pdf
Russell, W.M S., 1988. Population, swidden farming and the tropical environment. Population and Environment 10: 77-94. http://dx.doi.org/10.1007/bf01359134
Sanchez P.A., 1995. Science in agroforestry. Agroforest Systems 30: 5-55. http://dx.doi.org/10.1007/bf00708912
Sanchez, P.A., Palm, C.A., Vosti, S.A., Tomich, T. and Kasyoki, J., 2005. Alternatives to slash and burn: Challenges and approaches of an international consortium. In: Slash-and-burn Agriculture: The Search for Alternatives, edited by C.A. Palm, S.A. Vosti, P.A. Sanchez and P.J. Ericksen. New York: Columbia University Press.
Saroj, P.L. and Rupa, T.R., 2014. Cashew research in India: Achievements and strategies. Progressive Horticulture 46(1): 1-17.
Sauer, C.O., 1969. Agricultural Origins and Dispersals, 2nd edn. Cambridge and London: MIT Press.
Scherr, S.J., 1995. Economic factors in farmer adoption: Patterns observed in Western Kenya. World Development 23: 787-804. http://dx.doi.org/10.1016/0305-750x(95)00005-w
Schlager, E. and Ostrom, E., 1992. Property rights and natural resources: A conceptual analysis. Land Economics 68(3): 249-62. http://dx.doi.org/10.2307/3146375
Schlegel, S.A. and Guthrie, H.A., 1973. Diet and the tiruray shift from swidden to plow farming. Ecology of Food and Nutrition 2(3): 181-191. http://dx.doi.org/10.1080/03670244.1973.9990335
Schmidt-Vogt, D., Leisz, S.J., Mertz, O., Heinimann, A., Thiha, T. Messerli, P., Epprecht, M., Cu, P.V., Chi, V.K., Hardiono, M. and Dao, T.M., 2009. An assessment of trends in the extent of swidden in Southeast Asia. Human Ecology 37: 269-280. http://dx.doi.org/10.1007/s10745-009-9239-0
Schroth, G. and Harvey, C., 2007. Biodiversity conservation in cocoa production landscapes: An overview. Conservation and Biology 16(8): 2237-2244. http://dx.doi.org/10.1007/s10531-007-9195-1
Scott, J., 1999. Seeing Like a State: How Certain Schemes to Improve the Human Condition have Failed. New Haven, CT: Yale University Press.
Sinu, P.A., Kent, S.M. and Chandrashekara, K., 2012. Forest resource use and perception of farmers on conservation of a usufruct forest (Soppinabetta) of Western Ghats, India. Land Use Policy 29: 702-709. http://dx.doi.org/10.1016/j.landusepol.2011.11.006
Smith, D.A., 2005. Garden game: Shifting cultivation, indigenous hunting and wildlife ecology in western Panama. Human Ecology 33(4): 505-37. http://dx.doi.org/10.1007/s10745-005-5157-y
Smith, N.J.H., Williams, J.T., Plucknett, D.L. and Talbot, J.P., 1992. Tropical Forests and their Crops. Ithaca and London: Cornell University Press.
Spencer, J.E., 1966. Shifting Cultivation in Southeastern Asia. Berkeley and Los Angeles: University of California Press.
Stevens, C., Winterbottom, R., Springer, J. and Reytar, K., 2014. Securing Rights, Combating Climate Change: How Strengthening Community Forest Rights Mitigates Climate Change. Washington DC: World Resources Institute. http://www.wri.org/securingrights
Swift, M.J., Vandermeer, J., Ramakrishnan, P.S., Anderson, J.M., Ong, C.K. and Hawkins, B., 1996. Biodiversity and agroecosystem function. In: Functional Roles of Biodiversity: A Global Perspective, SCOPE Series, edited by H.A. Mooney, J.H. Cushman, E. Medina, O.E. Sala and E.-D. Schulze. Chichester, UK: Wiley.
Tejeda-Cruz, C., Silva-Rivera, E., Barton, J.R. and Sutherland, W.J., 2010. Why shade coffee does not guarantee biodiversity conservation. Ecology and Society 15: 13.
Thompson, I.D., Ferreira, J., Gardner, T., Guariguata, M., Koh, L.P., Okabe, K., Pan, Y., Schmitt, C.B., Tylianakis, J., Barlow, Kapos, V., Kurz, W.A., Spalding, M. and van Vliet, N., 2012. Forest biodiversity, carbon and other ecosystem services: Relationships and impacts of deforestation and forest degradation. In: Understanding Relationships Between Biodiversity, Carbon, Forests and People: The Key to Achieving REDD+ Objectives, IUFRO World Series No. 31, edited by J.A. Parrotta, C. Wildburger and S. Mansourian. Vienna: International Union of Forest Research Organizations.
Thrupp, L.A., Hecht, S.B. and Browder, J.O., 1997. The Diversity and Dynamics of Shifting Cultivation: Myths, Realities and Policy Implications. Washington, DC: World Resources Institute. http://pdf.wri.org/diversitydynamicscultivation_bw.pdf
Toledo, M. and Salick, J. 2006. Secondary succession and indigenous management in semideciduous forest fallows of the Amazon Basin. Biotropica 38(2): 161-170. http://dx.doi.org/10.1111/j.1744-7429.2006.00120.x
Tomalak, M., Rossi, E., Ferrini, F. and Moro, P.A., 2011. Negative aspects and hazardous effects of forest environments on human health. In: Forests, Trees and Human Health, edited by K. Nilsson, M. Sangster, C. Gallis, T. Hartig, S. de Vries, K. Seeland and J. Schipperijn. New York: Springer. http://dx.doi.org/10.1007/978-90-481-9806-1_4
Tontisirin, K., Nantel, G. and Bhattacharjee, L., 2002. Food-based strategies to meet the challenges of micronutrient malnutrition in the developing world. Proceedings of the Nutrition Society 61(2): 243-250. http://dx.doi.org/10.1079/pns2002155
Tscharntke, T., Klein, A.M., Kruess, A., Steffan-Dewenter, I. and Thies, C., 2005a. Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecology Letters 8: 857-874. http://dx.doi.org/10.1111/j.1461-0248.2005.00782.x
Tscharntke, T., Rand, T.A. and Bianchi, F.J.J.A., 2005b. The landscape context of trophic interactions:insect spillover across the crop-noncrop interface. Annual Zoology Fennici: 421-432. http://www.annzool.net/PDF/anzf42/anzf42-421.pdf
Tscharntke, T., Tylianakis, J.M., Rand, T.A., Didham, R.K., Fahrig, L. and Batáry, P., Bengtsson, J., Clough, Y., Crist, T.O., Dormann, C.F., Ewers, R.M., Fründ, J., Holt, R.D., Holzschuh, A., Klein, A.M., Kleijn, D., Kremen, C., Landis, D.A., Laurance, W., Lindenmayer, D., Scherber, C., Sodhi, N., Steffan-Dewenter, I., Thies, C., van der Putten, W.H. and Westphal, C., 2012. Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biological Reviews: 87: 661-685. http://dx.doi.org/10.1111/j.1469-185x.2011.00216.x
Turner, N.J., Łuczaj, Ł.J., Migliorini, P., Pieroni, A., Dreon, A.L., Sacchetti, L.E. and Paoletti, M.G., 2011. Edible and tended wild plants, traditional ecological knowledge and agroecology, Critical Reviews in Plant Sciences 30: 1-2, 198-225. http://dx.doi.org/10.1080/07352689.2011.554492
United Nations, 2009. The State of the World’s Indigenous People. New York: UN Department of Economic and Social Affairs, Permanent Forum on Indigenous Issues. http://www.un.org/esa/socdev/unpfii/documents/SOWIP/en/SOWIP_web.pdf
Uriarte, M., Pinedo-Vasquez, M., DeFries, R.S., Fernandes, K., Gutierrez-Velez, V., Baethgen, W. E. and Padoch, C., 2012. Depopulation of rural landscapes exacerbates fire activity in the western Amazon. Proceedings of the National Academy of Sciences 109(52): 21546-21550. http://dx.doi.org/10.1073/pnas.1215567110
Van Koppen, B., 1990. Women and the Design of Irrigation Schemes: Experiences from Two Cases in Burkina Faso. Paper presented at the International Workshop on Design for Sustainable Farmer-Managed Irrigation Schemes in Sub-Saharan Africa. Wageningen Agricultural University, The Netherlands, 5-8 February.
van Vliet, N., Mertz, O., Heinimann, A., Langanke, T., Pascual, U., Schmook, B., Adams, C., Schmidt-Vogt, D., Messerli, P., Leisz, S., Castella, J.-C., Jørgensen, L., Birch-Thomsen, T., Hett, C.,Bech-Bruun, T., Ickowitz, A., Vum, K.C., Yasuyuki, K., Fox, J., Padoch, C., Dressler, W. and Ziegler, A.D., 2012. Trends, drivers and impacts of changes in swidden cultivation in tropical forest-agriculture frontiers: A global assessment. Global Environmental Change 22: 418-429. http://dx.doi.org/10.1016/j.gloenvcha.2011.10.009
Veberic, R., Colaric, M. and Stampar, F., 2008. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chemistry 106(1): 153-157. http://dx.doi.org/10.1016/j.foodchem.2007.05.061
Velded, T., 2000. Village politics: Heterogeneity, leadership and collective action. Journal of Development Studies 36: 105-134. http://dx.doi.org/10.1080/00220380008422648
Wadley, R. L. and Colfer, C.J.P., 2004. Sacred forest, hunting, and conservation in West Kalimantan, Indonesia. Human Ecology 32: 313-338. http://dx.doi.org/10.1023/b:huec.0000028084.30742.d0
Warner, K., 1991. Shifting Cultivators: Local and Technical Knowledge and Natural Resource Management in the Humid Tropics. Community Forestry Note 8. Rome: FAO. ftp://ftp.fao.org/docrep/fao/u4390e/u4390e00.pdf
Watson, G.A., 1990. Tree crops and farming systems development in the humid tropics. Experimental Agriculture 26: 143-159. http://dx.doi.org/10.1017/s0014479700018147
Whitehead, A. 1985. Effects of technological change on rural women: A review of analysis and concepts. In: Technology and Rural Women: Conceptual and Empirical Issues, edited by I. Ahmed. London: George Allen and Unwin.
Wiersum, K.F., 1997. Indigenous exploitation and management of tropical forest resources: An evolutionary continuum in forest-people interactions. Agriculture, Ecosystems and Environment 63(1997): 1-16. http://dx.doi.org/10.1016/s0167-8809(96)01124-3
Wilcox, B.A. and Ellis, B., 2006. Forests and emerging infectious diseases of humans. Unasylva 57: 11-18. ftp://ftp.fao.org/docrep/fao/009/a0789e/a0789e03.pdf
Williams, T., 2014. Ebola’s silver lining. New Scientist 223: 26-27. http://dx.doi.org/10.1016/s0262-4079(14)61720-6
WOCAT, 2007. SWC Technology: Shade-grown Coffee. San José, Costa Rica: Ministerio de Agricultura y Ganadería.
World Bank, 2013. Forest, Trees and Woodlands in Africa. An Action Plan for World Bank Engagement. Washington, DC: World Bank. https://openknowledge.worldbank.org/bitstream/handle/10986/11927/730260REPLACEM0tion0Plan06014012web.pdf?sequence=1
World Bank, FAO and IFAD, 2009. Gender and Forestry, Module 15. Gender in Agriculture Sourcebook. Washington DC: World Bank. http://siteresources.worldbank.org/INTGEN
AGRLIVSOUBOOK/Resources/Module15.pdf
Youn, Y.-C., Liu, J., Daisuke, S., Kim, K., Ichikawa, M., Shin, J.-H. and Yuan, J., 2012. Northeast Asia. In: Traditional Forest-related Knowledge: Sustaining Communities, Ecosystems and Biocultural Diversity, edited by J.A. Parrotta and R.L. Trosper. Dordrecht, the Netherlands: Springer. http://dx.doi.org/10.1007/978-94-007-2144-9_8
Ziegler, A.D., Phelps, J., Yuen, J.Q., Webb, E.L., Lawrence, D., Fox, J.M., Bruun, T.B., Leisz, S.J., Ryan, C.M., Dressler, W., Mertz, O., Pascual, U., Padoch, C. and Koh, L.P., 2012. Carbon outcomes of major land-cover transitions in SE Asia: Great uncertainties and REDD+ policy implications. Global Change Biology 18(10): 3087-3099. http://dx.doi.org/10.1111/j.1365-2486.2012.02747.x
Zomer, R.J., Bossio, D.A., Trabucco, A., Yuanjie, L., Gupta, D.C. and Singh, V.P., 2007. Trees and Water: Smallholder Agroforestry on Irrigated Lands in Northern India. IWMI Research Reports, No. 122. Colombo, Sri Lanka: International Water Management Institute. http://indiaenvironmentportal.org.in/files/RR122.pdf
Zomer, R.J., Trabucco, A., Coe, R. and Place, F., 2009. Trees on Farm: Analysis of Global Extent and Geographic Pattern of Agroforestry. ICRAF Working Paper No. 89. Nairobi: World Agroforestry Centre. http://dx.doi.org/10.5716/wp16263.pdf
Zomer, R.J., Trabucco, A., Coe, R. Place, F., van Noordwijk, M. and Xu, J.C., 2014. Trees on Farms: An Update and Reanalysis of Agroforestry’s Global Extent and Socio-ecological Characteristics. Working Paper 179. Bogor, Indonesia: World Agroforestry Centre (ICRAF) Southeast Asia Regional Program. http://www.worldagroforestry.org/downloads/Publications/PDFS/WP14064.pdf
11 All terms that are defined in the glossary (Appendix 1), appear for the first time in italics in a chapter.