13. The Importance of Protected Areas
© 2019 J.W. Wilson and R.B. Primack, CC BY 4.0 https://doi.org/10.11647/OBP.0177.13
13.1 Establishing Protected Areas p. 462
13.2 Classification of Protected Areas p. 466
13.3 Prioritisation: What Should be Protected? p. 469
13.4 How Much Land Should We Protect? p. 478
13.5 Designing Protected Areas p. 480
13.6 Managing Protected Areas p. 494
13.7 Challenges for Protected Areas p. 500
13.9 Topics for Discussion p. 504

Sea anemones and cold-water corals are among the species that enjoy protection in the 1000 km2 Table Mountain National Park Marine Protected Area (MPA), South Africa. The MPA is divided into several no-take zones which act as breeding and nursery areas for marine life, as well as zones where harvesting is allowed under certain conditions. Photograph by Andrew Beard, https://www.flickr.com/photos/andrewbeard/13268749044, CC BY 2.0.
With its rich biological diversity, Africa plays a critical role in global conservation efforts. Yet, many of the continent’s most threatened species and ecosystems continue to face an uncertain future. In light of increasing human populations that need an increasing amount of natural resources each year, safeguarding the region’s biodiversity is a major challenge. One of the best ways to meet this challenge is to designate protected areas—regions where human activities are regulated or, at times, even prohibited by law.
Protecting existing wild populations in their natural ecosystems not only protects ecological communities and interactions, but also natural processes and ecosystem services.
Biodiversity conservation is most effective when we maintain healthy, functioning, and intact ecosystems. Although it is true that many species and populations live outside protected areas, and some wildlife populations (Craigie et al., 2010) and natural communities (Lindsey et al., 2014) are declining even when protected, well-managed protected areas continue to be the most effective method to safeguard biodiversity (Brooks et al., 2009; Ihwagi et al., 2015). Illustrating the point, a global meta-analysis, which included 952 locations across Sub-Saharan Africa, found that wildlife populations are 15% larger and species richness is 11% higher inside protected areas compared to populations directly outside (Gray et al., 2016). Differences may be even starker at individual sites: tea fields on Tanzania’s East Usambara Mountains held only 8% of the bird species present in the adjacent protected forest (Newmark, 2008), while some vultures in Eswatini now exclusively breed in protected areas (Monadjem and Garcelon, 2005). Studies from Tanzania have also shown how wildlife in protected areas are more resilient to climate change (Beale et al., 2013a), because habitat loss and fragmentation occur at four times their respective rates outside protected areas relative to inside them (see also Potapov et al., 2017). Consequently, until such a time that we can live more sustainably on unprotected lands, protected areas will remain an important cornerstone in our efforts to protect biodiversity. But how do we know what or where to protect, how much to protect, or how to effectively manage a protected area?
13.1 Establishing Protected Areas
A protected area is “a clearly defined geographical space (Figure 13.1), recognised, dedicated and managed through legal or other effective means to achieve the long-term conservation of nature with associated ecosystem services and cultural values” (Dudley, 2008). Given this broad definition, it comes as no surprise that governments, organisations, and local communities use a variety of mechanisms to establish protected areas. The most popular of these mechanisms are:

Figure 13.1 Land clearing and agricultural development pushes right up to the eastern edge of Bwindi Impenetrable National Park, Uganda. It is important for protected areas—and zones within those areas—to have clearly defined boundaries to avoid confusion on where and how human activities are regulated. Photograph by Jason Houston/USAID, https://www.flickr.com/photos/usaid-biodiversity-forestry/38484053220, CC0.
- Government action, which can occur at a national, regional, or local level.
- Community-based initiatives by local people and traditional groups.
- Land purchases and holdings by private individuals and organisations.
- Protected areas established through co-management agreements.
- Development of biological field stations or marine laboratories.
13.1.1 Government protected areas
Government actions are generally considered the most secure form of protection because they involve passage of laws and buy-in from multiple levels of society. Of course, legislation establishing a protected area does not guarantee that the species and ecosystems therein are adequately preserved. Small populations, especially those living in small protected areas, often require active management (Section 8.7.5) to ensure their continued survival. Another concern is that laws protecting national parks and other wildlife sanctuaries are not strictly enforced, leading to so-called paper parks—parks that appear on official government lists, but with wildlife monitoring, law enforcement, and ecosystem management lacking on the ground (Laurance et al., 2012). However, government-sanctioned protected areas do lay a solid foundation for partnerships among governments, international conservation organisations, multinational banks, research institutes, and educational organisations. Such partnerships can bring together funding, training, and scientific and management expertise to maximise the potential value of those protected areas.
13.1.2 Community conserved areas
In many areas, local people already protect biological communities, forests, wildlife, rivers, and coastal waters in the vicinity of their homes. Protection on these community conserved areas is enforced by village elders and councils to ensure the sustainable use of natural resources such as food supplies and drinking water. Natural areas have also been set aside by royal families and churches to provide a space for spiritual activities (see Box 2.1) and sustainable harvesting of medicinal plants (see Box 5.2). Because human activities are highly restricted in these sacred spaces, they provide an important refuge for biodiversity. Today, an increasing number of traditional communities link cultural advocacy directly to conservation through the establishment of protected areas on their lands as a safeguard against developments that would compromise their way of living. Other communities establish protected areas to attract tourists and ensure the protection of special wildlife. One such example is the Iyondji Bonobo Community Reserve in the DRC, which protects bonobos (Pan paniscus, EN), forest elephants (Loxodonta cyclotis), as well as one of the world’s most enigmatic birds, the Congo peafowl (Afropavo congensis, VU) (Dupain et al., 2013).
Traditional communities may link cultural advocacy to conservation by establishing protected areas as a safeguard against developments that would compromise their way of living.
13.1.3 Privately protected areas
Over the last few decades, many African countries have adopted a more Western form of land tenure under private ownership. Wealthy individuals or groups of people have taken advantage of this opportunity by acquiring large tracts of land for ecotourism purposes (de Vos et al., 2019). Because the ecotourism potential of these privately protected areas depends on how well the property is managed (Clements et al., 2016), private landowners often invest considerable effort to maintain and even increase wildlife populations on their land. Privately protected areas have unique advantages over government-protected areas. For example, they have local buy-in from landowners and their employees by design; this is often a significant stumbling block for government-protected areas. Private sites could also employ innovative funding mechanisms that allow them to fast-track land acquisition, perhaps in response to threats such as development. In some areas, privately protected areas may even employ more people, pay better wages, and contribute more to local economies that government protected areas (Sims-Castley et al., 2005). Privately protected areas can, therefore, play a significant role in overall conservation efforts (see Box 2.3), particularly in areas where threatened species (Cousins et al., 2010) and ecosystems (Gallo et al., 2009) are underrepresented in government-protected areas.
Because the ecotourism potential of private protected areas depends on how they are managed, landowners prioritize maintaining and even increasing wildlife populations on their land.
Despite the advantages of privately protected areas, we must also consider the drawbacks. Like many community conserved areas, privately protected areas are not permanently protected by the same mechanisms and oversight as government protected areas are. Ownership and management style can also change at the whim of the landowner, or perhaps the heirs. At times, management practices may be detrimental to the species and ecosystems these privately protected areas claim to protect, for example, through introduction of invasive species and harmful breeding practices (Milner et al., 2007), and by resisting regulatory controls (Cousins et al., 2010). Innovative strategies will thus be required to ensure that these areas do contribute to biodiversity protection, which include education, support, and methods that balance financial gains with conservation goals.
13.1.4 Co-managed protected areas
Local people who support conservation and the protection of their local natural resources are often inspired to take the lead in protecting their local biodiversity. Governments and conservation organisations can assist such initiatives by allowing local people to access specialist expertise and obtain financial assistance to develop conservation and ecotourism infrastructure. These conservation areas, characterised by partnerships between different levels of society that share decision-making responsibilities and consequences of management actions, have been termed co-managed protected areas. Tanzania, where the management of more than two million hectares of forests and woodlands have been transferred to local groups (Blomley et al., 2019), has been particularly active in this regard. One of the biggest strengths of co-management is that, with proper consultation and engagement, it avoids eco-colonialism—the unfortunate practice by some governments and conservation organisations of disregarding the rights and practices of local people during the establishment and management of new conservation areas or environmental laws and regulations.
Contractual parks offer a good model on how to avoid eco-colonialism. These protected areas are established and managed through agreements with private or communal landowners whose land forms part of a protected area (usually a national park). This not only allows a larger area to be protected, but also allows local people to benefit from biodiversity conservation through benefit sharing and job generation initiatives. Contractual parks play an important role, especially in South Africa, where it is used as a tool to meet both conservation goals and restitution of previously dispossessed land. One such example is the |Ai-|Ais/Richtersveld TFCA (Figure 13.2), which protects a huge number of succulent plant species and a variety of desert ecosystems at the border between Namibia and South Africa. Much of this national park is make up of communal lands, with the landowners—the local Nama people—having co-management and benefit-sharing agreements with the South African government (Reid et al., 2004). Incorporating activities of the landowners in sections of the park enriches tourism experiences, such as boating, hiking, and birdwatching, and contributes to preserving the Namas’ cultural identity, pastoral lifestyle, and threatened local languages (Chennels, 1999).

Figure 13.2 A lone giant quiver tree (Aloidendron pillansii, CR) stands guard over a desert valley in |Ai-|Ais/Richtersveld TFCA, on the border between South African and Namibia. This TFCA is special in that it is an agreement park, established through the cooperation between governments and private landowners. Photograph by Vincent van Oosten, https://pixabay.com/en/richtersveld-south-africa-desert-758235, CC0.
13.1.5 Field stations and marine laboratories
Biological field stations and marine laboratories are a special kind of protected area that provide a dedicated stable space for scientists, students, and even the general public to pursue research projects on all kinds of natural phenomena in an intact environment (Tydecks et al., 2016). By facilitating collaboration and long-term observation, work done at field stations in Africa has led to several fundamental scientific advances, including improved understanding of environmental responses to climate change and acid rain, as well as advances in social development through conservation activities. Today, there are biological field stations in at least 24 Sub-Saharan African countries (Tydecks et al., 2016). Among them are Namibia’s Gobabeb Research and Training Centre which focuses on desert conservation, Kenya’s Mpala Research Centre (Box 13.1) which investigates the potential for wildlife and livestock to coexist, Nigeria’s A.P. Leventis Ornithological Research Institute (see Box 15.4) which focuses on bird conservation, and Uganda’s Makerere University Biological Field Station which has a long, distinguished record of primate research.
Box 13.1 Mpala Research Centre: A Living Laboratory for (More than Just) Scientists
Mpala Research Centre,
Nanyuki, Kenya.
http://www.mpala.org
In the heart of Kenya’s Laikipia district, Mpala Conservancy stretches over 200 km2 of semi-arid savannah, acacia bushland, wooded grassland, rocky escarpments and riverine communities along the Ewaso Nyiro and Ewaso Narok rivers. The area is home to an abundance of wildlife, including all the classic savannah mammals: impala (Aepyceros melampus, LC), Grant’s gazelles (Nanger granti, LC), reticulated giraffe (Giraffa camelopardalis reticulata, EN), leopards (Panthera pardus, VU), lions (P. leo, VU), spotted hyenas (Crocuta crocuta, LC), and some of the largest savannah elephant (Loxodonta africana, VU) and African wild dog (Lycaon pictus, EN) populations in Kenya. There are also a few species typical of the northern regions of the Somali-Maasai centre of endemism, such as Grevy’s zebra (Equus grevyi, EN) and gerenuk (Litocranius walleri, NT). Mpala also functions as a working cattle ranch, with upwards of 2,000 cattle, camels, and sheep that are available for use by researchers.
This “multiple use” landscape and its neighbouring ranches provide exceptional opportunities for researchers to study interactions among humans, their domestic herds and wildlife in an area where they coexist. Since much of East Africa’s wildlife is found in similar areas outside formal protection, such research could provide essential and widely applicable knowledge for conservation efforts. They will be particularly important as conservation managers will increasingly have to balance wildlife and rangeland management needs to remain effective, in a context of human population growth and economic development. The Mpala Research Centre, established here in 1994, attracts hundreds of scientists every year who use this “living laboratory” to pursue projects varying in scope from the population biology of individual species to community-level dynamics and ecosystem functioning (Rubenstein and Rubinoff, 2014).
Having a research station in this area facilitates long-term and large-scale field experiments, including the Kenya Long-term Exclosure Experiment (KLEE). The 18 KLEE plots are designed to keep out different groups of animals: some plots only exclude megaherbivores (e.g. elephants and giraffes); others exclude all large herbivores; still others only exclude domestic cattle, among other combinations. This allows for controlled studies of the effects of different groups of herbivores on the vegetation and on each other. This research reveals that while domestic stock and wild grazers compete for forage during the dry season, the presence of zebras enhances cattle weight gain during the wet season—perhaps because zebras consume dead grass parts, improving forage quality for cattle (Riginos et al., 2012). Other studies at Mpala have also shown that wildlife and livestock can coexist and facilitate each other’s success, given the right approaches in management (Odadi et al., 2011; Ogutu et al. 2016).
Another long-time focus of research at Mpala is the threatened Grevy’s zebra. Dr. Daniel Rubenstein (Princeton University) and his research group examine the influence of environmental features on competitive behaviour and reproductive patterns in plains zebra and Grevy’s zebra. In turn, they are interested in how these social processes influence zebra population size. Their findings have the potential to inform management strategies in areas where Grevy’s numbers are too low to be self-sustaining (Rubenstein, 2010).
Involving the non-scientific community, especially those living around conservancies, is crucial to the long-term success of conservation efforts. Recognising this, Mpala has hosted several citizen science initiatives. For example, The Great Grevy’s Rally was a photographic census that relied on inputs from both scientists and members of the public, who travelled to conservancies, such as Mpala, to take pictures of every Grevy’s zebra they could find. Researchers processed these images using the Image Based Ecological Information System (IBEIS, http://ibeis.org) to differentiate individuals using their stripe patterns. This allows them to determine population size and structure, and assess whether zebra numbers are stable, increasing, or decreasing.

Figure 13.A Participants in the Kid’s Twiga Tally trying to differentiate individual giraffes using photos to understand how the IBEIS software works. Photograph by Danielle Martin, CC BY 4.0.
Also hosted at Mpala, the Kids Twiga Tally (Kahumbu et al., 2016) was a similar “sight-resight” survey of reticulated giraffes that relied on IBEIS software to distinguish between individuals and determine population structure. Its 70 young participants (Figure 13.A) came from both city schools and nearby pastoralist communities, spanning a range of socio-economic backgrounds. After spending two days taking pictures of giraffes on GPS-enabled cameras, the children returned to their schools having contributed meaningfully to conservation science.
13.2 Classification of Protected Areas
Protected areas vary greatly in how they are managed. For some, particularly those that protect very sensitive and/or recovering wildlife populations and ecosystems, human activity—even activities, such as photography, hiking, or bird watching (which can cause trampling and disturb shy animals)—may at times need to be forbidden except for specially arranged guided tours. For others, extraction of natural resources may be permitted albeit regulated.
To distinguish how protected areas are managed, the IUCN developed six categories to classify protected areas based on how the land is used (Table 13.1). Of these categories, the first five can be defined as true protected areas, because the environment is managed primarily for biological diversity. The sixth category, Protected area with sustainable use of natural resources, refers to extractive reserves that are managed primarily for the sustainable production of natural resources, such as timber and grazing lands. Nevertheless, extractive reserves can play an important role in conservation: (1) they frequently protect much larger areas than do other types of protected areas; (2) they still provide habitat for many species that were present pre-extraction; and (3) they often border and can thus provide a buffer around, and wildlife linkage between, category I–V protected areas.
Managers of extractive reserves must seek balance between the harvest of natural resources and risking environmental degradation from unsustainable practices.
Table 13.1 Description of Categories I–VI of the IUCN’s classification of protected areas.
|
Category |
Description |
|
|
Ia |
Strict nature reserve |
Managed strictly for biodiversity conservation. Serves as reference sites for research and monitoring. Human visitations and impacts highly regulated. |
|
Ib |
Wilderness area |
Generally large and relatively unmodified natural areas without significant human habitations. Managed to preserve the area’s natural character and ecological integrity. |
|
II |
National Park |
Large natural areas set aside for protection of biodiversity and ecosystem processes. Also managed to support human activities (spiritual, education, scientific, recreation) compatible with biodiversity protection. |
|
III |
National monument of feature |
Managed to protect a natural feature (e.g. seamount, geological feature, ancient grove) with outstanding cultural and/or natural significance. Can cover a small area, and often have high visitor value. |
|
IV |
Habitat/species management area |
Protected area dedicated to the protection of a specific species of habitat. May at times required regular and active intervention to ensure primary management goals are met |
|
V |
Protected landscape/seascape |
An area with a significant natural or cultural value, created by the interaction between people and nature. Managed to safeguard the interactions that sustains the area’s value. Often act as model for sustainability |
|
VI |
Managed-resource protected area |
Managed primarily for the low-level, non-industrial, sustainable use of natural resources. Generally large, with most of its ecosystems intact. |
Source: After Dudley, 2008
It is important to note that not all protected areas are covered under the IUCN’s six-category system. Prominently are RAMSAR wetlands (Section 12.1.2) which are not incorporated under formal protected areas, but still protected under international law. Other examples include locally-managed marine areas and indigenous reserves, some of which are as effectively managed as formal protected areas. The IUCN is currently working on a new classification, called ‘other effective area-based conservation measures’ (OECM; IUCN WCPA, 2018), to officially recognise the contribution of areas falling outside formal protected area networks to biodiversity conservation efforts.
13.3 Prioritisation: What Should be Protected?
Historically, the boundaries of protected areas was often determined through pragmatic considerations, such as the availability of funds and land, and political influence, rather than ecological considerations. Many conservation areas were thus established on “lands that nobody wants”: marginal areas with little agriculture and development potential, or areas that were too remote to have high commercial value (a trend that continues even today: Venter et al., 2018). Other protected areas were established in locations with charismatic megafauna, so ecosystems without those species remained unprotected. Consequently, some of Africa’s most threatened species and ecosystems remain under-protected (Beresford et al., 2011).
In a crowded world with finite natural resources and limited funding, it is increasingly important to be strategic about where protected areas are established.
In a crowded world with finite natural resources and limited funding, it is becoming increasingly important to be strategic about where protected areas are established. To do this, conservation biologists and policy makers must answer three key questions: (1) What is most important to protect? (2) Where would it be best protected? (3) How could it be most effectively protected? Three criteria can be used to answer the first two of these questions:
- Distinctiveness (or irreplaceability): Ecosystems with species that are distinct in their taxonomy (e.g. ecosystems that contain the only species in a taxonomic group) or geographic distribution (e.g. endemic species), or ecosystems with unique attributes (e.g. scenic landscapes, unusual geological features).
- Endangerment (or vulnerability): Areas that contain concentrations of species threatened with extinction, or ecosystems in danger of being destroyed.
- Utility: Species and ecosystems that people value, including culturally significant species, economically valuable species or ecosystems, or areas that can contribute to combating climate change.
Using these criteria, scientists have developed several broadly complementary methods to prioritise areas for protection. The approaches differ more in what traits they emphasise rather than in their fundamental principles. Thus, although some people may argue about which approach is better, each approach contributes to the protection of biodiversity.
13.3.1 Species approach
Many protected areas are created to protect (e.g. threatened, culturally significant, or keystone) species. Species that provide the motivation to establish a protected area are known as focal species. As a prominent example using the focal species concept, the Alliance for Zero Extinction (http://www.zeroextinction.org) identified 67 priority sites across Sub-Saharan Africa (853 sites globally) that contain the last remaining populations of one or more Endangered or Critically Endangered species. Flagship species, such as gorillas, are a special kind of focal species because they capture public attention, have symbolic value, and are important for ecotourism purposes. Many flagship species and focal species are also umbrella species, because their protection indirectly benefits other species and ecosystem components with which they share their landscape.
Protected areas are often established to protect threatened or charismatic species, unique ecosystems, and or wilderness areas.
13.3.2 Ecosystem approach
There is debate among conservation biologists over whether ecosystems rather than individual species should be the primary target of conservation efforts. Supporters of an ecosystem approach argue that protecting and managing ecosystems can preserve more species and provide more value to people than spending the same amount of money to protect individual species. Focusing on ecosystems also allows for greater flexibility in justifying conservation efforts, because it can be easier to demonstrate the economic value of ecosystems for helping to control floods, filtering water, and providing opportunities for recreation. To that end, the WWF has identified 238 ecoregions across the globe (the “Global 200”)—57 of them in Sub-Saharan Africa—that are most crucial to the biodiversity conservation (Olson et al., 2002). This Global 200 analysis formed the basis of a more recent global assessment that identified 41 at-risk ecoregions—areas of high conservation priorities because they are undergoing high levels of habitat conversion and have low protected areas coverage (Watson et al., 2016). Africa has several at-risk ecoregions, particularly in Angola, South Africa, the DRC, and West Africa’s Sahel region. The IUCN Red List of Ecosystems (RLE, Section 8.5.1) is another example of an ecosystem-focused prioritisation for conservation. While the ecosystems approach overcomes several limitations of the species approach, some conservationists argue that focussing on distinct ecosystems may, in itself, be detrimental, and that the scope of conservation should be expanded, for example by also including biogeographic transition zones (van Rensburg et al., 2013).
13.3.3 Wilderness approach
Wilderness areas are large areas where people have had little influence on the environment (relative to other areas), they have few people living in them, and are unlikely places for human development in the short term. These areas are conservation priorities because they may be the only places where animals that require large home ranges can continue to survive in the wild. Further, wildernesses can serve as controls or benchmarks for researchers to measure the effect of human disturbance on nature. The most popular way to identify wilderness areas is to identify areas without roads. While very few roadless areas remain, many of the world’s most important roadless wildernesses, some larger than 10,000 km2, are in Africa (Ibisch et al., 2016). Of concern is that, second to South America, Africa also leads the world in wilderness losses over the past decade (Potapov et al., 2017). It is worth emphasising that even wilderness areas have had a long history of human activity (Roberts et al., 2017). It is not always necessary or even possible to eliminate all human activity from such areas, if those activities do not obstruct conservation goals.
13.3.4 Hotspot approach
Multiple prominent initiatives have prioritised conservation in areas where large concentrations of species can be protected in a relatively small area. Perhaps the most prominent example is the Global Biodiversity Hotspots initiative. Combining a species approach with an ecosystem approach, Global Biodiversity Hotspots are areas with exceptionally high levels of biological diversity and endemism—that is, irreplaceable biodiversity—that are threatened with imminent habitat destruction (Table 13.2). Norman Myers, a British biologist who launched his conservation career as a wildlife photographer in Kenya, originally proposed the Biodiversity Hotspot concept (Myers, 1988). Working with a team of prominent scientists, Myers identified 25 Hotspots (five of them in Sub-Saharan Africa), which contained 44% of all vascular plant species and 35% of all terrestrial vertebrate species on only 1.4% of the Earth’s land surface (Myers et al., 2000). More recently, Conservation International (CI) identified an expanded set of 36 Biodiversity Hotspots (Mittermeier et al., 2005), eight of which are in Sub-Saharan Africa (Figure 13.3). This expanded set of Biodiversity Hotspots covers only 2.3% of Earth’s surface yet contains over 50% of all plant species and over 40% of all terrestrial vertebrate species.
Table 13.2 A natural history comparison of Sub-Saharan Africa’s eight Global Biodiversity Hotspots.
|
Location |
Original extent (× 1,000 km2) |
Remaining undisturbed vegetation (%) |
Number of species |
||
|
Plants |
Birds |
Mammals |
|||
|
Guinean Forests of West Africa |
620 |
15 |
9,000 |
917 |
390 |
|
Succulent Karoo |
103 |
29 |
6,356 |
225 |
75 |
|
Cape Floristic Region |
90 |
20 |
9,000 |
320 |
127 |
|
Maputaland-Pondoland-Albany |
274 |
25 |
8,100 |
631 |
202 |
|
Coastal Forests of Eastern Africa |
291 |
10 |
4,050 |
633 |
198 |
|
Eastern Afromontane |
1,018 |
11 |
7,600 |
1,300 |
490 |
|
Indian Ocean Islandsa |
601 |
10 |
13,500 |
503 |
211 |
|
Horn of Africa |
1,659 |
5 |
5,000 |
697 |
220 |
Source: Mittermeyer et al., 2004; https://www.cepf.net/our-work/biodiversity-hotspots.
a Includes Madagascar and Mascarene islands
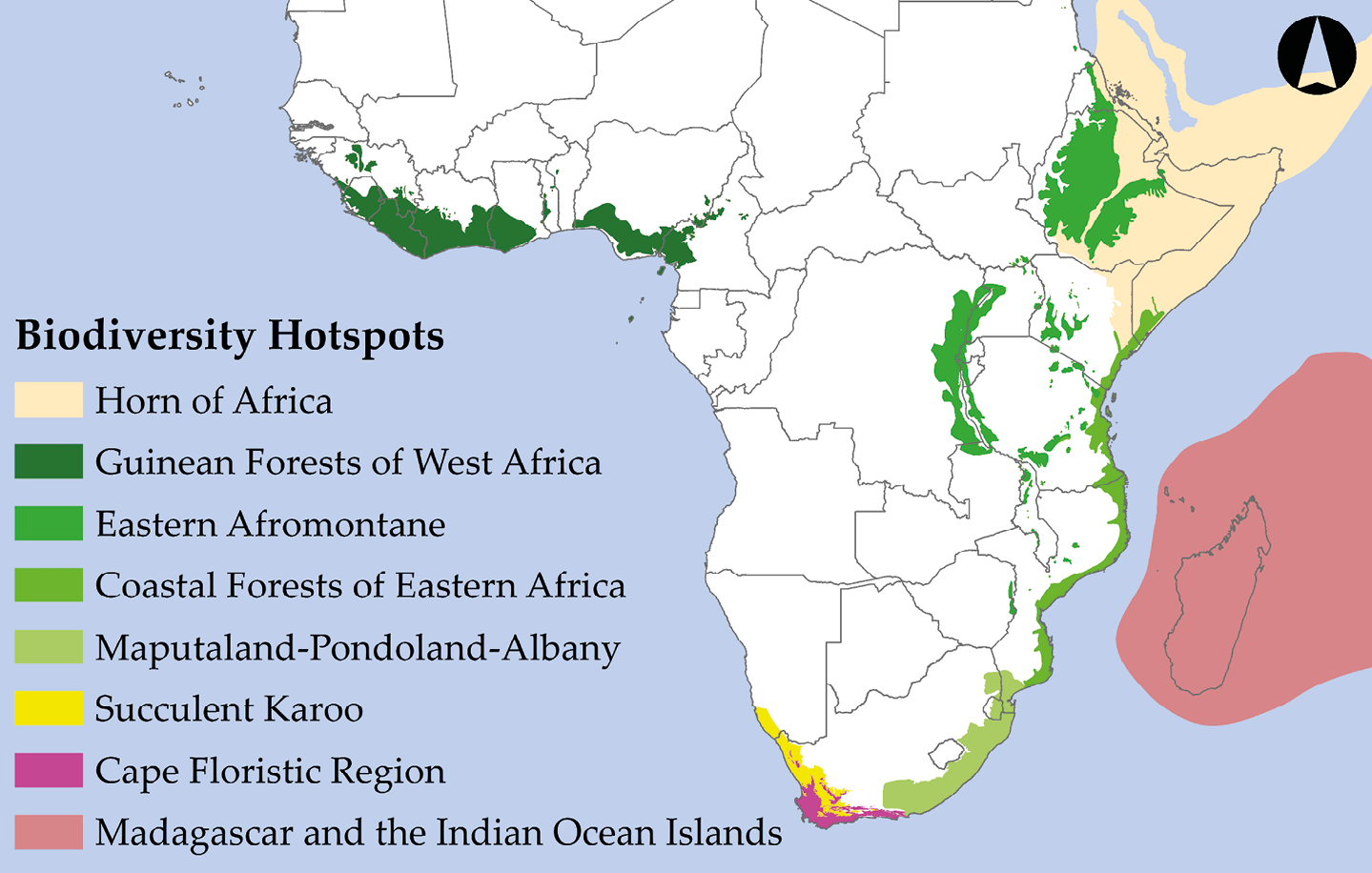
Figure 13.3 Sub-Saharan Africa’s eight Global Biodiversity Hotspots. These areas are targets for protection because of their high biodiversity, endemism, and significant threat of imminent extinctions. After Mittermeier et al., 2005. Map by Johnny Wilson, CC BY 4.0.
While the Global Biodiversity Hotspots highlight some of the most important global conservation priorities, none of these Hotspots are small enough to be contained in a single protected area—in fact, most of these Hotspots identify whole regions, not projects, requiring conservationists to still make decisions for prioritising protection within them. To create actionable priorities from within regional hotspots, several initiatives aim to identify local hotspots of species richness that can be conserved as one protected area of a manageable size. One such approach is the Key Biodiversity Areas (KBA) programme (Eken et al., 2004), which identifies conservation priorities using standardised criteria and thresholds that account for concentrations of threatened species and/or globally significant population aggregations. The KBA programme functions as an umbrella designation for several taxon-specific approaches, most prominently BirdLife International’s Important Bird and Biodiversity Areas (IBA) programme (Fishpool and Evans, 2011). Other KBA programmes include PlantLife International’s Important Plant Areas programme (e.g. Smith and Smith 2004), as well as the Important Sites for Freshwater Biodiversity programme (Darwall et al., 2005). One example from Guinea used KBA criteria and thresholds regarding threatened mammals to provide suggestions for expanding the country’s protected areas network (Brugiere and Kormos, 2009).
13.3.5 Gap analysis approach
Assessing the performance of existing protected areas can be done by spatially comparing their footprint to prioritised conservation areas (as above). Such an assessment offers not only an assessment of existing protected areas performance, but also offers a means to identify conservation gaps—important areas that still need to be protected to meet broader conservation goals. Such assessments, which systematically evaluate whether different aspects of biodiversity are adequately protected, are collectively known as systematic conservation planning assessments (McIntosh et al., 2017). Perhaps the most popular systematic conservation planning method is gap analysis, during which scientists overlay maps of species (or ecosystem) distributions with maps of protected areas to identify species (called gap species, see also Figure 10.3) or ecosystems that are not adequately protected in existing protected areas networks (Box 13.2).
Gap analysis enables conservation planners to identify species or ecosystems that are not adequately protected in existing protected areas networks.
Box 13.2 Identifying Key Sites for Conservation in the Albertine Rift
1Albertine Rift Program,
Wildlife Conservation Society,
Kampala, Uganda.
2Current Address:
Key Biodiversity Area Secretariat,
c/o BirdLife International,
Cambridge, UK.
aplumptre@keybiodiversityareas.org
The Albertine Rift is one of the richest regions on Earth for vertebrate diversity (Figure 13.B). Spanning about 100 km either side of the international border of the eastern DRC, it includes forests, wetlands and savannahs from eastern DRC and western Uganda, Rwanda, Burundi, and Tanzania, and runs from the northern end of Lake Albert to the southern end of Lake Tanganyika. It contains more than 40% of Africa’s mammals, 52% of Africa’s birds, as well as 19% of its amphibians and plants, in only 1% of the continent’s surface area. It also contains more endemic and globally threatened species than any other ecoregion in Africa (Plumptre et al., 2007). Endemic large charismatic species include the eastern gorilla (Gorilla beringei, CR), golden monkey (Cercopithecus kandti, EN), Congo bay owl (Phodilus prigoginei, EN), and Ruwenzori turaco (Ruwenzorornis johnstoni, LC). The lakes in the Albertine Rift each also contain several hundred unique fish species. Unfortunately, this rich biodiversity also occurs in one of the most densely populated parts of Africa, and the threats to existing protected areas are high.


Figure 13.B (Top) Mubwindi Swamp, in Bwindi Impenetrable National Park, an important site for mountain gorillas and the Albertine Rift endemic Grauer’s Rush Warbler (Bradypterus grayeri, EN). (Bottom) A Grauer’s gorilla, the largest of the four gorilla subspecies and a flagship for conservation efforts in the Albertine Rift. Photographs by A.J. Plumptre/WCS, CC BY 4.0.
The Wildlife Conservation Society (WCS) has been working to support the conservation of six key landscapes in the Albertine Rift (ARCOS, 2004), particularly focusing on (a) identifying critical areas for conservation of threatened and endemic species; (b) undertaking research and monitoring of species and key landscapes; and (c) supporting the conservation of critical sites and the creation of new protected areas to conserve large and small mammals, birds, reptiles, amphibians and plants in all protected areas, as well as sites where new protected areas might be established. These surveys have identified critically important areas in eastern DRC, such as the Itombwe and Kabobo Massifs where new species have been identified and some species were rediscovered, having been last seen more than 50 years ago. Working with local communities, the surveys have been used to design the boundaries of newly established protected areas to ensure that they capture as much of the biodiversity as feasible. Once the local people in the area are presented with survey results and options for protection discussed, they often realise the importance of their site and propose more stringent protection measures than conservationists initially thought possible.
Using species distribution models (SDM) of the region’s endemic and globally threatened species, WCS gained an understanding of where these species should occur both now and under future climate change scenarios (Ayebare et al., 2018). Using Marxan software (Possingham et al., 2000), WCS then identified those areas that would conserve all the species of conservation interest at minimum cost (Plumptre et al., 2019). This procedure identified the Itombwe and Kabobo Massifs together with the Sitebi Hills east of Mahale Mountains National Park in western Tanzania as being critical for conservation of species that are currently not adequately protected (Figure 13.C).
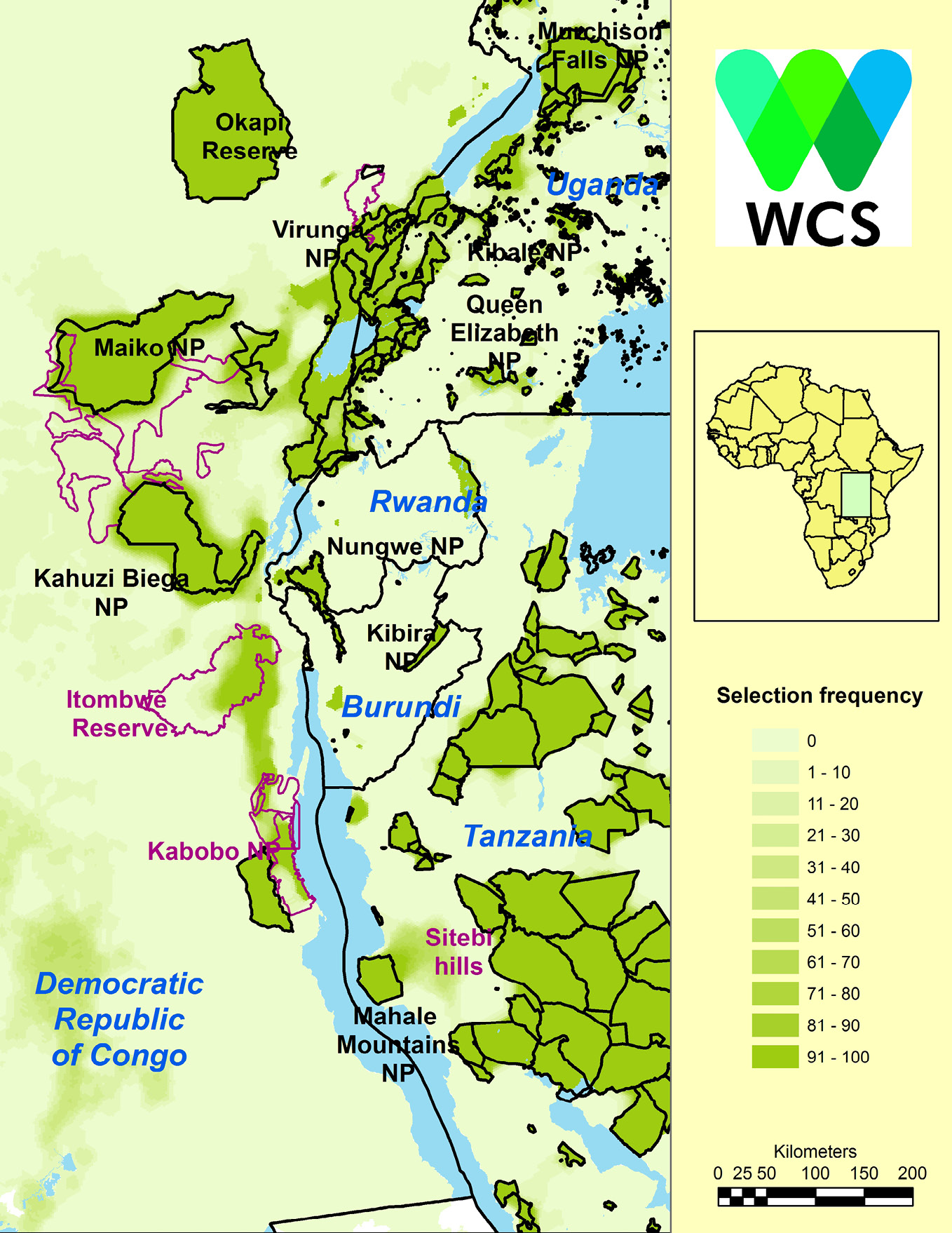
Figure 13.C Selection frequency of 5 km2 cells in the Albertine Rift from Marxan analysis, indicating priority areas for the conservation of endemic and threatened mammals, birds, reptiles, amphibians, and plants. Existing protected areas (all highlighted) were locked in but proposed protected areas such as Itombwe and Kabobo and community reserves (purple boundary) were not. Darker green areas indicate priority conservation sites. Image courtesy of WCS Albertine Rift Program, CC BY 4.0.
These results were used to develop an Albertine Rift Action Plan (Plumptre et al., 2016), together with detailed conservation action plans for the preservation of the six core landscapes and their unique and threatened species, both inside and outside of protected areas, now and into future.
When identifying conservation gaps, it is important to think carefully about the taxa or ecosystem used to make the assessment. Many conservation assessments assume that one well-known species group can act as a biodiversity indicator (also known as a biodiversity surrogate or surrogate species) for lesser-known taxa, so establishing a protected area to protect one gap species will also afford protection to other under-protected taxa. While this is true to some level, several studies have shown that this may not always be the case (Rodrigues and Brooks, 2007; Carwardine et al., 2008; Jones et al., 2016).
13.3.6 Optimisation approach
Prioritisation efforts typically need to consider multiple factors in addition to biodiversity, such as cost-effectiveness, socio-economics, site condition, and potential threats that may impact a proposed protected area. Technical computer software known as “decision support tools” are providing a new way to identify conservation priorities that meet a suite of conservation objectives. One of the most popular packages is Marxan (http://marxan.org), a freely available program that identifies the optimal location for protected areas based on flexible user-defined criteria (Watts et al., 2009). The user-defined criteria can be complex; for example, one can set the model parameters to choose the areas that best protect certain aspects of biodiversity (e.g. protect at least 25% of each vegetation type) while reducing costs and minimising impact on other stakeholders; model input can include measured data, as well as expert input. In one such example, conservation biologists from South Africa, Eswatini, and Mozambique used Marxan to identify potential locations for new protected areas in the Maputaland Centre of Endemism which the three countries share. They found that adding 4,291 km2 to the existing protected areas network could generate US $18.8 million in revenues while fulfilling their conservation objectives: protecting 44 landcover types, 53 species, and 14 ecological processes (Smith et al., 2008).
Decision support tools help identify conservation priorities that meet a suite of objectives, including cost-effectiveness, socio-economics, and site condition.
Regardless which prioritisation approach one follows, it is important to remember that prioritising species and ecosystems in need of protection does not amount to “doing conservation”. Real conservation only happens when a conservation plan that will implement those suggestions is drawn up and put in place. A review of eight different systematic conservation assessments in South Africa provides a good foundation to guide conservation biologists in the process from prioritisation to implementation (Knight et al., 2006).
13.4 How Much Land Should We Protect?
As of mid-2019, there were just over 7,500 protected areas covering over 4.5 million km2 of land and ocean surface (UNEP-WCMC, 2019) scattered across Sub-Saharan Africa (Figure 13.4). The country with the largest number of protected areas is South Africa with over 1,500 protected areas, while the country with the largest total area under protection is Tanzania, with over 360,000 km2. While these statistics may seem impressive, seeing these numbers in perspective is important before performance is judged. Currently, one of the most prominent sets of targets used to measure conservation progress is laid out in the international Aichi Biodiversity Targets (https://www.cbd.int/sp/targets). The global conservation area target reads:
“By 2020, at least 17 per cent of terrestrial and inland water areas and 10 per cent of coastal and marine areas, … are conserved … and integrated into the wider landscape and seascape.
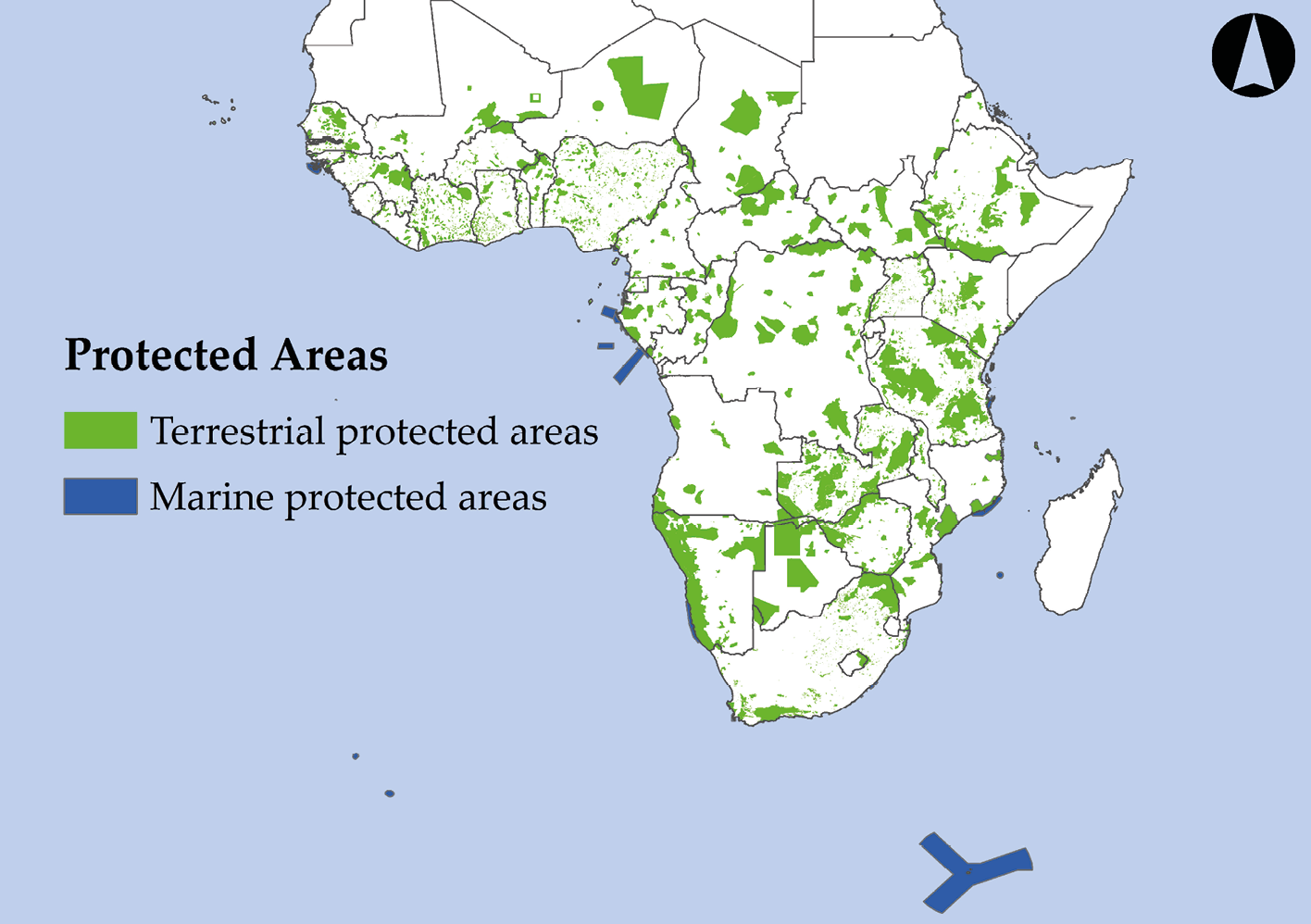
Figure 13.4 The location of Sub-Saharan Africa’s terrestrial and marine protected areas (MPAs), which falls under the IUCN’s categories I–VI classification for protected areas. Note that many small protected areas do not clearly show up at this scale. Source: UNEP-WCMC, 2019. Map by Johnny Wilson, CC BY 4.0.
While Sub-Saharan Africa as a region is well on its way to achieving its goal of protecting 17% of terrestrial areas, the percentage of land protected is very uneven among countries.
The good news is that as a region, Sub-Saharan Africa is well on its way to achieving the Aichi terrestrial target, since just under 17% of the region’s total land and inland water surfaces were protected as of mid-2019 (UNEP-WCMC, 2019). Further good news is that 22 Sub-Saharan African countries have protected more than 17% of their land area, with Seychelles (42%), Republic of the Congo (41%), and Tanzania (38%) leading the way. Sub-Saharan Africa’s protected areas network is also one the best performers globally in affording protection to migratory birds (Runge et al., 2015) and terrestrial megafauna (Lindsey et al., 2017).
Despite this progress, some notable gaps remain. Foremost, the percentage of land protected is very uneven among countries. While a few countries have reached the Aichi protected areas target, there were also 10 countries with less than 5% of their land protected, and an additional six countries which protect less than 10%. Furthermore, the amount of land protected does not necessarily translate to adequate protection for all ecosystems (Watson et al., 2016). For example, despite having the most protected areas, South Africa protects only 8% of its land, well below the Aichi target. Many protected areas also qualify as paper parks (Tranquilli et al., 2012, 2014), with a questionable contribution towards achieving conservation goals.
13.4.1 A neglected system: marine protected areas
When thinking about conservation in Africa, many people’s minds will wander towards images of a charismatic terrestrial mammal, like an elephant, lion, or gorilla. But what about all the whales, dolphins, sea urchins, starfish, nudibranchs, and other wonderful marine creatures? Perhaps due to the outsized influence of Africa’s famous land mammals on the ecotourism sector, Africa’s marine conservation efforts have always lagged behind terrestrial conservation efforts. In total, just over 700,000 km2 (7%) of Sub-Saharan Africa’s marine environment is protected (UNEP-WCMC, 2019). The gaps in marine conservation are even more obvious when one considers that as of mid-2019, only six countries have achieved the 10% Aichi Target, with Gabon (29%) and St. Helena (28%) leading the way. Marine protection is particularly lacking along the Atlantic coast (Klein et al., 2015), where many of 15 coastal countries protect less than 1% of their coastal and oceanic waters. It is also worth keeping in mind that the 10% coverage target (a modest goal that many countries may fail to achieve), may not be enough to achieve key conservation and sustainable development goals (Spalding et al., 2008). For example, to reverse declining commercially important fish populations, it is estimated that as much as 30% of the marine environment may need to be protected (O’Leary et al., 2016).
There is clearly an urgent need to establish more marine protected areas (MPAs), protected areas within oceanic and coastal environments (Box 13.3). There is also an urgent need to scale up law enforcement in the marine environment (Brashares et al., 2004). Increasing our marine protection efforts—which even local communities can initiate (Rocliffe et al., 2014)—is well worth it: it strengthens local fisheries (Kerwath et al., 2009; Lester et al. 2009) and offers educational and recreational opportunities, such as swimming and diving, which in turn generates ecotourism revenue. For example, Africa’ oldest MPA, Tsitsikamma National Park in South Africa (established in 1964), attracts over 170,000 visitors each year (Chadwick et al., 2014); the tourism revenues support numerous jobs and are a major stimulant of the local economy (Oberholzer et al., 2010). This is in stark contrast to the marine environment off West Africa, where unregulated fisheries are putting tremendous strain on local economies amid a lack of ecotourism infrastructure (Agnew et al., 2009; Gremillet et al., 2015).
Box 13.3 Marine Protected Areas in East Africa and the Western Indian Ocean
Acadia National Park, US National Park Service,
Bar Harbor, ME, USA.
How can MPAs in the Western Indian Ocean best enhance the preservation of biodiversity and the economies in this Global Biodiversity Hotspot? The ecosystems of the East African coast and nearby islands are diverse—mangrove forests, river deltas, coastal lagoons, rocky shores, sandy beaches, coral reefs, mud flats, seagrass beds, and open water. These areas are also economically important, with millions of people dependent on these waters’ shrimp, fish, and other natural resources for their livelihoods.
How effective are these MPAs, both in protecting biodiversity and people’s livelihoods? In 2006, an assessment of eight MPAs in Kenya, Tanzania, and Seychelles found several shortcomings, including inadequacies in staffing, funding, stakeholder engagement, and articulation of goals and management practices. Also, there needed to be additional monitoring and research to inform management and policy (Hockings et al., 2006). Despite these faults, the abundance and size of fish increased dramatically in several MPAs within 10 years of implementing fishing restrictions (McClanahan et al., 2007). The size and quality of fish caught in surrounding fishing grounds also increased substantially, probably due to fish dispersing from the MPAs.
Following these successes, the number and management of MPAs in the area have steadily increased and improved, at least, in part, due to cultivating better relationships with local stakeholders. One such example comes from the Quirimbas archipelago, just off the coast of northern Mozambique, where the Quirimbas National Park (over 1,000 km2) is managed through a cooperative effort of 40 villages, the government of Mozambique, and WWF. At the northern end of the Quirimbas archipelago, a few kilometres north of Quirimbas National Park, the Vamizi Conservation Project (Figure 13.D) protects an additional 230 km2 around the islands of Vamizi, Rongui and Macaloe. The Vamizi Project was initiated in 2002 as an innovative community-based management project involving local communities, international NGOs, and a group of individual investors. After protection, fish populations quickly began rebounding and had positive spill-over effects on fish around the reserve (da Silva et al., 2015). The stories of the abundant fish have contributed to a challenge for the project—attracting commercial fishermen from outside the area. To help ensure the financial and scientific sustainability of the project, partners developed a luxury ecotourism site and a research centre on Vamizi Island.

Figure 13.D Vamizi Island has some of the world’s richest and most pristine coral reefs, as well as the last population of the grey reef shark (Carcharhinus amblyrhynchos, NT) in Mozambique. The reefs are now protected thanks to a collaborative conservation effort that includes the local community. Photograph by Isabel Marques da Silva, CC BY 4.0.
Other protected areas have met variable degrees of success, as conservation managers and communities in the region test different approaches and figure out how best to sustain MPAs in a challenging environment. Different approaches are likely to work in different situations, depending on availability of resources, local stakeholders, and other constraints. As MPAs in the region continue to develop, coordination among countries could improve the value of the MPAs to biodiversity conservation. Already there are examples of multiple pathways to improving and expanding MPAs to protect biodiversity and achieve sustainable fisheries in this region (McClanahan et al., 2016). The future is hopeful.
13.5 Designing Protected Areas
The unplanned way in which protected areas have historically been established means that their design may at times impede rather than aid their goals. For example, many protected areas are too small to sustain viable populations of the species they are meant to protect. To avoid and mitigate such mistakes, conservation biologists are increasingly exploring methods to design more efficient protected areas networks.
Conservation biologists often start the process of designing protected areas networks by considering “the four Rs”:
- Representation: A network of protected areas should protect as much of the biodiversity (including species, ecosystems, genetic diversity, ecosystem processes, etc.) of a region, country, or subcontinent (depending on the scale of planning) as possible.
- Resiliency: Protected areas should be large enough that they can maintain biodiversity (including species, ecosystems, genetic diversity, etc.) for the foreseeable future, including in the face of climate change and natural disasters such as cyclones/hurricanes and uncontrollable wildfires.
- Redundancy: A network of protected areas should not rely on a single protected area to conserve key aspects of a region’s biodiversity; rather important aspects of biodiversity should be included in multiple protected areas to ensure their long-term existence.
- Reality: Each protected area requires sufficient funding, political will, defensibility, and local buy-in to support biodiversity over the long term.
In addition to the four Rs (which can also be applied to species protection), the following questions can also help guide planning of protected area networks (Figure 13.5):
- How large of an area must be protected and what landscape features must it include to effectively and sustainably protect biodiversity that may not be able to persist outside it?
- Is a single large protected area better, or are multiple smaller reserves more effective?
- What shape should a protected area be?
- When creating multiple protected areas, should conservation managers create them near one another or far apart? Should they be connected in some way, or should they be isolated from one another?
- How should human activities be accommodated? What activities should be allowed?
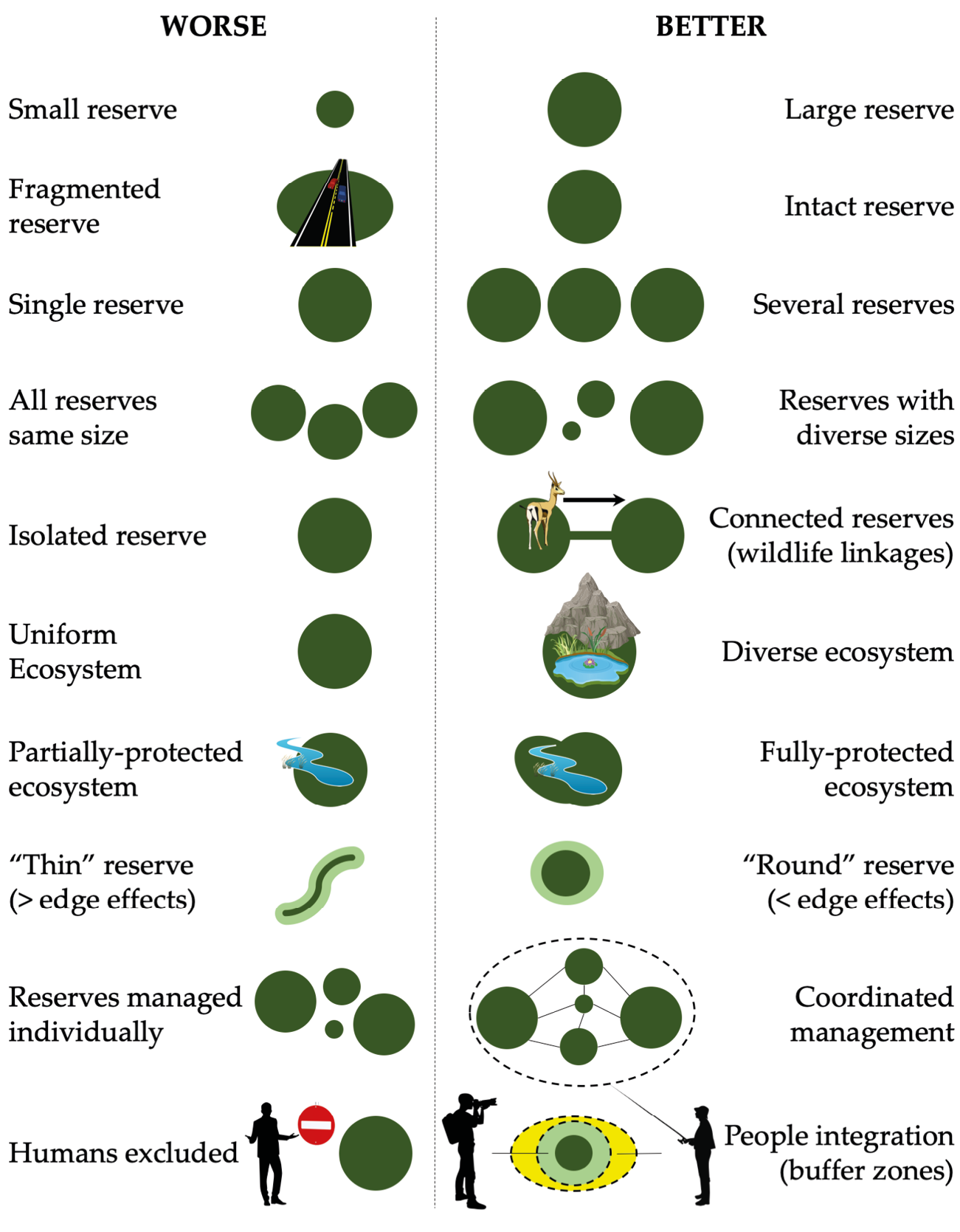
Figure 13.5 There are several major principles of reserve design to consider when establishing a new protected area or redrawing the boundaries of an existing protected area. While addressing all these principles is not always possible, the designs on the right are generally considered preferable to those on the left. After Shafer, 1997, CC BY 4.0.
To prepare readers for discussions with land managers, conservation planners, and policy makers who are in the process of developing new protected areas, the next section introduces some of the most important principles related to protected areas design. It is important to note that this introduction is not meant to serve as a universal set of rules for the design of protected areas. Because every project presents a special and unique set of circumstances, a single set of simplistic or overly general guidelines will not suffice. Also, the principles discussed below have been explored mainly in terms of protecting terrestrial vertebrates, vascular plants, and large invertebrates, so it is still uncertain how they apply to freshwater and marine nature protected areas.
13.5.1 What size should a protected area be?
The design of protected areas, and their size, is often addressed through the lens of the island biogeography model that states that large islands can accommodate more species and larger populations than small islands (Section 5.1). Research on extinction rates of populations (Newmark, 1996; Woodroffe and Ginsberg, 1998) and species richness (Harcourt et al., 2001; Brashares et al., 2001) has shown that protected areas function very much like islands. Specifically, because large protected areas contain greater habitat diversity than small protected areas, larger protected areas can accommodate (a) more species, (b) a larger range of ecosystem processes, and (c) viable populations of large species that range over large areas and live at low densities.
Large protected areas are generally preferred over small ones because they can support a greater variety of ecosystems and larger wildlife populations.
Given the range of costs and benefits of establishing large protected areas, conservation biologists have debated whether creating a single large reserve or several small reserves of the same total area—known as the SLOSS (Single Large Or Several Small) debate—is better. As discussed in Section 5.1.1, habitat fragmentation is currently one of the main drivers of species extinctions; it divides large populations into more vulnerable subpopulations, leads to undesirable edge effects, creates barriers to dispersal, and provides entry points for invasive species. These negative impacts are also of concern for protected areas, especially those that are small and fragmented (leading to larger perimeter:area ratios). For example, fragmentation concentrates elephants (Vanak et al., 2010) and apex predators (Cozzi et al., 2013) in the core of protected areas, greatly limiting the effective protected area for these taxa. However, these same impacts do not alter ungulate foraging (Kiffner et al., 2013), leading, potentially, to overgrazing near reserve borders. Studies have also shown how wildlife experience higher levels of mortality near protected area boundaries (Balme et al., 2010). Ignoring such edge effects could disrupt the long-term conservation value of a protected area, particularly small ones that could effectively function as edge habitat in its entirety. Because one big fragmented reserve has many of the characteristics of several small protected areas, conservation planners should aim to establish properly-placed large protected areas and to keep them as intact as possible. It is thus good practice to restrict and even remove highways, fences, farms, logging operations, and other human activities inside protected areas because of how they fragment habitats and reduce habitat availability overall.
But how do we know when a protected area is big enough? Ultimately, optimal size depends on the area over which important natural processes take place, which varies depending on the ecosystem. In some cases, the functional size may be quite small, such as a desert spring, a mountain bog, or a rocky outcrop. In contrast, the functional size of tropical forests, seasonal drylands, and desert communities are typically quite large, possibly spanning across country borders. Understanding and planning for protecting these different targets thus requires a familiarity with the functioning and ecology of each ecosystem.
When considering the size of a proposed protected area, conservation managers must also consider how well the area can be monitored and defended from threats. In some instances, an entire community may be incorporated into a relatively small protected area that is easy to monitor and defend against pollution, invasive species, and so forth. More often however, only a portion of the target community can be protected. In such cases it is important to consider how secure the conservation target will ultimately be. For example, if an aquatic organism needs protection, clearly the protection of its immediate habitat is critical. However, if a major threat is upstream from its habitat, then protection of the immediate habitat alone will be insufficient. Instead, managers would need to find ways to prevent outside threats from impacting populations inside the protected area. One option could be to discuss the threats and how to mitigate them with surrounding landowners, perhaps by facilitating their adoption of sustainable land-use practices. If the magnitude of the threats cannot be reduced to acceptable levels, a prioritisation programme might be used to identify critical sub-components of a larger ecosystem that will still accomplish the necessary protection. These kinds of considerations can become very complex and involved. But they are also very important to consider as options, especially when dealing with ecosystems situated between a variety of stakeholders.
13.5.2 Zoning as a solution to conflicting demands
While the general consensus seems to be that larger protected areas are better than smaller ones, establishing a properly-placed large protected area can be an imposing challenge. In a few special cases, large protected areas may be established through cooperation between multiple levels of society. More often, however, conservation biologists are faced with limited resources, and stakeholders can reasonably ask why a large park is required in an area that can otherwise be used to support agriculture or other types of businesses that may provide quick profits and jobs.
One way to deal with such conflicting demands while still achieving the target of protecting a large area is through a method called mixed-use zoning. Mixed-use zoning prioritises the overall conservation objectives for a protected area but also sets aside designated areas where certain regulated human activities are permitted (Box 13.4). In this way, some areas (or zones) may be designated for subsistence agriculture, shade-grown crops, timber production, hunting, ecotourism, or water management. Other areas are designated are dedicated to recovery of threatened species, ecotourism, ecosystem restoration, and scientific research. This is the case at the W-Arly-Pendjari (WAP) Complex, which straddles the border zone between Benin, Burkina Faso, and Niger. The core of the complex consists of three national parks covering 14,948 km2, set aside for strict biodiversity conservation. These national parks are surrounded by as many as 16 additional reserves, partial reserves, and hunting concessions, bringing the total area of protected Sudano-Sahelian savannah to 26,000 km2 (WHC, 2018).
Mixed-use zones sets aside areas for certain regulated human activities within a larger conservation area. This approach helps abate conflicting land use pressure.
Box 13.4 Zoning: Something for Everyone in the Forests of Dzangha-Sangha
1World Wildlife Fund,
Washington DC, USA.
2Current address:
The Pimm Group, Nicholas School of the Environment,
Duke University, Durham, NC, USA.
richardwcarroll@hotmail.com
Located in the dense forests of southwestern Central African Republic (CAR), in a wedge between neighbouring Cameroon to the West and the Republic of the Congo to the East, the Dzanga-Sangha Project (DSP) aims to conserve CAR’s last lowland tropical forest by integrating conservation and regional development. The DSP, which formally began in 1988 with the establishment of a cooperative agreement between WWF and the CAR government, is an integrated conservation and development project (ICDP); its activities are focused on protected area management, rural development, tourism, and project administration, as well as sustainable use of natural resources and applied ecological and social research. The focal area of the DSP is the Dzanga-Sangha Complex of Protected Areas (Figure 13.E), an area of 4,589 km2 comprising the Dzanga-Sangha Special Reserve (3,359 km2) and Dzanga Ndoki National Park (1,143 km2). The Complex is home to healthy populations of forest elephant (Figure 13.F), western lowland gorilla (Gorilla gorilla gorilla, CR), chimpanzee, and other wildlife characteristic of the Northwest Congolian Moist Lowland Forest (Carroll, 1992). The forest also shelters the BaAka Pygmies, a hunter-gatherer community whose traditional livelihood is directly linked to the forest and its plant and wildlife resources (Robinson and Remis, 2014).
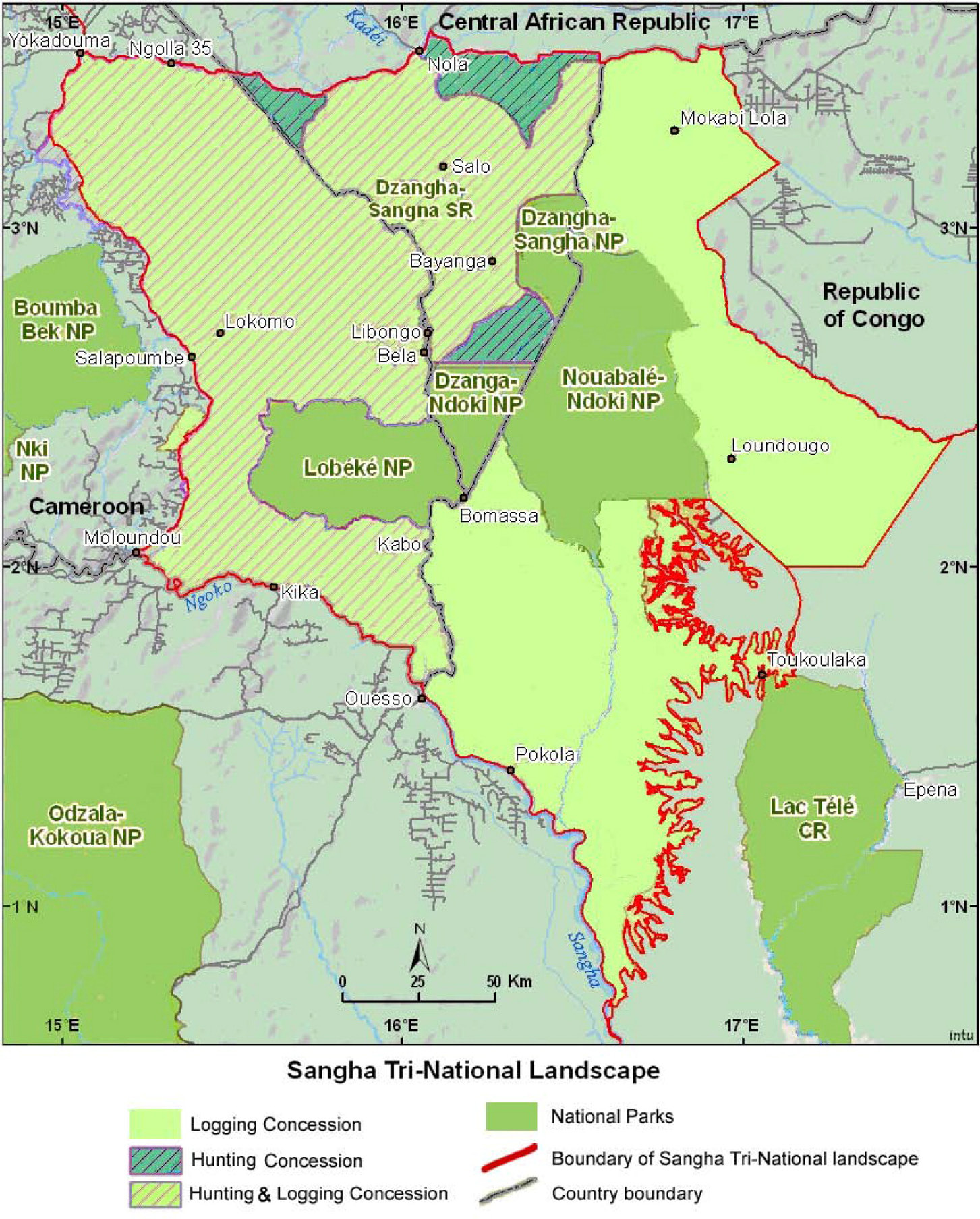
Figure 13.E The location of Dzanga-Sangha Special Reserve and Dzanga-Ndoki National Park, CAR, in relation to the Sangha Tri-National Landscape. Source: Endamana et al., 2010, CC BY 3.0.

Figure 13.F Forest elephants burrow for nutrients in Dzanga-Ndoki National Park’s mineral rich pools. Photograph by Ana Verahrami/Elephant Listening Project, CC BY 4.0.
Many of the WWF-supported programmes in Central Africa have sought to create the conditions for traditional peoples, such as Pygmies to maintain their lifestyles, and to adapt to changing social conditions should they choose. In the case of the DSP, two-thirds of the Complex area is classified as a “Special Reserve”, a designation that the CAR government created to accommodate traditional peoples’ use of the forest. While traditional hunting and gathering are broadly allowed in the Special Reserve, national laws specifically prohibit hunting of “integrally protected species”, such as gorillas, chimpanzees and elephants, in the Complex and elsewhere in CAR.
To establish a “safe zone” where wildlife can reproduce away from human pressures (Blom et al., 2004), and to accommodate tourism, one third of the Complex is designated as a national park. Hunting is not allowed in the national park; as compensation, 40% of all tourist receipts go to a village association, which includes BaAka, and 50% pays salaries for local employees of the park and special reserve. In other words, 90% of the dividends earned from tourism activities goes to the local people affected by conservation activities. The local community by and large supports the designation of this no-hunting zone, both to sustain their traditional activities and those of tourists.
Building on the successes in CAR, the DSP is also an active partner in the 36,000 km2 transboundary Sangha Tri-National (STN) initiative. Reflecting the Peace Park concept, the initiative is a multi-national effort to protect a large block of contiguous forests, the heart of which lies at the meeting point of the Congo-CAR-Cameroon boundaries. This initiative includes CAR’s Dzanga-N’Doki National Park, as well as two adjacent national parks: Cameroon’s Lobéké National Park (430 km2) and Republic of the Congo’s Nouabalé-Ndoki National Park (4,190 km2). These three national parks are surrounded by extensive buffer zones that include the Dzanga-Sangha Special Reserve, forests around Lobeke (700 km2) and the peripheral zone in Republic of the Congo with almost 12,000 km2 of logging concessions. STN was declared as the first landscape level World Heritage Site in 2012.
In summary, the Dzanga-Sangha Project is an ambitious, long-term effort of the CAR government, WWF, and other participating partners to save the largest and most biologically diverse tract of forest remaining in the region. Moreover, the evolution of the STN initiative demonstrates the shift from site-focused conservation to a more eco-regional or landscape strategy that incorporates the impact of human activities and the movement of animal populations across international boundaries.
Through its Biosphere Reserves programme, UNESCO has pioneered a formal zoning approach that integrates human activities, scientific research, biodiversity conservation, and tourism at a single location (Coetzer et al., 2014). A biosphere reserve is divided into three zones to delineate different levels of human use (Figure 13.6). The core of a typical biosphere reserve is a no-take zone (also called a core zone), strictly protected for biodiversity and ecosystem functioning. Around the core area is a restricted-use buffer zone, where people can conduct traditional, low-impact activities, such as collecting edible plants and small amounts of wood for fuel, and scientists can conduct non-destructive research. Outside of the buffer zone is a transition zone that allows some sustainable development (such as small-scale farming) and some medium-impact natural resource extraction (such as selective logging and fishing). As of mid-2019, there were 73 UNESCO Biosphere Reserves in 26 different Sub-Saharan African nations (http://www.unesco.org/new/en/natural-sciences); new reserves are regularly being added.
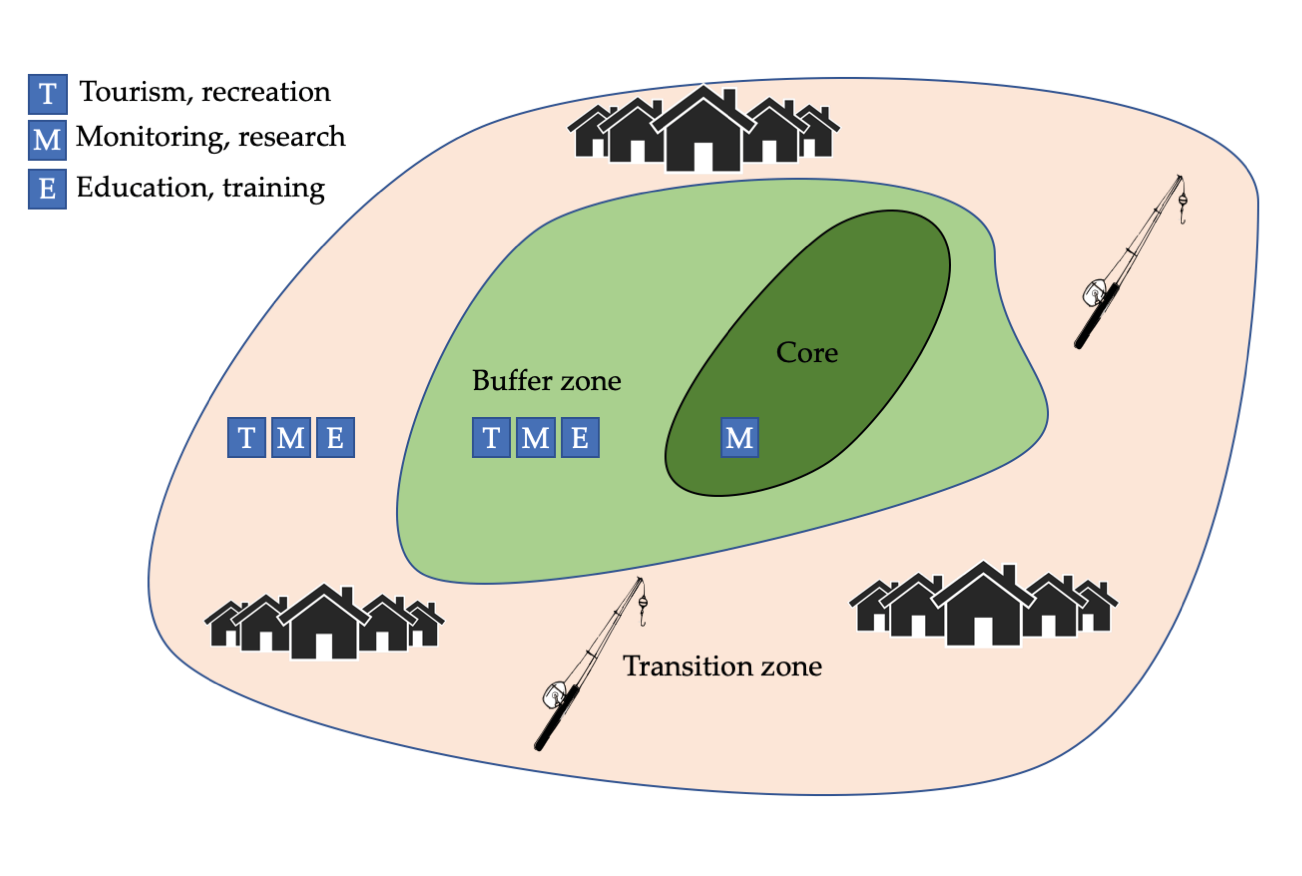

Figure 13.6 (Top) The general zones of a biosphere reserve: a core area set aside strictly for biodiversity conservation; a restricted-use buffer zone where human activities compatible with conservation are carried out; and a buffer zone dedicated to sustainable development. (Bottom) Fishermen on their traditional fishing boats in the buffer zone of Ethiopia’s Lake Tana Biosphere Reserve. Photograph by Alan Davey, https://www.flickr.com/photos/adavey/2260748777, CC BY 2.0.
Zoning is also proving effective in resolving conflicting demands over marine environments. Like terrestrial biosphere reserves, zoned MPAs consist of core zones where marine organisms can escape and recover from human disturbances, and multiple-use zones where activities such as harvesting of natural resources are permitted. Of course, harvesting fish and other marine species is not the only human activity that needs to be regulated. For example, many marine organisms are sensitive to anthropogenic noise, which interferes with communication and other important behaviours (Shannon et al., 2015). Creating multiple types of multiple-use areas can allow for different intensities of human activities. The is well illustrated at Eritrea’s Sheik Said Marine National Park; here, only approved scientific research is allowed in the restricted zone, low-impact ecotourism activities such as snorkelling and spiritual activities are allowed in the sanctuary zone, while noisy motorboats and limited take are allowed in the habitat protection zone (Habtemariam and Fang, 2016).
Zoned marine protected areas include core zones where marine organisms can escape human disturbance, and multiple-use zones where certain activities are permitted.
While resolving conflicting demands for space, zoning also provides benefits to biodiversity and people. For example, when compared to nearby unprotected sites, zoned MPAs typically have greater total weight of commercially important fish, greater numbers of individual fish, and greater coral reef cover (Lester et al., 2009). Conditions that allow marine organisms within MPAs to thrive, in turn, create opportunities for fish and other sea creatures to spill from the MPA into adjacent unprotected areas where they can be caught by local fishers, with a goal of a more sustainable harvest overall. A study from South Africa evaluated this hypothesis by attaching radio transmitters onto several white stumpnose (Rhabdosargus globiceps VU), an important fish for both commercial and recreational fisheries (Kerwath et al., 2009). This study showed that tagged fish spent 50% of their time outside the MPA, which would make them theoretically available to fishermen half of the time, while fish that did not leave protected waters produced offspring that could later disperse into multiple-use areas.
Despite the clear benefits of zoning, enforcing restrictions remains a major challenge. Even with good public outreach efforts and the threat of fines, harvesters of natural resources may still move toward and sometimes even into restricted zones to access more abundant or accessible natural resources. The resultant overharvesting at the margins of protected areas may prevent wildlife from dispersing into a wider area, which then make it hard for people that abide by the rules to access natural resources. The primary challenge in zoning is thus to find a compromise that the various stakeholders are willing to accept, and that provides opportunities for the long-term sustainable use of natural resources. Once those compromises have been agreed upon, a combination of local involvement, public outreach, clear posting of information signs, and visible enforcement of zoning restrictions can significantly increase the success of a zoning plan.
13.5.3 Connectivity among protected areas
Although large protected areas are preferable to smaller ones, sometimes small protected areas are the only available option, and conservation biologists must figure out how to protect biodiversity in these small areas. This is important in an African context, where most protected areas are very small, and only very few are sufficiently large to truly fulfil biodiversity needs (Table 13.3). To help conservation biologists meet this challenge, there is a growing body of evidence showing that small protected areas, even ones less than a hectare, can in fact be effective at maintaining viable wildlife populations. But how can that be? Does it suggest that small conservation areas are also useful for conservation purposes?
Table 13.3 A size comparison of Sub-Saharan Africa’s 10 largest protected areas.
|
Name |
Location |
Size (km2) |
Established |
|
Prince Edward Island Marine Protected Area |
South Africa |
181,230 |
2013 |
|
Termit & Tin Toumma National Nature and Cultural Reserve |
Niger |
97,000 |
2012 |
|
Ouadi Rimé-Ouadi Achim Faunal Reserve |
Chad |
77,950 |
1969 |
|
Air and Ténéré Reserves |
Niger |
77,360 |
1988 |
|
Central Kalahari Game Reserve |
Botswana |
52,800 |
1961 |
|
Namib-Naukluft National Park |
Namibia |
49,768 |
1979 |
|
Borana Controlled Hunting Area |
Ethiopia |
45,366 |
1973 |
|
Selous Game Reserve |
Tanzania |
44,000 |
1905 |
|
Ngiri-Tumba-Maindombe* |
DRC |
65,696 |
2008 |
|
Okavango Delta system* |
Botswana |
55,374 |
1996 |
Source: https://www.protectedplanet.net
* Ramsar wetlands
One of the main reasons why some wildlife populations can persist in small protected areas is that these areas violate an important assumption—that protected areas are isolated from one another. But we now know that wildlife populations often disperse between protected areas through the surrounding habitat matrix (Pryke et al., 2015). This dispersal maintains both metapopulation dynamics (Section 11.3) and reduces the risk of deleterious genetic effects (Section 8.7.1), allowing a network of small protected areas to effectively function as one large conservation area (Wegmann et al., 2014). In contrast, reserve isolation create population sinks for wildlife meant to be protected (Newmark, 2008). Consequently, re-establishing or maintaining connectivity within protected areas networks, and particularly among small reserves, has become an important strategy for enhancing their conservation value
Landscape connectivity may enable a network of small protected areas to effectively function as one large conservation area.
Many of the strategies used to maintain and restore ecosystem connectivity (Section 11.3) can be applied to protected areas management. However, this can be challenging given that administrative boundaries seldom consider natural ecosystem boundaries (Dallimer and Strange, 2015). Consequently, many ecosystems are artificially divided between different countries, each with its own development needs and management styles. Furthermore, many border barriers meant to restrict movement of people also restrict wildlife movement.
Transfrontier conservation areas enable two or more countries to collaboratively manage a shared ecosystem for mutual benefit.
Bioregional management seeks to conserve such large ecosystems that cross political borders. One way to accomplish this is to establish a transfrontier conservation area (TFCA) (also known as Peace Park or transboundary protected area), in which two or more countries collaboratively manage a shared ecosystem for mutual benefit (Hanks, 2008; see also Box 2.2 and Box 11.3). In addition to pooling scarce resources, this cooperative management style often includes removal of human-made physical barriers such as fences to allow free movement of animals (and sometimes also people, such as pastoralists) within the TFCA (Section 11.3.1). Sub-Saharan Africa first transboundary protected area was created in 1954, with the establishment of W National Park in Benin, Burkina Baso, and Niger, so named because the River Niger is shaped like letter “W” in this area. But it was only after the creation of the Peace Parks Foundation in 1997, and the Kgalagadi Transfrontier Park 2000, on the border between Botswana and South Africa (Anderson et al., 2013), that the concept gained widespread popularity in the region.
13.5.4 What about small isolated reserves?
At times, there will be no other choice than to accept that a small reserve is the only option available to achieve in situ conservation. In those cases, it is certainly better to accept the challenge. For many species, especially plants, a small protected area is the only buffer they have against extinction (Wintle et al., 2019). Biologists in South Africa have also pioneered an initiative to maintain species that require large home ranges in small, isolated protected areas by artificially managing dispersal dynamics (see Box 8.3). Small reserves, especially those located in or near populated areas (see Box 14.2), can also serve as locations for public outreach, conservation education, recreation, and citizen science that can improve public engagement with nature and awareness of conservation issues (Miller and Hobbs, 2002). Lastly, in addition to serving as stepping stones (Section 11.3.1), even small protected areas in urban areas provide various ecosystem services, including mitigating the urban heat island effect and reducing flooding (Feyisa et al., 2014, see also Section 7.1.6). In each of these cases, conservation biologists must creatively consider how to replicate natural processes across a small and/or fragmented protected areas network to ensure that they function on a scale that will maintain the target populations and communities.
13.6 Managing Protected Areas
Many people today have a misconception that the job of a conservation manager is done once a protected area is established. This might have been true if nature were “in balance” (a flawed concept in today’s human-dominated world, see e.g. Pimm, 1991). However, reality is very different. In many cases, humans have modified the environment so much that important populations and ecosystem processes cannot be maintained without at least some intervention, even inside protected areas. It is also important to regulate the activities of people who enter protected areas, particularly those who feel that reserves and national parks are shared public spaces that should be open to hunting, fishing, logging, farming, or mining activities. If we ignore these threats by leaving protected areas unmanaged and regulations unenforced, the biodiversity they are supposed to protect will almost certainly be lost over time.
Protected areas management should ideally be guided by a carefully-designed management plan assembled and regularly reviewed by a team of experts.
Every single protected area on Earth requires some form of management to be effective. Ideally, a protected area’s management is guided by a carefully-designed management plan assembled and reviewed by a team of experts (Henschel et al., 2014). While the details of each protected area’s management plan will be different, important aspects to address include monitoring and maintaining complex and adaptive ecosystems (Chapter 10), managing threatened species (Chapter 11), and providing resources, training, and memorable experiences to local people and visitors (discussed below). Management plans should also address which activities are prohibited (e.g. hunting or campfires) which activities are encouraged (e.g. wildlife photography, citizen science projects), and how rules and regulations will be enforced (Chapter 12). Lastly, the best management plans have a system in place to ensure that goals and activities are regularly reviewed and updated to account for new knowledge and experiences, and changing priorities.
In some protected areas, particularly small ones, it may be necessary to artificially maintain conditions that enable local wildlife to persist. One such example is the maintenance of natural fire regimes in fire-adapted ecosystems (Section 10.2.1). Another example is the temporary (or sometimes permanent) supply of limiting resources, such as exposed mineral licks, carcasses for scavengers, and nest boxes for bats and birds. Conservation managers might also establish artificial water sources or plant native fruit trees to support local (or translocated) wildlife. When taking such steps, it is important to strike a balance between establishing protected areas free from human influence and creating semi-natural areas in which plants and animals become so dependent on people that their persistence is not sustainable over the long term.
Management actions are generally implemented without completely understanding how the action will influence local ecosystem processes and wildlife populations. In light of this uncertainty, and despite good intentions, it should come as no surprise that some management actions may not achieve conservation goals. Some management actions may even later show to have unintended consequences that harm local biodiversity. While some actions are easy to reverse, some may put conservation managers on a cycle of reactionary management that is hard to escape. For that reason, it is important to carefully consider both the benefits and drawbacks of a management action before implementation. It is also important to be ready and willing to adapt management protocols as and when needed (see adaptive management, Section 10.2.3).
13.6.1 The importance of monitoring
An important aspect of a protected area management plan involves setting up a well-designed, long-term monitoring plan to assess whether conservation goals are being met. The exact types of information gathered will depend on the goals and objectives of each protected area, but can include tracking threatened wildlife populations, monitoring ecosystem health, or evaluating whether a threat is increasing or decreasing. These assessments may involve a wildlife survey (Section 9.1), taking regular measurements of various ecosystem indicators (Section 10.1), and/or conducting regular law enforcement monitoring (Section 12.3). In recognition of the linkages between the wellbeing of people and success of conservation (Oberholzer et al., 2010; Oldekop et al., 2016; Hauenstein et al., 2019), many conservation biologists are now also combining biodiversity monitoring with monitoring local peoples’ well-being.
A protected area management plan should include a long-term monitoring plan to assess whether conservation goals are being met.
Monitoring may highlight uncomfortable realities for conservation managers. An example could be management actions that prove to harm biodiversity (discussed above). Another uncomfortable reality is when one species needs to be prioritised over another. This is the case on protected islands off Southern Africa’s west coast, where biologists have resorted to selectively culling Cape fur seals (Actocephalus pusillus, LC) that predate on three species of threatened seabirds; in one case, this predation led to the abandonment of an entire seabird breeding colony (Makhado et al., 2009). Even more problematic is when one threatened species causes significant harm to another. This is the case in Uganda’s Kibale National Park, where chimpanzees kill as much as 12% of the area’s Ugandan red colobus monkeys (Procolobus tephrosceles, EN) each year (Watts and Mitani, 2002; Lwanga et al., 2011). It is however important to not confuse sustainable levels of predation with real threats that can lead to extinction. For example, in Ethiopia, the big-headed African mole rat (Tachyoryctes microcephalus, EN) is the favoured prey of the similarly-threatened Ethiopian wolf (Canis simensis, EN). However, rather than predation by the wolves, habitat loss from agriculture and overgrazing is the most important threat to the survival of the mole rat (Lavrenchenko and Kennerley, 2016), as well as the wolf (Marino and Sillero-Zubiri, 2011).
The control of any wildlife population, even invasive species in protected areas, can become very emotional for the public. It may even give rise to animal rights advocacy groups that oppose or even impede conservation. Such is the case in South Africa, where a well-organised group of local citizens opposed the eradication of invasive Himalayan tahrs (Hemitragus jemlahicus, NT), relatives of goats, which threatened imperilled Fynbos plants in a World Heritage Site (Gaertner et al., 2016). To avoid unnecessary conflict with such citizen groups, which can quickly turn into a public relations nightmare, it is important to consider whether drastic management actions are necessary. If so, it is wise to involve and educate the public for the need of such actions at an early stage.
Because monitoring can be resource-intensive, it is important to ensure the scale and methods of monitoring are appropriate for management needs. For small reserves, tracking only a few ecosystem components during periodic site visits might be sufficient. In contrast, for large or remote protected areas, geospatial analysis with environmental data obtained through remote sensing methods (Section 10.1.1) may be a more feasible way to monitor legal and illegal human impacts, such as logging (Figure 13.7), shifting cultivation, hunting, and mining. Many protected areas are also increasingly reliant on local people, researchers, tourists, and other groups of people to contribute to monitoring, particularly through citizen science projects (Section 15.4.1).
Figure 13.7 Satellite imagery provides a cost-effective method for monitoring ecosystem conditions, both inside and outside protected areas. These freely available NASA Landsat images show how Rwanda’s Gishwati Forest lost 99.4% of its 1,000 km2 forest cover between 1986 (left) and 2001 (right). The area was declared a national park in 2016, and wildlife populations have started increasing thanks to habitat protection and restoration efforts. Photographs by NASA, https://earthobservatory.nasa.gov/images/38644/gishwati-forest-rwanda, CC BY 4.0.
13.6.2 The importance of working with local people
The future of a protected area almost always depends on the degree of support, neglect, or hostility it receives from people who may be living inside the protected area, or in the surrounding area. Local people are unlikely to support conservation areas where there is a history of mistrust or disagreement between them and conservation authorities, or where park managers have not worked with and/or discussed conservation goals with them. This is particularly true when local people have been displaced by conservation actions (Cross, 2015; Baker et al., 2012) or are victims of human-wildlife conflict (Section 14.4). Such victims will understandably be angry and frustrated and may even reject conservation regulations altogether. Escalating cycles of hostility due to enforcement efforts can even lead to outright violence, during which protected areas staff, residents, and tourists can be threatened, hurt, or even killed.
The future of a protected area depends on the degree of support, neglect, or hostility it receives from people who live inside the protected area, or in the surrounding area.
To avoid such an ugly scenario, a central part of any protected area’s management plan must be a policy to ensure that local communities value, and benefit from, conservation activities. The ultimate goal of such a policy should not only be to ensure that local people become strong supporters of conservation efforts, but that they later also willingly contribute to them. At a very basic level, this can be accomplished by developing a range of ecotourism opportunities, particularly those that encourage participation in citizen science projects (Section 15.4.1), and those that afford opportunities where the goals and benefits of a protected area can be explained to local people. South Africa’s SANParks does this by encouraging school visits and accommodating a variety of income groups through a multi-tiered fee system (Beale et al., 2013b). When conservation displaces local people or limits activities previously allowed, it might also be worth investigating whether there is room to practice traditional activities in a sustainable way. Such is the case in South Africa, where the regional conservation authority Ezemvelo KZN Wildlife allows local people to sustainably harvest plant resources, such as thatching grass and medicinal plants, from protected areas they manage (Beale et al., 2013b; see also Section 13.5.2).
The next level of involvement includes benefit sharing. This often takes the form of compensatory payments for people who have lost assets due to conservation actions (Hall et al., 2014; see also Section 14.4). Some park managers also provide educational and employment support to local communities. One example comes from Botswana’s Okavango Delta region, where employment opportunities generated through ecotourism ventures at Moremi Game Reserve greatly improved relationships between local communities and park managers (Mbaiwa and Strongza, 2011; see also Section 14.3). African Parks, who manages 15 national parks across 10 African countries, have made local involvement (Figure 13.8) and community development a core part of their mission, which they accomplish by constructing schools, facilitating entrepreneurship, and funding healthcare services. The third level of involvement involves co-management partnerships, where local people directly participate in park management and planning (discussed in Section 13.1.4).

Figure 13.8 Wildlife experts working with African Parks fitting an elephant in Garamba National Park, DRC, with a satellite tracking device. Garamba’s management staff sometimes invites chiefs and other local villagers to take part in park events; touching a live elephant and seeing how biologists, veterinarians, and other experts operate allows the visitors to connect to conservation on a very personal level. Photograph by Naftali Honig/African Parks, CC BY 4.0.
13.6.3 The importance of accommodating visitors
Developing plans that accommodate outside visitors is also an important aspect of protected areas management. Tourists are some of the most important outside visitors to attract. Their spending stimulates local economies, and provides funds for salaries, maintenance, and other conservation initiatives (Ferraro and Hanauer, 2014). When tourism activities are combined with citizen science projects (Section 15.4.1), visitors can also contribute to monitoring, further expanding the capacity of protected areas staff. Accommodating university students and other researchers is also important, as they could provide valuable information to park managers and training to staff at a steeply discounted price, compared to work by expensive outside consultants who may not always understand local dynamics.
While ecotourism provides opportunities for employment, income, and monitoring, it is important to manage the multiple threats introduced by visitors.
While visitors provide significant benefits, it is important to monitor harmful elements they may knowingly or unknowingly introduce (Buckley et al., 2016). For example, visitors may introduce invasive species (Spear et al., 2013; Foxcroft et al., 2019) or induce behavioural changes in the animals they observe (Geffroy et al., 2015). Visitors may also directly damage protected ecosystems: frequent boating and diving among reefs can degrade reef communities when divers’ flippers, boat hulls, and anchors crush fragile corals. Visitors may even kill wildlife directly when they trample wildflowers, disrupt nesting birds, collide into animals that are crossing roads, or spread diseases to wildlife (Ryan and Walsh, 2011). When visitor activities are restricted, especially previously-allowed activities, park managers need to be able to explain reasons for the current policies and ensure that reasonable alternatives are available. For example, if the number of tourists visiting a special wildlife spot must be restricted to prevent damage to a site, the tourists could be offered the chance to visit a different site or participate in another activity.
13.6.4 The IUCN Green List of Protected Areas
A challenge that park managers frequently face is objectively determining how well their protected areas are managed. While profit margins, tourist numbers, species diversity, and population indices offer some form of evaluation criteria, it is not a fool-proof system: some well-managed protected areas are not very accessible to tourists, while carelessly increasing species richness or wildlife populations will likely have detrimental consequences. Tools such as the Management Effectiveness Tracking Tool (Stolton et al., 2007), Spatial Monitoring and Reporting Tool (Moreto, 2015), and Rapid Assessment and Prioritisation of Protected Areas Management (Ervin, 2003) have helped park managers assess whether the goals of their management plans were being achieved. But with no global standard of best practices against which protected areas are objectively assessed, park managers are mostly left to evaluate success based on their own subjective criteria and goals.
To fill this gap, the IUCN recently established the Green List of Protected Areas (http://www.iucn.org/greenlist) which aims to increase the number of protected areas that are effectively and fairly managed (Figure 13.9). Nominated protected areas will be evaluated against a set of standards which attest to management structures that can achieve long-term positive impacts on biodiversity and people. This list of standards, adapted to reflect local contexts within which evaluated protected areas operate, is divided into four higher level components: (1) good governance, (2) sound design and planning, (3) effective management, and (4) successful conservation outcomes (Figure 13.10). There are even plans, through a “Fair Finance” initiative, to reward protected areas that receive Green List status by making resources available to further strengthen their accomplishments.

Figure 13.9 Ethiopia’s Simien Mountains National Park, where Gelada baboons (Theropithecus gelada, LC) roam in packs of hundreds, and globally threatened species such as the Walia ibex (Capra walie, EN) and Ethiopian wolf (Canis simensis, EN) hang on to the edge of existence. The continued persistence of these and other species endemic to this World Heritage Site depends on effective management of protected areas such as this. Photograph by Hulivili, https://en.wikipedia.org/wiki/File:Semien_Mountains_13.jpg, CC BY 2.0.
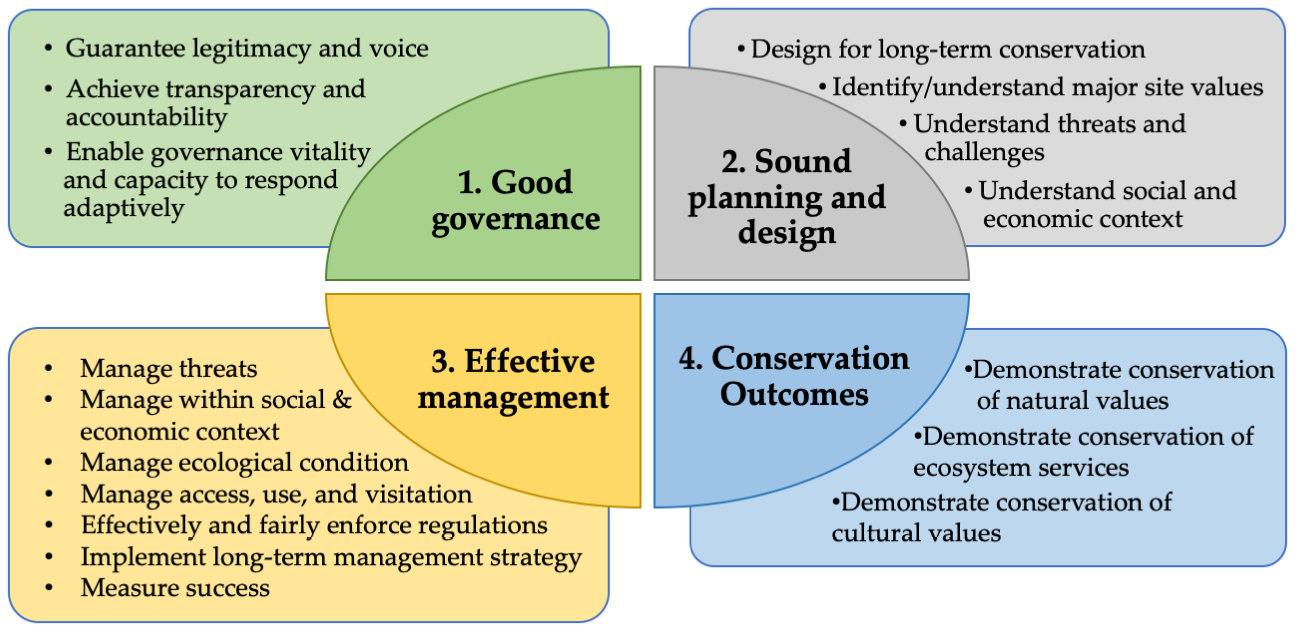
Figure 13.10 The list of generic standards, to be adapted for local contexts, against which protected areas will be evaluated before achieving IUCN Green List of Protected Areas status. After IUCN and WCPA, 2017, CC BY 4.0.
The Green List has only recently been established; hence, not many protected areas have been evaluated by the time this book was written. Sub-Saharan Africa’s first Green List sites were Kenya’s Lewa Wildlife Conservancy and Ol Pejeta Conservancy, both which formed part of the 2014 initial trial period. Both sites were re-certified in 2018, when Kenya’s Ol Kinyei Conservancy was also added to the Green List. Hopefully many more sites will follow suit in the near future.
13.7 Challenges for Protected Areas
The biggest challenges that park managers will face in the coming decades stem from a growing human population. When key natural resources, such as firewood and bushmeat, become harder to find, conflict is inevitable as more people look for new lands where they can fulfil their needs. As more people encroach into protected areas, so too will habitat loss, pollution, invasive species, and diseases. Despite conservationists’ best efforts to build collaborations with nearby communities, park managers need to anticipate that this ever-greater demand for space and natural resources will add additional challenges to their work plans. Below, we discuss three challenges that will likely continue to pose threats in future, and for which there are not always easy solutions.
13.7.1 Funding limitations
To enable protected areas to achieve their full potential, there must be adequate funding to support a team of well equipped, properly trained, and motivated staff (James et al. 2001; Gill et al., 2017). There is also a need for buildings, vehicles, communications equipment, and other appropriate infrastructure and resources to enable the staff to fulfil their duties, and for tourists to have a memorable time. The cost of these resources can quickly add up; for example, researchers estimated that more than $1 billion is needed each year to manage Africa’s protected areas that include lion populations (Lindsey et al., 2018). Yet, Africa’s protected areas are frequently understaffed, lack basic equipment, and face funding shortages (Tranquilli et al., 2014; Watson et al., 2014). Without the means to travel, communicate, and protect themselves, even motivated staff may find themselves stuck at their duty stations, unaware of what is happening elsewhere in their park. Some of these challenges can be solved with an adequate ecotourism plan, which can be facilitated from the grassroots level up or government level down. A growing number of funding mechanisms, including private and international donors, have also started to fill funding gaps (Section 15.3) which, in turn, has allowed more NGOs to assist in conservation areas management (Tranquilli et al., 2012; Lindsey et al., 2014). Above all, a carefully assembled management and monitoring plan, which is adequately funded, is key to the success of protected areas.
13.7.2 Planning for climate change
Because protected areas are fixed in space and time, many species that are currently protected will adjust their ranges beyond the borders of existing protected areas due to climate change. One study from South Africa found that 62% of bird species will lose some degree of protection over the next few decades, with five species losing at least 85% of their protected ranges (Coetzee et al., 2009). Studies in West Africa yielded remarkably similar results, where 63% of amphibians, 63% of mammals, and 55% of bird species face decreased protection due to changing climate (Baker et al., 2015). The situation is even worse for taxa with too little protection as it is. For example, suitable habitat for only 5% of African bat species is currently protected; due to climate change, it will further decrease by 2050 (Smith et al., 2016).
To ensure the future protection of species vulnerable to climate change, we must incorporate species’ predicted distribution ranges into the planning of protected areas networks. For species that disperse easily, this requires protecting gaps in their current and future ranges (Hole et al., 2011), as well as protecting, maintaining, and restoring potential dispersal pathways (Section 11.3). For poor dispersers, conservationists could start experimenting with assisted colonisations, or identify and protect their climate refugia (Section 11.4). For many species, however, establishing protected areas in their future ranges will be nearly impossible simply because no land is available. These species will greatly depend on conservation efforts outside protected areas, which we will discuss in Chapter 14.
13.7.3 Facing degazettement
It may be reasonable to assume that protected areas (especially government protected areas, established by law) afford permanent protection to biodiversity on those lands. Unfortunately, that is not the case—between 1950 and 2017, at least 227 different protected areas in Sub-Saharan Africa lost (partially or fully) lost their legal protected status (WWF and CI, 2016), in a process formally known as protected area downgrading, downsizing, and degazettement (PADDD, http://www.padddtracker.org). There are a variety of reasons behind PADDDs. For example, some protected areas have been PADDDed because of environmental degradation caused by conflicting land uses, including illegal logging, illegal agriculture, and land invasions; in such cases governments (in consultation with conservation managers) may determine that the resources needed for land rehabilitation are better spent protecting other sites (Fuller et al., 2010). Others have been PADDDed because incorrect procedures were followed during establishment—in such cases, it might be prudent to carefully consider if a compromise could be reached that combines the goals of conservation and development (Section 14.3). However, the vast majority of African PADDDs are enacted because of more sinister motives, such as to undercut conservation restrictions (Mascia and Pailler, 2011). For example, when examining each threat individually, data from WWF and CI (2016) suggest that mining pressure was the leading cause of previous African PADDDs. Considering that nearly 30% of African protected areas are still earmarked for oil and gas exploration (Leach et al., 2016), the threat from mining will likely also continue in the foreseeable future (Durán et al., 2013; Edwards et al., 2013).
Mining pressure is currently the leading cause for downgrading and degazettement of African protected areas.
Most conservationists consider the PADDD process a bad precedent that should be avoided unless necessary. While there are legitimate reasons behind some PADDDs (Fuller et al., 2010), few are enacted with conservation goals in mind. In many cases, government officials remove the protected status of lands without even consulting conservation scientists and park managers. Such decisions are particularly frustrating when important areas that protect threatened species and ecosystems are affected. Combatting the continuing threat of PADDDs will depend on national and international conservation organisations partnering with vigilant citizens who take ownership of their natural treasures. Until citizenry can trust that government officials have the interests of their natural heritage at heart, protected areas PADDDs will remain a highly controversial topic.
13.8 Summary
- Establishing protected areas is the most effective method for safeguarding biodiversity. Seventeen percent of Sub-Saharan Africa’s land surface is included in over 7,500 protected areas, with new reserves and parks regularly designated. In contrast, only 7% of the region’s marine and coastal environments are protected, with protection highly uneven among countries.
- Government agencies and conservation organisations set priorities for establishing new protected areas based on the relative distinctiveness, endangerment, and utility of a species or ecosystems. Many protected areas are established to preserve species of special significance, unique ecosystems, wilderness areas, and concentrations of threatened species. Gap analysis is used to identify elements of biodiversity not accommodated in existing protected area networks.
- While protected areas have previously been designed haphazardly, conservation biologists are developing guidelines for designing more effective protected areas. As general guidelines, protected areas should be large whenever possible, and should not be fragmented. Conservation planners should also aim to create linked networks of conservation areas to encourage wildlife dispersal.
- Protected areas must be actively managed to maintain biodiversity. Monitoring provides much needed information to evaluate whether management activities are achieving their intended objectives or need to be adapted.
- Managing interactions with local people and visitors is critical to the success of protected areas and should be part of a management plan. To obtain and maintain local support, managements plans should consider benefit sharing and co-management partnerships.
13.9 Topics for Discussion
- Obtain a map of your region’s protected areas (e.g. nature reserves and national parks) and multiple-use managed areas (e.g. hunting and logging concessions). (https://protectedplanet.net is a good source.) If you could designate an additional protected area, where would it be? What shape would your protected area be? What would the management goals be for these additional areas? Explain all your answers.
- Think about a protected area that you have visited. What is the main goal of this protected area? Do you think park management is succeeding in the goal? What are they doing particularly well? What could they do to manage the protected area better?
- Think of a protected area near you that safeguards an aquatic environment, such as a beach, estuary, or lake. What unique challenges do you think the people managing that protected area face that managers of a terrestrial protected area do not face?
- Many countries are developing protected areas that cross international borders. What are the main goals of these parks? Are they achieving their goals? What are the main challenges?
- How can national parks continue to function optimally in countries where the central governments have largely ceased to function, and where corruption is rampant?
13.10 Suggested Readings
de Vos, A., H.S. Clements, D. Biggs, et al. 2019. The dynamics of proclaimed privately protected areas in South Africa over 83 years. Conservation Letters 12: e12644. https://doi.org/10.1111/conl.12644 Tracking the growth of privately protected areas in South Africa as a function of national legislation.
Ferro, P.J., M.M. Hanauer, and K.R.E. Sims. 2011. Conditions associated with protected area success in conservation and poverty reduction. Proceedings of the National Academy of Sciences 108: 13913–18. https://doi.org/10.1073/pnas.1011529108 Protected areas can provide many benefits for poor people living nearby.
Fox, H.E., M.B. Mascia, X. Basurto, et al. 2012. Reexamining the science of marine protected areas: Linking knowledge to action. Conservation Letters 5: 1–10. https://doi.org/10.1111/j.1755-263X.2011.00207.x Biological, social, and policy issues are all important in effective marine protected areas.
Gross, J.E., S. Woodley, L.A. Welling, et al. 2016. Adapting to Climate Change: Guidance for Protected Area Managers and Planners (Gland: IUCN). https://portals.iucn.org/library/node/46685 Best practices guidelines for managing protected areas under climate change.
Ihwagi, F.W., T. Wang, G. Wittemyer, et al. 2015. Using poaching levels and elephant distribution to assess the conservation efficacy of private, communal and government land in northern Kenya. PLoS ONE 10: e0139079. https://doi.org/10.1371/journal.pone.0139079 A comparison of different types of protected areas in protecting biodiversity
Knight, A.T., A. Driver, R.M. Cowling, et al. 2006. Designing systematic conservation assessments that promote effective implementation: Best practice from South Africa. Conservation Biology 20: 739–50. https://doi.org/10.1111/j.1523-1739.2006.00452.x An overview of eight systematic conservation plans from design to implementation.
Mascia, M.B., and S. Pallier. 2011. Protected areas downgrading, downsizing, and degazettement (PADDD) and its conservation implications. Conservation Letters 4: 9–20. https://doi.org/10.1111/j.1755-263X.2010.00147.x In many areas of the world, the official protection of national parks and other conservation areas is being withdrawn.
Mbaiwa, J.E., and A.L. Stronza. 2011. Changes in resident attitudes towards tourism development and conservation in the Okavango Delta, Botswana. Journal of Environmental Management 92: 1950–59. https://doi.org/10.1016/j.jenvman.2011.03.009 Local people being positive about conservation.
Olds, A.D., R.M. Connolly, K.A. Pitt, et al. 2012. Habitat connectivity improves reserve performance. Conservation Letters 5: 56–63. https://doi.org/10.1111/j.1755-263X.2011.00204.x Networks of protected areas benefit from connectivity.
Survival International. 2014. Parks Need People (London: Survival International). https://assets.survivalinternational.org/documents/1324/parksneedpeoples-report.pdf Integrating cultural protection with biodiversity conservation.
Bibliography
Agnew, D.J., J. Pearce, G. Pramod, et al., 2009. Estimating the worldwide extent of illegal fishing. PLoS ONE 4: e4570. https://doi.org/10.1371/journal.pone.0004570
Andersson, J., M. de Garine-Wichatitsky, D. Cumming, et al. 2013. Transfrontier Conservation Areas: People Living on the Edge (New York: Routledge).
ARCOS (Albertine Rift Conservation Society) 2004. A framework for conservation in the Albertine Rift: 2004–30 (Kampala: ARCOS).
Ayebare, S., A.J. Plumptre, D. Kujirakwinja, and D. Segan. 2018. Conservation of the endemic species of the Albertine Rift under future climate change. Biological Conservation 220: 67–75. https://doi.org/10.1016/j.biocon.2018.02.001
Baker, D.J., A.J. Hartley, N.D. Burgess, et al. 2015. Assessing climate change impacts for vertebrate fauna across the West African protected area network using regionally appropriate climate projections. Diversity and Distributions 21: 991–1003. https://doi.org/10.1111/ddi.12337
Baker, J., E.J. Milner-Gulland, and N. Leader-Williams. 2012. Park gazettement and integrated conservation and development as factors in community conflict at Bwindi Impenetrable Forest, Uganda. Conservation Biology 26: 160–70. http://doi.org/10.1111/j.1523-1739.2011.01777.x
Balme, G.A., R.O.B. Slotow, and L.T.B. Hunter. 2010. Edge effects and the impact of non‐protected areas in carnivore conservation: Leopards in the Phinda–Mkhuze Complex, South Africa. Animal Conservation 13: 315–23. https://doi.org/10.1111/j.1469-1795.2009.00342.x
Beale, C.M., S. van Rensberg, W.J. Bond, et al. 2013b. Ten lessons for the conservation of African savannah ecosystems. Biological Conservation 167: 224–32. https://doi.org/10.1016/j.biocon.2013.08.025
Beale, C.M., N.E. Baker, M.J. Brewer, et al. 2013a. Protected area networks and savannah bird biodiversity in the face of climate change and land degradation. Ecology Letters 16: 1061–68. https://doi.org/10.1111/ele.12139
Beresford, A.E., G.M. Buchanan, P.F. Donald, et al. 2011. Poor overlap between the distribution of protected areas and globally threatened birds in Africa. Animal Conservation 14: 99–107. https://doi.org/10.1111/j.1469-1795.2010.00398.x
Blake, S., S.L. Deem, S. Strindberg, et al. 2008 Roadless wilderness area determines forest elephant movements in the Congo Basin. PloS ONE 3: e3546. https://doi.org/10.1371/journal.pone.0003546
Blom, A., R. van Zalinge, E. Mbea, et al. 2004. Human impact on wildlife populations within a protected Central African forest. African Journal of Ecology 42: 23–31. https://doi.org/10.1111/j.0141-6707.2004.00441.x
Blomley, T., F. Nelson, H. Doulton, et al. 2019. Scaling up community forest enterprises in Tanzania. IIED Briefing, April 2019. https://pubs.iied.org/pdfs/17701IIED.pdf
Brashares, J.S., P. Arcese, and M.K. Sam. 2001. Human demography and reserve size predict wildlife extinction in West Africa. Proceedings of the Royal Society B 268: 2473–78. https://doi.org/10.1098/rspb.2001.1815
Brashares, J.S., P. Arcese, M.K. Sam, et al. 2004. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science 306: 1180–83. https://doi.org/10.1126/science.1102425
Brooks, T.M., S.J. Wright, and D. Sheil. 2009. Evaluating the success of conservation actions in safeguarding tropical forest biodiversity. Conservation Biology 23: 1448–57. https://doi.org/10.1111/j.1523-1739.2009.01334.x
Brugiere, D., and R. Kormos. 2009. Review of the protected area network in Guinea, West Africa, and recommendations for new sites for biodiversity conservation. Biodiversity and Conservation 18: 847–68. https://doi.org/10.1007/s10531-008-9508-z
Buckland, S.T., and A. Johnston. 2017. Monitoring the biodiversity of regions: Key principles and possible pitfalls. Biological Conservation 214: 23–34. https://doi.org/10.1016/j.biocon.2017.07.034
Buckley, R.C., C. Morrison, and J.G. Castley. 2016. Net effects of ecotourism on threatened species survival. PloS ONE 11: e0147988. https://doi.org/10.1371/journal.pone.0147988
Carroll, R. 1992. Central African Republic. In: The Conservation Atlas of Tropical Forests Africa, ed. by J.A. Sayer, et al. (London: Palgrave Macmillan). https://portals.iucn.org/library/sites/library/files/documents/1992-063.pdf
Carwardine, J., K.A. Wilson, M. Watts, et al. 2008. Avoiding costly conservation mistakes: The importance of defining actions and costs in spatial priority setting. PLoS ONE 3: e2586. https://doi.org/10.1371/journal.pone.0002586
Chadwick. P., J. Duncan, and K. Tunley. 2014. State of management of South Africa’s marine protected areas (Cape Town: WWF South Africa). http://awsassets.wwf.org.za/downloads/final_wwf_marine_report_02_dec_2014_web_1.pdf
Chennels, R. 1999. What have we achieved? (Cape Town: South African San Institute).
Clements, H., J. Baum, and G.S. Cumming. 2016. Money and motives: An organizational ecology perspective on private land conservation. Biological Conservation 197: 108–15. https://doi.org/10.1016/j.biocon.2016.03.002
Coetzee, B.W.T., M.P. Robertson, B.F.N. Erasmus, et al. 2009. Ensemble models predict Important Bird Areas in southern Africa will become less effective for conserving endemic birds under climate change. Global Ecology and Biogeography 18: 701–10. https://doi.org/10.1111/j.1466-8238.2009.00485.x
Coetzer, K.L., E.T.F. Witkowski, and B.F.N. Erasmus. 2014. Reviewing Biosphere Reserves globally: Effective conservation action or bureaucratic label? Biological Reviews 89: 82–104. https://doi.org/10.1111/brv.12044
Cousins, J., J. Sadler, and J. Evans. 2010. The challenge of regulating private wildlife ranches for conservation in South Africa. Ecology and Society 15: 28. https://www.ecologyandsociety.org/vol15/iss2/art28
Cozzi, G., F. Broekhuis, J.W. McNutt, et al. 2013. Comparison of the effects of artificial and natural barriers on large African carnivores: Implications for interspecific relationships and connectivity. Journal of Animal Ecology 82: 707–15. https://doi.org/10.1111/1365-2656.12039
Craigie, I.D., J.E.M. Baillie, A. Balmford, et al. 2010. Large mammal population declines in Africa’s protected areas. Biological Conservation 143: 2221–28. https://doi.org/10.1016/j.biocon.2010.06.007
Cross, H. 2016. Displacement, disempowerment and corruption: Challenges at the interface of fisheries, management and conservation in the Bijagós Archipelago, Guinea-Bissau. Oryx 50: 693–701. https://doi.org/10.1017/S003060531500040X
da Silva, I.M., N. Hill, H. Shimadzu, et al. 2015. Spillover effects of a community-managed marine reserve. PloS ONE 10: e0111774. https://doi.org/10.1371/journal.pone.0111774
Dallimer, M., and N. Strange. 2015. Why socio-political borders and boundaries matter in conservation. Trends in Ecology and Evolution 30: 132–39. https://doi.org/10.1016/j.tree.2014.12.004
Darwall, W., K. Smith, T. Lowe, et al. 2005. The status and distribution of freshwater biodiversity in Eastern Africa (Gland: IUCN). https://www.iucn.org/downloads/the_status_and_distribution_of_freshwater_biodiversity_in_eastern_africa.pdf
de Vos, A., H.S. Clements, D. Biggs, et al. 2019. The dynamics of proclaimed privately protected areas in South Africa over 83 years. Conservation Letters 12: e12644. https://doi.org/10.1111/conl.12644
Dudley, N. 2008. Guidelines for applying protected area management categories (Gland: IUCN). https://www.iucn.org/theme/protected-areas/about/protected-area-categories
Dupain, J., A. Fowler, P. Kasalevo, et al. 2013. The process of creation of a new protected area in the Democratic Republic of Congo: The case of the Iyondji Community Bonobo Reserve (DRC). Pan Africa News 20: 10–13.
Durán, A.P., J. Rauch, and K.J. Gaston. 2013. Global spatial coincidence between protected areas and metal mining activities. Biological Conservation 160: 272–78. https://doi.org/10.1016/j.biocon.2013.02.003
Edwards, D.P., S. Sloan, L. Weng, et al. 2014. Mining and the African environment. Conservation Letters 7: 302–11. https://doi.org/10.1111/conl.12076
Endamana, D., A.K. Boedhihartono, B. Bokoto, et al. 2010. A framework for assessing conservation and development in a Congo Basin Forest Landscape. Tropical Conservation Science 3: 262–81. https://doi.org/10.1177%2F194008291000300303
Ervin, J. 2003. Rapid assessment of protected area management effectiveness in four countries. BioScience 53: 833–41. https://doi.org/10.1641/0006-3568(2003)053[0833:RAOPAM]2.0.CO;2
Ferraro, P.J., and M.M. Hanauer. 2014. Quantifying causal mechanisms to determine how protected areas affect poverty through changes in ecosystem services and infrastructure. Proceedings of the National Academy of Sciences 111: 4332–37. https://doi.org/10.1073/pnas.1307712111
Feyisa, G.L., K. Dons, and H. Meilby. 2014. Efficiency of parks in mitigating urban heat island effect: An example from Addis Ababa. Landscape and Urban Planning 123: 87–95. https://doi.org/10.1016/j.landurbplan.2013.12.008
Fishpool, L.D.C., and M.I. Evans. 2001. Important Bird Areas in Africa and Associated Islands (Cambridge: BirdLife International).
Foxcroft, L.C., D. Spear, N.J. van Wilgen, et al. 2019. Assessing the association between pathways of alien plant invaders and their impacts in protected areas. NeoBiota 43: 1–25. https://doi.org/10.3897/neobiota.43.29644
Fuller, R.A., E. McDonald-Madden, K.A. Wilson, et al. 2010. Replacing underperforming protected areas achieves better conservation outcomes. Nature 466: 365–67. https://doi.org/10.1038/nature09180
Gaertner, M., B.M.H. Larson, U.M. Irlich, et al. 2016. Managing invasive species in cities: A framework from Cape Town, South Africa. Landscape and Urban Planning 151: 1–9. https://doi.org/10.1016/j.landurbplan.2016.03.010
Gallo, J.A., L. Pasquini, B. Reyers, et al. 2009. The role of private conservation areas in biodiversity representation and target achievement within the Little Karoo region, South Africa. Biological Conservation 142: 446–54. https://doi.org/10.1016/j.biocon.2008.10.025
Geffroy, B., D.S.M. Samia, E. Bessa, et al. 2015. How nature-based tourism might increase prey vulnerability to predators. Trends in Ecology and Evolution 30: 755–65. https://doi.org/10.1016/j.tree.2015.09.010
Gill, D.A., M.B. Mascia, G.N. Ahmadia, et al. 2017. Capacity shortfalls hinder the performance of marine protected areas globally. Nature 543: 665–69. https://doi.org/10.1038/nature21708
Gray, C.L., S.L. Hill, T. Newbold, et al. 2016. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nature Communications 7: 12306. https://doi.org/10.1038/ncomms12306
Gremillet, D., C. Peron, P. Provost, et al. 2015. Adult and juvenile European seabirds at risk from marine plundering off West Africa. Biological Conservation 182: 143–47. https://doi.org/10.1016/j.biocon.2014.12.001
Habtemariam, B.T., and Q. Fang. 2016. Zoning for a multiple-use marine protected area using spatial multi-criteria analysis: The case of the Sheik Seid Marine National Park in Eritrea. Marine Policy 63: 135–43. https://doi.org/10.1016/j.marpol.2015.10.011
Hall, J.M., N.D. Burgess, S. Rantala, et al. 2014. Ecological and social outcomes of a new protected area in Tanzania. Conservation Biology 28: 1512–21. https://doi.org/10.1111/cobi.12335
Hanks, J. 2003. Transfrontier Conservation Areas (TFCAs) in Southern Africa: Their role in conserving biodiversity, socioeconomic development and promoting a culture of peace. Journal of Sustainable Forestry 17: 127–48. https://doi.org/10.1300/J091v17n01_08
Harcourt, A.H., S.A. Parks, and R. Woodroffe. 2001. Human density as an influence on species/area relationships: Double jeopardy for small African reserves? Biodiversity and Conservation 10: 1011–26. https://doi.org/10.1023/A:1016680327755
Hauenstein, S., M. Kshatriya, J. Blanc, et al. 2019. African elephant poaching rates correlate with local poverty, national corruption and global ivory price. Nature Communications 10: 2242. https://doi.org/10.1038/s41467-019-09993-2
Henschel, P., L. Coad, C. Burton, et al. 2014. The lion in West Africa is critically endangered. PLoS ONE 9: e83500. https://doi.org/10.1371/journal.pone.0083500
Hockings, M., S. Stolton, F. Leverington, et al. 2006. Evaluating Effectiveness: A Framework for Assessing Management Effectiveness of Protected Areas (Gland: IUCN). https://portals.iucn.org/library/efiles/documents/PAG-014.pdf
Hole, D.G., B. Huntley, J. Arinaitwe, et al. 2011. Toward a management framework for networks of protected areas in the face of climate change. Conservation Biology 25: 305–15. https://doi.org/10.1111/j.1523-1739.2010.01633.x
Ibisch, P.L., M.T. Hoffmann, S. Kreft, et al. 2016. A global map of roadless areas and their conservation status. Science 354: 1423–27. https://doi.org/10.1126/science.aaf7166
Ihwagi, F.W., T. Wang, G. Wittemyer, et al. 2015. Using poaching levels and elephant distribution to assess the conservation efficacy of private, communal and government land in northern Kenya. PLoS ONE 10: e0139079. https://doi.org/10.1371/journal.pone.0139079
IUCN WCPA (World Commission on Protected Areas). 2017. IUCN Green List of Protected and Conserved Areas: Standard, v. 1.1 (Gland:IUCN).
IUCN WCPA (World Commission on Protected Areas). 2018. PARKS 24: Special Issue: Standard (Gland:,IUCN). https://doi.org/10.2305/IUCN.CH.2018.PARKS-24-SI.en
Jones, K.R., A.J. Plumptre, J.E.M. Watson, et al. 2016, Testing the effectiveness of surrogate species for conservation planning in the Greater Virunga Landscape, Africa. Landscape and Urban Planning 145: 1–11. https://doi.org/10.1016/j.landurbplan.2015.09.006
Kahumbu, P., D. Martins, J. Schieltz, et al. 2016. Transforming the next generation through citizen science in Kenya: The kids twiga tally Swara April–June 2016: 52–56. https://dir.princeton.edu/pdf_dir/Kids%20Twiga%20Tally.pdf
Kerwath, S.E., E.B. Thorstad, T.F. Næsje, et al. 2009. Crossing invisible boundaries: The effectiveness of the Langebaan Lagoon Marine Protected Area as a harvest refuge for a migratory fish species in South Africa. Conservation Biology 23: 653–61. https://doi.org/10.1111/j.1523-1739.2008.01135.x
Kiffner, C., C. Stoner, and T. Caro. 2013. Edge effects and large mammal distributions in a national park. Animal Conservation: 97–107. https://doi.org/10.1111/j.1469-1795.2012.00577.x
Klein, C.J., C.J. Brown, B.S. Halpern, et al. 2015. Shortfalls in the global protected area network at representing marine biodiversity. Scientific Reports 5: 17539. https://doi.org/10.1038/srep17539
Knight, A.T., A. Driver, R.M. Cowling, et al. 2006. Designing systematic conservation assessments that promote effective implementation: Best practice from South Africa. Conservation Biology 20: 739–50. https://doi.org/10.1111/j.1523-1739.2006.00452.x
Laurance, W.F., D.C. Useche, J. Rendeiro, et al. 2012. Averting biodiversity collapse in tropical forest protected areas. Nature 489: 290–94. https://doi.org/10.1038/nature11318
Lavrenchenko, L., and R. Kennerley. 2016. Tachyoryctes microcephalus. The IUCN Red List of Threatened Species 2016: e.T21293A115161321. http://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T21293A22276163.en
Leach, K, S.E. Brooks, and S. Blyth. 2016. Potential threat to areas of biodiversity importance from current and emerging oil and gas activities in Africa (Cambridge: UNEP-WCMC). https://www.unep-wcmc.org/system/dataset_file_fields/files/000/000/394/original/African_threat_mapping_270716.pdf
Lester, S.E., B.S. Halpern, K. Grorud-Colvert, et al. 2009. Biological effects within no-take marine reserves: A global synthesis. Marine Ecology Progress Series 384: 33–46. https://doi.org/10.3354/meps08029
Lindsey P.A., V.R. Nyirenda, J.L. Barnes, et al. 2014. Underperformance of African protected area networks and the case for new conservation models: Insights from Zambia. PLoS ONE 9: e94109. https://doi.org/10.1371/journal.pone.0094109
Lindsey, P.A., G. Chapron, L.S. Petracca, et al. 2017. Relative efforts of countries to conserve world’s megafauna. Global Ecology and Conservation 10: 243–52. https://doi.org/10.1016/j.gecco.2017.03.003
Lindsey, P.A., J.R.B. Miller, L.S. Petracca, et al. 2018. More than $1 billion needed annually to secure Africa’s protected areas with lions. Proceedings of the National Academy of Sciences: E10788-E10796. https://doi.org/10.1073/pnas.1805048115
Lwanga, J.S., T.T. Struhsaker, P.J. Struhsaker, et al. 2011. Primate population dynamics over 32.9 years at Ngogo, Kibale National Park, Uganda. American Journal of Primatology 73: 997–1011. http://doi.org/10.1002/ajp.20965
Makhado, A.B., M.A. Meÿer, R.J.M. Crawford, et al. 2009. The efficacy of culling seals seen preying on seabirds as a means of reducing seabird mortality. African Journal of Ecology 47: 335–40. https://doi.org/10.1111/j.1365-2028.2008.00966.x
Marino, J., and C. Sillero-Zubiri. 2011. Canis simensis. The IUCN Red List of Threatened Species 2011: e.T3748A10051312. http://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T3748A10051312.en
Mascia, M.B., and S. Pailler. 2011. Protected area downgrading, downsizing, and degazettement PADDD and its conservation implications. Conservation Letters 4: 9–20. https://doi.org/10.1111/j.1755-263X.2010.00147.x
Mbaiwa, J.E., and A.L. Stronza. 2011. Changes in resident attitudes towards tourism development and conservation in the Okavango Delta, Botswana. Journal of Environmental Management 92: 1950–59. https://doi.org/10.1016/j.jenvman.2011.03.009
McClanahan, T.R., J.M. Maina, N.A. Graham et al. 2016. Modeling reef fish biomass, recovery potential, and management priorities in the western Indian Ocean. PloS ONE 11: e0154585. https://doi.org/10.1371/journal.pone.0154585
McClanahan, T.R., N.A.J. Graham, J.M. Calnan, et al. 2007. Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecological Applications 17: 1055–67. https://doi.org/10.1890/06-1450
McIntosh, E.J., R.L. Pressey, S. Lloyd, et al. 2017. The impact of systematic conservation planning. Annual Reviews of Environment and Resources 42: 677–97. https://doi.org/10.1146/annurev-environ-102016-060902
Miller, J.R., and R.J. Hobbs. 2002. Conservation where people live and work. Conservation Biology 16: 330–37. https://doi.org/10.1046/j.1523-1739.2002.00420.x
Milner, J.M., E.B. Nilsen, and H.P. Andreassen. 2007. Demographic side effects of selective hunting in ungulates and carnivores. Conservation Biology 21: 36–47. https://doi.org/10.1111/j.1523-1739.2006.00591.x
Mittermeier, R.A., P. Robles-Gil, M. Hoffman, et al. 2005. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions (Chicago: University of Chicago Press).
Monadjem, A., and D.K. Garcelon. 2005. Nesting distribution of vultures in relation to land use in Swaziland. Biodiversity and Conservation 14: 2079–93. https://doi.org/10.1007/s10531-004-4358-9
Moreto, W.D. 2015. Introducing intelligence-led conservation: Bridging crime and conservation science. Crime Science 4: 15. https://doi.org/10.1186/s40163-015-0030-9
Myers, N. 1988. Threatened biotas: “Hot spots” in tropical forests. Environmentalist 8: 187–208. https://doi.org/10.1007/BF02240252
Myers, N., R.A. Mittermeier, C.G. Mittermeier, et al. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–58. https://doi.org/10.1038/35002501
Newmark, W.D. 1996. Insularization of Tanzanian parks and the local extinction of large mammals. Conservation Biology 10: 1549–56. https://doi.org/10.1046/j.1523-1739.1996.10061549.x
Newmark, W.D. 2008. Isolation of African protected areas. Frontiers in Ecology and the Environment 6: 321–28. https://doi.org/10.1890/070003
O’Leary, B.C., M. Winther-Janson, J.M. Bainbridge, et al. 2016. Effective coverage targets for ocean protection. Conservation Letters 9: 398–404. https://doi.org/10.1111/conl.12247
Oberholzer, S., M. Saayman, A. Saayman, et al. 2010. The socio-economic impact of Africa’s oldest marine park. Koedoe 52: 879. https://doi.org/10.4102/koedoe.v52i1.879
Odadi, W.O., M.K. Karachi, S.A. Abdulrazak, et al. 2011. African wild ungulates compete with or facilitate cattle depending on season. Science 333: 1753–55. https://doi.org/10.1126/science.1208468
Ogutu, J.O., H.-P. Piepho, M.Y. Said, et al. 2016. Extreme wildlife declines and concurrent increase in livestock numbers in Kenya: What are the causes? PLoS ONE 11: e0163249. https://doi.org/10.1371/journal.pone.0163249
Oldekop, J.A., G. Holmes, W.E. Harris, et al. 2016. A global assessment of the social and conservation outcomes of protected areas. Conservation Biology 30: 133–41. https://doi.org/10.1111/cobi.12568
Olson, D.M., and E. Dinerstein. 2002. The Global 200: Priority ecoregions for global conservation. Annals of the Missouri Botanical Garden 89: 199–24. https://doi.org/10.2307/3298564
Pimm, S.L. 1991. The Balance of Nature? (Chicago: University of Chicago Press).
Plumptre, A.J., S. Ayebare, D. Kujirakwinja, and D. Segan. 2019. Conservation planning for Africa’s western Rift: Conserving a biodiverse region in the face of multiple threats. Oryx 53: in press.
Plumptre, A.J., S. Ayebare, D. Segan, et al., 2016. Conservation action plan for the Albertine Rift. WCS Report. https://albertinerift.wcs.org/About-Us/News/articleType/ArticleView/articleId/10950/Conservation-Action-Plan-for-Albertine-Rift-identifies-key-sites-and-species.aspx
Plumptre, A.J., T.R.B. Davenport, M. Behangana, et al. 2007. The biodiversity of the Albertine Rift. Biological Conservation 134: 178–94. https://doi.org/10.1016/j.biocon.2006.08.021
Possingham, H.P., I.R. Ball, and S. Andelman. 2000. Mathematical methods for identifying representative reserve networks. In: Quantitative Methods for Conservation Biology, ed. by S. Ferson and M. Burgman (New York: Springer). https://doi.org/10.1007/b97704
Potapov, P., M.C. Hansen, L. Laestadius, et al. 2017. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Science Advances 3: e1600821. https://doi.org/10.1126/sciadv.1600821
Pryke, J.S., M.J. Samways, and K. de Saedeleer. 2015. An ecological network is as good as a major protected area for conserving dragonflies. Biological Conservation 191: 537–45. https://doi.org/10.1016/j.biocon.2015.07.036
Reid, H., D. Fig, H. Magome, et al. 2004. Co-management of contractual national parks in South Africa: Lessons from Australia. Conservation and Society 2: 377.
Riginos, C., L.M. Porensky, K.E. Veblen, et al. 2012. Lessons on the relationship between livestock husbandry and biodiversity from the Kenya Long-term Exclosure Experiment (KLEE). Pastoralism 2: 10. https://doi.org/10.1186/2041-7136-2-10
Roberts, P., C.O. Hunt, M. Arroyo-Kalin, et al. 2017. The deep human prehistory of global tropical forests and its relevance for modern conservation. Nature Plants 3: 17093. https://doi.org/10.1038/nplants.2017.93
Robinson, C.A.J., and M.J. Remis. 2014. Entangled Realms: Hunters and Hunted in the Dzanga-Sangha Dense Forest Reserve (APDS), Central African Republic. Anthropological Quarterly 87: 613–33. https://doi.org/10.1353/anq.2014.0036
Rocliffe, S., S. Peabody, M. Samoilys, et al. 2014. Towards a network of locally managed marine areas (LMMAs) in the Western Indian Ocean. PLoS ONE 9: e103000. https://doi.org/10.1371/journal.pone.0103000
Rodrigues, A.S.L., and T.M. Brooks. 2007. Shortcuts for biodiversity conservation planning: The effectiveness of surrogates. Annual Review of Ecology, Evolution, and Systematics 38: 713–37. https://doi.org/10.1146/annurev.ecolsys.38.091206.095737
Rubenstein, D.I. 2010. Ecology, social behavior, and conservation in zebras. Advances in the Study of Behavior 42: 231–58. https://doi.org/10.1016/S0065-3454(10)42007-0
Rubenstein, D.I., and I. Rubinoff. 2014. Mpala’s Beginnings. Mpala Memos Apr: 5–6 2014. http://www.mpala.org/documents/Get_our_Newsletter_33_3158696297.pdf
Runge, C.A., J.E.M. Watson, S.H.M. Butchart, et al. 2015. Protected areas and global conservation of migratory birds. Science 350: 1255–58. https://doi.org/10.1126/science.aac9180
Ryan, S.J., and P.D. Walsh. 2011. Consequences of non-intervention for infectious disease in African great apes. PloS ONE 6: e29030. https://doi.org/10.1371/journal.pone.0029030
Shafer, C.L. 1997. Terrestrial nature reserve design at the urban/rural interface. In: Conservation in Highly Fragmented Landscapes, ed. by M.W. Schwartz (New York: Chapman and Hall). https://doi.org/10.1007/978-1-4757-0656-7_15
Shannon, G., M.F. McKenna, L.M. Angeloni, et al. 2015. A synthesis of two decades of research documenting the effects of noise on wildlife. Biological Reviews 91: 982–1005. https://doi.org/10.1111/brv.12207
Sims-Castley, R., G.I. Kerley, B. Geach, et al. 2005. Socio-economic significance of ecotourism-based private game reserves in South Africa’s Eastern Cape Province. Parks 15: 6–18.
Smith, A., M.C. Schoeman, M. Keith, et al. 2016. Synergistic effects of climate and land-use change on representation of African bats in priority conservation areas. Ecological Indicators 69: 276–83. https://doi.org/10.1016/j.ecolind.2016.04.039
Smith, R.J., J. Easton, B.A. Nhancale, et al. 2008. Designing a transfrontier conservation landscape for the Maputaland centre of endemism using biodiversity, economic and threat data. Biological Conservation 141: 2127–38. https://doi.org/10.1016/j.biocon.2008.06.010
Smith, T.J., and G.F. Smith. 2004. Selecting important plant areas in southern Africa: News and views. South African Journal of Science 100: 434–35. https://hdl.handle.net/10520/EJC96312
Spalding, M.D., L. Fish, and L.J. Wood. 2008. Toward representative protection of the world’s coasts and oceans—progress, gaps, and opportunities. Conservation Letters 1: 217–26. https://doi.org/10.1111/j.1755-263X.2008.00030.x
Spear, D., L.C. Foxcroft, H. Bezuidenhout, et al. 2013. Human population density explains alien species richness in protected areas. Biological Conservation 159: 137–47. https://doi.org/10.1016/j.biocon.2012.11.022
Stolton, S., M. Hockings, N. Dudley, et al. 2007. Management effectiveness tracking tool: Reporting progress at protected area sites (Gland: WWF). http://assets.panda.org/downloads/mett2_final_version_july_2007.pdf
Tranquilli, S., M. Abedi-Lartey, F. Amsini, et al. 2012. Lack of conservation effort rapidly increases African great ape extinction risk. Conservation Letters 5: 48–55. https://doi.org/10.1111/j.1755-263X.2011.00211.x
Tranquilli, S., M. Abedi-Lartey, K. Abernethy, et al. 2014. Protected areas in tropical Africa: Assessing threats and conservation activities. PloS ONE 9: e114154. https://doi.org/10.1371/journal.pone.0114154
Tydecks, L., V. Bremerich, I. Jentschke, et al. 2016. Biological field stations: A global infrastructure for research, education, and public engagement. BioScience 66: 164–71. https://doi.org/10.1093/biosci/biv174
UNEP-WCMC. 2019. World Database on Protected Areas. https://www.protectedplanet.net
van Rensburg, B.J., S. Hugo, N. Levin, et al. 2013. Are environmental transitions more prone to biological invasions? Diversity and Distributions 19: 341–51. https://doi.org/10.1111/ddi.12026
van Wilgen, B.W., and H.C. Biggs. 2011. A critical assessment of adaptive ecosystem management in a large savanna protected area in South Africa. Biological Conservation 144: 1179–87. https://doi.org/10.1016/j.biocon.2010.05.006
Vanak, A.T., M. Thaker, and R. Slotow. 2010. Do fences create an edge-effect on the movement patterns of a highly mobile mega-herbivore? Biological Conservation 143: 2631–37. https://doi.org/10.1016/j.biocon.2010.07.005
Venter, O., A. Magrach, N. Outram, et al., 2018. Bias in protected‐area location and its effects on long‐term aspirations of biodiversity conventions. Conservation Biology 32: 127–34. https://doi.org/10.1111/cobi.12970
Watson, J.E., N. Dudley, D.B. Segan, et al. 2014. The performance and potential of protected areas. Nature 515: 67–73. https://doi.org/10.1038/nature13947
Watson, J.E.M., K.R. Jones, R.A. Fuller, et al. 2016. Persistent disparities between recent rates of habitat conversion and protection and implications for future global conservation targets. Conservation Letters 9: 413–21. https://doi.org/10.1111/conl.12295
Watts, D.P., and J.C. Mitani. 2002. Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. International Journal of Primatology 23: 1–28. https://doi.org/10.1023/A:1013270606320
Watts, M.E, I.R. Ball, R.R. Stewart, et al. 2009. Marxan with Zones: Software for optimal conservation-based land- and sea-use zoning. Environmental Modelling and Software 24: 1513–21. https://doi.org/10.1016/j.envsoft.2009.06.005
Wegmann M., L. Santini, B. Leutner, et al. 2014 Role of African protected areas in maintaining connectivity for large mammals. Philosophical Transactions of the Royal Society B 369: 20130193. https://doi.org/10.1098/rstb.2013.0193
WHC (World Heritage Committee). 2018. W-Arly-Pendjari Complex. http://whc.unesco.org/en/list/749
Wintle, B.A., H. Kujala, A. Whitehead, et al. 2019. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proceedings of the National Academy of Sciences 116: 909–14. https://doi.org/10.1073/pnas.1813051115
Woodroffe, R., and J.R. Ginsberg. 1998. Edge effects and the extinction of populations inside protected areas. Science 280: 2126–28. https://doi.org/10.1126/science.280.5372.2126
WWF, and CI (Conservation International). 2016. PADDDtracker.org Data Release v. 1.1, http://www.padddtracker.org