2. Introduction to Sub-Saharan Africa
© 2019 J.W. Wilson and R.B. Primack, CC BY 4.0 https://doi.org/10.11647/OBP.0177.02

The forested hills of Bwindi Impenetrable National Park, Uganda, are home to Africa’s iconic mountain gorillas and over 350 bird species. The park, a World Heritage Site, is situated in the Albertine Rift, a Biodiversity Hotspot. Photograph by Jason Houston/USAID, https://www.flickr.com/photos/usaid-biodiversity-forestry/25422552517, CC0.
Not only is Africa the second most-populous continent in the world, its human population is also incredibly diverse. Consider, for example, that over 2,000 native languages are spoken across the continent (Lewis et al., 2014). (Interestingly, there are strong positive correlations between linguistic diversity and biodiversity, as well as between the loss of species and languages; Gorenflo et al., 2012). Africa is also economically diverse; the continent contains some of the poorest nations in the world but also some of the fastest growing economies (World Bank, 2017). Herein also lies a major challenge: Africa’s diverse human population—already over 1 billion people—is expected to double over the next 25 years (World Bank, 2019). To stimulate economic growth and provide resources for a growing and upwardly mobile human population, once unending wildernesses are constantly being cleared for agriculture, timber, expanding cities, and other human activities. In the process, the remaining natural areas are being polluted, overharvested, and fragmented, particularly in areas of outstanding conservation value (Balmford et al., 2011).
This environmental destruction we are witnessing across Africa holds negative consequences for all people on the continent. Among the most vulnerable are traditional peoples who rely on natural products such as firewood, wild animals, and wild edible fruits and roots to maintain their way of life. The destruction of the environment also makes it more challenging for city dwellers to access basic needs such as clean drinking water, clean air, and wilderness areas where they can fulfil their spiritual and emotional needs. With Africa’s human population and consumption expected to grow substantially for many years to come, there is an urgent need to find ways to ensure that the region’s unique environmental treasures are preserved, for the benefit of current and future generations.
2.1 Sub-Saharan Africa’s Natural Environment
Much of the African continent encompasses the Afrotropical ecoregion, which is separated from other ecoregions by the Indian Ocean to the East, the Atlantic Ocean to the West, and the Saharan Desert to the North. These major geographic features have acted as barriers to movement since the African continent first took its current shape, enabling species and ecosystems characteristic of the region to evolve in relative isolation from those of other ecoregions. The Afrotropical ecoregion can be further subdivided into eight terrestrial biomes (Figure 2.1), each with its own distinct climate, geology, and biota (Burgess et al., 2004):
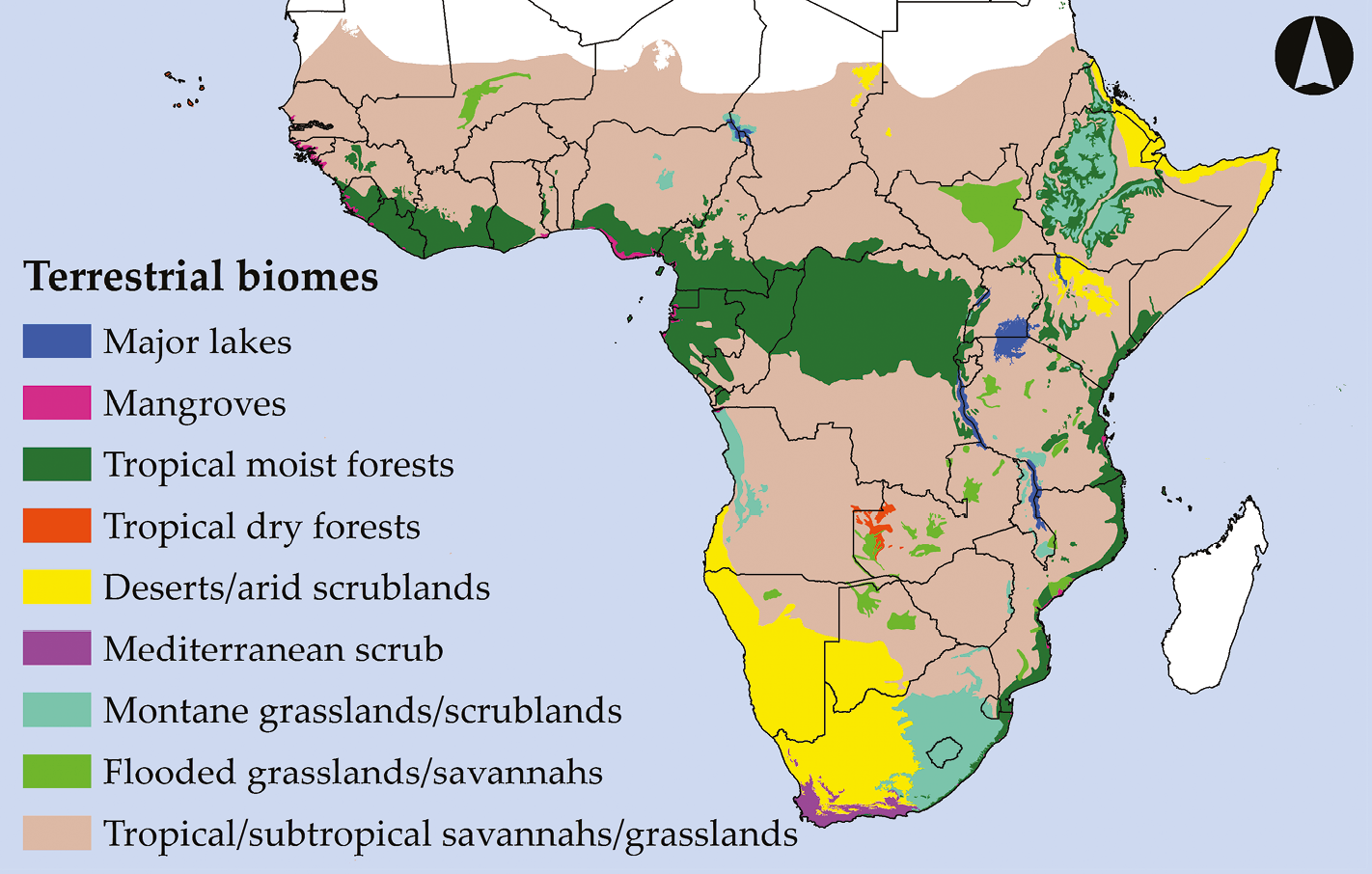
Figure 2.1 Simplified map of Sub-Saharan Africa’s eight terrestrial biomes. The region’s topographic complexity, the diversity of biomes, and the multiple ecological transition zones between the different biomes have given rise to a rich biodiversity. After Olson et al., 2001. Map by Johnny Wilson, CC BY 4.0.
- Tropical and subtropical savannahs and grasslands: Sub-Saharan Africa’s largest biome is a mosaic of grasslands, woodlands, bushlands, thickets, and semi-arid drylands that are maintained by fire and grazing. East and Southern Africa’s miombo and mopane savannah-woodland ecosystems are included in this ecosystem.
- Deserts and arid scrublands: A biome of areas where evaporation exceeds precipitation, generally with rainfall < 250 mm/year. Generally associated with searing daytime temperatures and wind-swept sand dunes, this biome contains scrub deserts rich in succulent plants, rocky mountain deserts, and arid grassland-savannah mosaics, such as the Sahel region located just south of the Sahara.
- Tropical moist forests: Lowland broadleaf ecosystems with near-continuous canopies that run as a broad band across equatorial Africa. This biome is characterised by high rainfall (> 2 m/year), low variability in temperatures, and very high species diversity.
- Montane grasslands and scrublands: A patchily distributed biome that occurs at altitudes > 800 m and has enough rainfall that a variety of grasses can thrive. Generally lacking trees except along some rivers and streams, it includes high altitude heathlands and other Afro-alpine areas.
- Mediterranean scrub: A scrubland ecosystem of limited extent, better known as the Fynbos or Cape Floristic Region, that is situated at Africa’s southwestern tip. Characterised by hot dry summers and cool moist winters, it contains one of Earth’s richest concentrations of endemic plant species.
- Flooded grasslands and savannahs: Grasslands, marshes, and shallow lakes that are periodically flooded by water that can be fresh, brackish, or hypersaline. When flooded, these areas host some of the largest water bird congregations in the region.
- Tropical dry forests: A highly restricted forest type that can be found in western Zambia and adjacent Angola, as well as on Cabo Verde. While these areas may receive high rainfall, they are characterised by seasonal droughts that can last several months.
- Mangroves: Coastal wetlands of tropical climates characterised by distinctive woody plants with aerial roots that can tolerate saltwater. Typically associated with intertidal zones and muddy bottoms, mangroves provide nursery grounds for many aquatic animal species.
In addition to these terrestrial biomes, Sub-Saharan Africa also contains several aquatic biomes. Prominent freshwater biomes include several large rivers along with their headwaters and deltas, numerous small rivers, multiple large and small lakes, as well as a variety of wetland ecosystems such as swamps, bogs, and salt marshes (WWF/TNC, 2013). Prominent marine biomes include tropical coral reefs along Africa’s east coast, as well as temperate continental shelves and seas along South Africa and Namibia (Spalding et al., 2007). There are also several important oceanic upwellings—areas of high productivity where surface waters are fertilised by nutrient-rich waters that “wells up” from below; these include the tropical Gulf of Guinea upwelling along West Africa, and the Benguela upwelling ecosystem along Africa’s southwest coast.
The variety of biomes present in Sub-Saharan Africa is the result of variable geology and a long history of changes in climate and ecological communities. For example, when Earth’s climate was warmer, tropical moist forests were more widely distributed. As the planet cooled during glacial periods, forests contracted and became fragmented while grasslands expanded; some new biomes developed as the climate changed and species moved around. Even today, biome boundaries are still shifting: for example, over the last few decades the boundary between the Sahara Desert and Sahel has shifted by hundreds of kilometres southward (Foley et al., 2003). The development, fragmentation, and movement of these and other biomes, as well as the influence of major dispersal barriers, such as large rivers and mountain ranges, have stimulated speciation, as different populations became specialised to conditions that were restricted to their particular elevations or on certain sides (wet or dry, sunny or shady) of mountain ranges.
Sub-Saharan Africa boasts tremendous species richness, the result of a complex geological and environmental history.
Due to this dynamic geological, climatic, and environmental history, as well as all the factors that have promoted speciation, Sub-Saharan Africa boasts tremendous species richness. The region is particularly well known for its mammals, particularly its charismatic terrestrial megafauna and other large mammals that attract millions of tourists from all around the world each year (Figure 2.2). Among the most famous are the Big Five animals—lions (Panthera leo, VU), savannah elephants (Loxodonta africana, VU), African buffalo (Syncerus caffer, NT), African leopards (P. pardus, VU), and black rhinoceros (Diceros bicornis, CR). Other notable mammals include cheetahs (Acinonyx jubatus, VU), the fastest mammal on Earth; Maasai giraffes (Giraffa camelopardalis tippelskirchii, VU), the world’s tallest mammal; the giant eland (Tragelaphus derbianus, VU), the world’s largest antelope; and Africa’s four species of great apes. Many small mammals are also noteworthy. For example, East Africa’s naked mole-rat (Heterocephalus glaber, LC) is the world’s only mammalian thermoconformer—meaning it is almost entirely cold-blooded; like reptiles their body temperature tracks ambient temperatures (Buffenstein and Yahav, 1991). The naked mole-rat and Southern Africa’s Damaraland mole-rat (Fukomys damarensis, LC) are the only known eusocial mammals; like some ants and bees, only one female (the queen) reproduces with one to three breeding males, while all the other colony members are sterile workers (Jarvis et al., 1994).

Figure 2.2 The thrill to go on a guided safari walk with the Big Five in a protected area, such as Zambia’s South Luangwa National Park pictured here, is a major drawcard to foreigners visiting Africa. Dangerous mammals calm down significantly when they are not persecuted. Photograph by Time + Tide, CC BY 4.0.
While Africa’s large mammals are a major tourist drawcard, the region hosts many other rich and noteworthy wildlife assemblages. With more than 2,100 bird species, 1,400 of them found nowhere else on Earth (Sinclair and Ryan, 2011), the Afrotropics may be the most taxonomically diverse bird region on Earth (Lotz et al., 2013). Among the many bird species that call Africa home is the world’s largest extant species of bird, the red-necked ostrich (Struthio camelus camelus); standing up to 2.74 m tall, it is in dire need of conservation attention (Miller et al., 2011). Africa is also home to the world’s heaviest extant flying animal, the kori bustard (Ardeotis kori, NT), which can weigh over 20 kg (Dunning, 2008). Over 100,000 insects have been described in Sub-Saharan Africa (Miller and Rogo, 2001), which include the world’s smallest butterfly, the dwarf blue (Oraidium barberae, LC) of Southern Africa, and the aptly named goliath beetles (Goliathus spp.), which can be found throughout much of tropical Africa. The region also hosts a great number of noteworthy endemic amphibians and reptiles, which include the world’s largest frog, the Goliath frog (Conraua goliath, EN) of Cameroon and Equatorial Guinea, and the black mamba (Dendroaspis polylepis, LC), arguably the world’s most feared snake, which is widespread across Africa’s savannahs. Lastly, Africa is home to Jonathan the Aldabra giant tortoise (Aldabrachelys gigantea, VU); having hatched in 1832, he is considered the oldest living terrestrial animal in the world.
Species that have survived previous mass extinction events are unable to withstand the current onslaught of human activities.
The region’s plant richness, estimated at over 45,000 species (Klopper et al., 2007), is also important from a global perspective. Many plant species have high economic value, particularly those that have been domesticated in the region, and are now important crops across the world. Primary among these are coffee—second only to tea in worldwide popularity as a beverage—which is native to West and Central Africa (Coffea robusta) and Ethiopia (Coffea arabica). Other important crops that originated in in the Afrotropics include okra, black-eyed peas, watermelon, and African oil palm. Conserving the wild genetic diversity of these domesticated plants in their native ranges is important because they may serve as “insurance” for today’s crops that may be less productive in future due to anthropogenic climate change (Davis et al., 2012). Others, such as the wide variety of plants utilised in traditional medicine to treat malaria, may one day lead to new antimalarial drugs (Chinsembu, 2015). Similarly, many plant species also have high evolutionary value. These include relict species that survived previous mass extinction events, such as cycads (Encephalartos spp.) (unfortunately several cycad species are now Extinct in the Wild), and Lazarus species that were once believed to be extinct, such as the unique jellyfish tree (Medusagyne oppositifolia, CR) of the Seychelles.
A few small and isolated African ecosystems are particularly rich in species. Particularly noteworthy is the Rift Valley lakes, such as Lake Victoria, Lake Malawi, and Lake Tanganyika, which hold the richest freshwater fish diversity in the world. For example, nearly 14% of the world’s freshwater fish species occur in Lake Malawi (also known as Lake Nyasa). Moreover, over 90% of Lake Malawi’s 500–1,000 (numbers vary by source) fish species (Figure 2.3) are endemic, and thus found nowhere else on Earth. The Cape Floristic Region is home to the greatest concentration of non-tropical endemic species in the world, including speciose well-known plant genera like Protea and Erica. The Succulent Karoo, directly north of the Cape Floristic Region, may be the most floristically rich desert in the world (Mittermeier et al., 2004). Africa has deservedly received international acclaim for these and many other natural wonders. Prominently, more than 37 sites in Sub-Saharan Africa have already been recognised as natural World Heritage Sites. One such site is also Africa’s oldest national park, Virunga National Park in eastern DRC, which contains at least 218 mammal and 706 bird species (WHC, 2007).

Figure 2.3 Lakes in Central Africa’s Rift Valley hold the richest freshwater fish communities on Earth. Many species, such as these brightly coloured cichlids from Lake Malawi, face extinction because of overfishing, pollution, and invasive species. Photograph by OakleyOriginals, https://www.flickr.com/photos/oakleyoriginals/8589738572, CC BY 2.0.
2.2 History of Conservation in Sub-Saharan Africa
Traditional communities have long held a belief that humans are physically and spiritually connected to nature, and that communal needs outweighed individual desires. This also extended to natural resources, which were considered communal property that must also be shared with the spirits of the ancestors and future generations. Managing natural resources this way required strict adherence to customary law systems that imposed controls on the collection of animal and plant products. Some animals and plants were also worshiped, which leads to mythical superstitions and taboos that prohibited the killing of culturally and spiritually important animals, as well as totem species that bond families and villages together. Customary laws also created Africa’s first protected areas, such as royal hunting grounds (areas where kings and traditional chiefs had exclusive hunting rights) and areas of spiritual significance (Box 2.1), where access and harvesting of natural resources were restricted.
Traditional African communities have long shared the belief that humans are physically and spiritually connected to nature, and that communal needs outweigh individual desires.
Box 2.1 Sacred Spaces: A Tradition of Forest Conservation in Benin
School of Agribusiness and Agricultural Policies,
National University of Agriculture,
Cotonou, Republic of Benin.
enomh2@yahoo.fr
The importance of forests for human life has been recognised for millennia. That is why public approaches have historically been adopted for their protection. Today, some of the most effective programmes are those that integrate local communities and their traditional knowledge with scientific forest management. The Convention on Biological Diversity (CBD) recognises the value of the cultural practices of traditional peoples for (a) practicing conservation and maintaining biodiversity and (b) promoting sustainable use. The sacred forests of Benin are recognised as a tangible heritage, both natural and cultural; their management by the local community is a major achievement in modern conservation.
Forest protection, an ancient reality in Benin
The life of traditional communities of Benin is closely linked to conservation of its forests, also known as Zoun in the local Goun language. Many social practices of Beninese communities rely on leaves, animals, water, stones, and other resources; the areas that provide these natural resources are called sacred forests because they are inhabited by deities or spirits, serve as spaces for rituals, or represent the seat of past kings. Monitoring of sacred forests is often entrusted to members of a certain lineage. For example, custody of the forest of the city of Abomey is the responsibility of traditional chief Dah Djagba, whose ancestors were installed near the sacred spring Didonou by King Houegbadja of Abomey in the 17th century. A sacred forest is a point of contact between a community and a spirit or deity, and between the visible and the invisible. The value and protection of the sacred forest is passed down from generation to generation, as are the rules and regulations. Typically hunting and setting fires in sacred forests are prohibited, while logging for timber and gathering plants for food and medicine are strictly regulated, with these products shared between priests and caretakers of the site (Juhe-Beaulaton and Roussel, 2002). The Aloe vera plant, for example, has long been used by vodun (spirit) priests during religious ceremonies to heal the wounds of new initiates.
Sacred forests today
Sacred forests have significant spiritual capital, or the power to influence the communities that revere them. They influence the collective consciousness regarding experiences as basic as rain, health, and the collection of spring water (in the case of the Abomey forest), or as complex as religious ceremonies, fertility, and overall happiness. Sacred sites also play an important role in cult practices (Roussel, 1994): funeral rites, ceremonies for dead infants, rites for accidental deaths (Laine, 1990; Sokpon et al., 1998), and healing ceremonies with medicinal plants. Meetings of secret societies such as the Zangbeto, Kuvito, and Oro, and religious or social ceremonies and ordeals are held in sacred forests. They also play an important role in the exercise of justice and social cohesion; disobeying the traditional rules and damaging the sacred forest can cause harm to the whole community (bad harvests, epidemics, drought, and mosquito infestations) or the person responsible (accidents, illness, or misfortune). The wrongdoer may need to perform a rite of reparation, such as an animal sacrifice or offering to repair the damage that they have caused.

Figure 2.A Tomato farm on the periphery of the Gbevozoun sacred forest, Benin. Farming encroachment has reduced this sacred forest from 1.6 km2 to 0.5 km2 in recent years; other sacred forests face a similar fate. Photograph by Emile N. Houngbo, CC BY 4.0.
Resistance to human pressures
One difficulty of managing sacred forests today is that they are often not well delineated. Under the influence of population growth, the area occupied by a sacred forest sometimes diminishes to a minimum size under communal protection. Some sacred forests in Benin, such as the 32 km2 Birni forest, 11 km2 Tanekas forest, and 2 km2 Natitingou forest, have vanished due to human pressure on the land. The peripheral zone of Gbevozoun sacred forest in which the Gbevo deity is believed to dwell is currently encroached by agriculture (Figure 2.A), and only a central core of 0.5 km2 of the forest’s original 1.6 km2 is still protected. The Honhoue sacred forest, meanwhile, still retains an area of 0.04 km2 that has not shrunk over time. This is due the local community’s belief in the power of the Honhoue divinity and 40 other deities that dwell in the forest.
Sacred forests are based on traditions of safeguarding religious ceremonies and nature for the future, and they continue to be a means of protecting biodiversity. They may be a resource for conservation of rare plant species for medicinal purposes, and even future improvement of agro-biodiversity. The preservation of sacred forests is crucial to community involvement in conservation.
This culturally driven system of checks and balances was greatly disrupted with the arrival of European settlers in the 17th century. Armed with guns, and little thought given to sustainability, the earliest colonists killed thousands of animals for food, trophies, sport, and profit. Following concerns about declining wildlife populations, particularly at the southern tip of South Africa, Sub-Saharan Africa’s first formal environmental legislation was introduced in 1657, followed by the region’s first formal environmental law in 1684 (MacKenzie, 1997). Significantly, this first law separated protected species, such as the common hippopotamus (Hippopotamus amphibious, VU), from pest species (which at the time included lions). Unfortunately, these early laws and regulations were of little consequence as an increasing number of colonists, lured by the promise of unlimited hunting on unexplored lands, arrived in the region. Consequently, by 1700, populations of every animal over 50 kg within 200 km from Cape Town were extirpated (Rebelo, 1992). These developments also led to Africa’s first modern human-caused mammal extinctions. First to disappear was the bluebuck (Hippotragus leucophaeus, EX) around 1798. Nearly a century later, in 1871, the Cape warthog (Phacochoerus aethiopicus aethiopicus, EX)—more closely related to East Africa’s desert warthog (Phacochoerus aethiopicus delamerei LC) than the widespread common warthog (Phacochoerus africanus LC)—disappeared, followed by the quagga (Equus quagga quagga, EX) around 1878 (the last captive individual died in 1883). Elsewhere, bontebok (Damaliscus pygargus pygargus, NT), Cape mountain zebra (Equus zebra zebra, VU), southern white rhinoceros (Ceratotherium simum simum, NT), and black wildebeest (Connochaetes gnou, LC) were all reduced to about a dozen individuals at one or two locations.
Ecosystems—forests in particular—near early European settlements similarly suffered as early colonists perceived them as an “inexhaustible” supply of fuel and timber. This widespread overharvesting prompted the Cape Colony’s Governor in 1778 to appoint its first professional nature conservator, Johann Fredrick Meeding, to exercise some control over deforestation. But, like controls on hunting large mammals, these efforts generally only had a local and temporary impact.
2.2.1 The 1800s and launching of formal conservation efforts
Interest in the formal protection of Africa’s biodiversity started to intensify during the 19th century. Most of the initial steps were taken in South Africa, which had the largest early colonial settlements and, hence, the most species threatened by human activities. First, in 1822, the Game Law Proclamation introduced hunting licence fees and closed seasons for selected species, followed by regulations to protect ‘open spaces’ in 1846 and forests in 1859. A major step towards ecosystem protection was taken in 1876 with the creation of the Cape Colony’s Department of Forests and Plantations, while the appointment of a Superintendent of Woods and Forests in 1881 led to initial efforts towards the scientific management of ecosystems. Then, in 1886, the British government passed the Cape Act for the Preservation of Game (in 1891 extended to other British South African Territories), followed by the Cape Forest Act of 1888. The Cape Forest Act played an instrumental role in the proclamation of the Cape Colony’s first formally protected areas, namely the Tsitsikamma and Knysna Forest Reserves, in 1888; today these lands are incorporated into South Africa’s Garden Route National Park (Figure 2.4). These were followed by the appointment of Southern Africa’s first formal game warden, H. F. van Oordt, in 1893, to manage Pongola Nature Reserve, proclaimed in 1894. (Pongola was degazetted and converted into agriculture land in 1921 but re-established in 1979). Thereafter, protected areas were established at regular intervals across South Africa, starting with Groenkloof Nature Reserve in February 1895, then Hluhluwe Valley and Umfolozi Junction Game Sanctuaries (today the Hluhluwe-iMfolozi Park) in April 1895. (St Lucia Game Reserve, today part of iSimangaliso Wetland Park, was also established sometime in 1895.)

Figure 2.4 Sub-Saharan Africa’s first formally protected area was established to stop logging of the Tsitsikamma coastal forests, South Africa. Photograph by Androstachys, https://commons.wikimedia.org/wiki/File:Knysna_Forest00.jpg, CC BY 4.0.
West and Central Africa saw its first steps towards formal conservation efforts in 1885, with the establishment of forest reserves to protect valuable timber products (Brugiere and Kormos, 2009). The region’s first game reserves were gazetted as early as 1889 in the DRC to protect elephants. Unfortunately, these efforts were of little consequence as ivory hunters continued to slaughter the region’s elephant populations. It was only after colonial governments raised concerns about declining ivory revenues that the region passed its first formal environmental law in 1892, with the ratification of the Congo Basin Convention to regulate the ivory trade in French, Portuguese, and Belgian territories (Cioc, 2009).
In East Africa, colonial authorities passed its first formal environmental legislations in 1888. These initial laws called for game reserve establishment, hunting quotas for common species, strict protection for breeding females and immature animals, and hunting bans for rare species (Prendergast and Adams, 2003). While protected area establishment was initially slow, a circular from Lord Salisbury (the UK’s Prime Minister at the time) in which he called for protected areas and hunting restrictions to prevent large mammal extinctions, prompted the passing of the German East African Game Ordinance of 1896. That same year, East Africa saw the proclamation of its first modern protected areas, both in Tanzania: one along the Rufiji river (today included in Selous Game Reserve), and one west of Mount Kilimanjaro.
Initial laws and regulations to protect Africa’s environment were greatly expanded in 1900, with the signing of the Convention on the Preservation of Wild Animals, Birds, and Fish in Africa, during the International Conference of the African Colonial Powers held in London, UK. The most innovative agreement of this treaty was the establishment of Schedules that afforded different species different levels of protection. Species on Schedule 1 included rare and valuable species for which all hunting was prohibited; Schedule 2 and 3 included species for which hunting of young animals and accompanying females was prohibited; Schedule 4 included species for which hunting was allowed ‘in limited numbers’; and Schedule 5 included ‘harmful’ species whose populations needed to be reduced. While this convention never went into force (because not enough parties ratified it), several signatories continued to follow the convention’s agreements by establishing wildlife reserves. Among the first to act were Ghana and Sierra Leone, which took their first formal steps towards conserving the environment in 1901. Soon afterwards, in 1903, Africa’s first conservation non-governmental organisation (NGO) was established, namely the Society for the Preservation of Wild Fauna of the Empire (today known as Fauna & Flora International, or FFI).
In 1925, Africa’s first national park, the Albertine Rift’s Albert National Park (today divided into the DRC’s Virunga and Rwanda’s Volcanoes National Parks) was proclaimed. The following year, South Africa’s Sabie Game Reserve (which was originally gazetted in 1898) was renamed and expanded as Kruger National Park. Although most early laws focused on protecting rare and ‘valuable’ mammals, birds, tortoises, and timber forests, the welwitschia (Welwitschia mirabilis) (Figure 2.5) was the first African plant to enjoy formal protection after colonial powers ratified the 1933 Convention Relative to the Preservation of Fauna and Flora in the Natural State (often referred to as the London Convention).

Figure 2.5 The welwitschia, a primitive gymnosperm found only in the Namib Desert of Namibia and Angola, was the first African plant to enjoy formal protection. It is adapted to collect coastal fog on its single pair of leaves which appear as many, having been torn apart by harsh desert conditions. Considered a living fossil, some welwitschias may be over 2,000 years old. Photograph by nhelia, https://pixabay.com/photos/welwitschia-mirabillis-namibia-49479, CC0.
From the outset however, colonial governments managed Africa’s earliest protected areas with policies more representative of Western values, which emphasised the need for nature to be shielded from human activities, and conservation management to be centralised. This top-down, protectionist “fines and fences” strategy, also known as “fortress conservation”, showed little regard for the rights and cultural practices of local communities. In fact, local peoples were more likely seen as a threat to the environment. Consequently, many of Africa’s first formally protected areas were established on land forcibly taken from communal ownership, and access to natural resources on which the local peoples previously relied upon was prohibited. Paradoxically, hunting privileges were reserved for wealthy elites on protected areas set aside for colonists’ enjoyment (Figure 2.6). These practices, termed eco-colonialism for the similarity to the abuses of native rights by colonial powers, caused a growing rift between conservation authorities and deeply offended local peoples.

Figure 2.6 A photo from East Africa in the late 1800s, illustrating typical African conservation of the time: restricting hunting privileges and wildlife trade to rich colonists who shipped their bounties to Europe, with little if any benefit to Africa. From Wikipedia, https://en.wikipedia.org/wiki/File:Ivory_1880s.jpg, CC0.
2.2.2 Conservation efforts after colonialism
Following World War II (1939–1945), after which many African countries regained independence, there was an urgent need for new conservation treaties that also addressed the needs of local peoples. Tanzania’s first president, Julius Nyerere, most vividly expressed this at the 1961 Pan-African Symposium on the Conservation of Nature and Natural Resources in Modern African States (Watterson, 1963), in a speech that became known as the Arusha Manifesto:
“The survival of our wildlife is a matter of grave concern to all of us in Africa. These wild creatures amid the wild places they inhabit are not only important as a source of wonder and inspiration, but are an integral part of our natural resources and our future livelihood and well-being. In accepting the trusteeship of our wildlife we solemnly declare that we will do everything in our power to make sure that our children’s grand-children will be able to enjoy this rich and precious inheritance. The conservation of wildlife and wild places calls for specialist knowledge, trained manpower, and money, and we look to other nations to cooperate with us in this important task – the success or failure of which not only affects the continent of Africa but the rest of the world as well.
Soon after the Arusha Manifesto, the African Charter for the Protection and Conservation of Nature was established in 1963. This was followed by the African Convention on the Conservation of Nature and Natural Resources (Algiers Convention in short), which was adopted by member states of the Organisation of African Unity (which preceded the African Union) in 1968. The Algiers Convention provided a major break from colonial conservation models by acknowledging the principle that environmental management is a common responsibility among all Africans, while it also called for conservation of soil and water, and for environmental research and conservation (IUCN, 2004).
Despite the progress and extended scope of the Algiers Convention, conservation policies implemented by early post-colonial governments unfortunately continued to resemble those of colonial governments, notably the centralised and authoritarian style of decision-making. Similarly, the visions of well-funded international conservation organisations operating in the region generally reflected the perceptions and policies of developed nations, and thus lacked adequate consideration of local cultures (Abrams et al., 2009). Consequently, in the years following Africa’s decolonisation, conservation largely remained a polarising endeavour that continued to uproot the lives of tens of millions of conservation refugees over time (Geisler and de Sousa, 2001).
2.3 Conservation in Sub-Saharan Africa Today
Building on the environmental laws and protected areas system Africans have inherited from the tumultuous past has not been easy. The scars left in the collective psyche by forced relocations and exclusions have been difficult to mend, with many conservation initiatives still struggling to shake the unfortunate association. Nevertheless, Africa’s passionate conservation biologists and the broader public have shown tremendous fortitude and initiative in advancing the biodiversity conservation agenda over the last few decades. Much of this progress can be attributed to a growing realisation that conserving biodiversity is best achieved when combined with the social and economic upliftment of local people.
Conservation initiatives continue to struggle to shake the unfortunate association from past actions taken with a centralised and authoritarian style of decision-making.
Perhaps the first true step to conservation reform came at the 1975 World Parks Congress hosted in the DRC, when the International Union for Conservation of Nature and Natural Resources (IUCN) adopted its first resolution that recognised the rights and needs of traditional peoples. Over the next few decades, conservation policies of national governments followed, many of which included local people in very explicit terms. One example is Namibia’s Constitution, passed in 1990, stating that:
“The State shall actively promote and maintain the welfare of the people by adopting, inter alia, policies aimed at the following: maintenance of ecosystems, essential ecological processes and biological diversity of Namibia and utilisation of living natural resources on a sustainable basis for the benefit of all Namibians, both present and future”.
As the previous centralised and authoritarian style of conservation policy making has made way for more inclusive conservation activities (Abrams et al., 2009), an increasing number of local communities have become active participants in environmental programmes and policy development inside and on the periphery of protected areas. Two notable examples are biosphere reserves (Section 13.5.2) and transfrontier conservation areas (TFCA, Box 2.2), both pioneering strategies in promoting human-wildlife coexistence. Several governments are also expanding their protected areas networks by experimenting with private ownership of protected areas (Box 2.3) and co-management partnerships (Section 13.1.4), a land tenure model in which local people share the decision-making and other responsibilities of protected areas management with public institutions (Borrini-Feyerabend et al., 2004). In recent years, integrated conservation and development projects (ICDPs, Section 14.3) have also emerged as viable options to link conservation and socio-economic development.
Box 2.2 Why Go Transfrontier? (And Why Not?)
Biodiversity Conservation consultant.
tamarron@bezeqint.net
The past two decades have brought high praise and gaining momentum for TFCAs in Southern Africa, as in other parts of the world (e.g. Vasilijevic et al., 2015; Zunckel, 2014). While Africa’s first TFCA, the W National Park, was established already in 1954 by the governments of Benin, Burkina-Faso, and Niger, it was only after the Kgalagadi Transfrontier Park was established in 1999 (between the governments of South Africa and Botswana) that TFCAs have become a prominent component of the concepts driving biodiversity conservation and tourism development in Southern Africa, and across the continent.
TFCAs can support biodiversity conservation in several ways. They help protect large conservation areas and ecological corridors, facilitate cross-border knowledge exchange and cooperation in conservation and enforcement efforts, and promote mainstreaming conservation considerations into land-use planning. These benefits, in turn, offer socio-economic advantages through eco-tourism, sustainable use of natural resources, increased attraction for investors and donors, and, in some cases, supporting peace-building efforts.
Establishing a TFCA, however, entails challenges and risks (Vasilijevic et al., 2015; Zunckel, 2014; Ron, 2007). These processes are often top-down in nature, involving long and costly high-level negotiations between governments with critical conservation funds being spent on multiple cross-border meetings of senior officials and coordination efforts. Due to financial and political considerations, too often the focus remains at the central governments’ level, with limited engagement with local stakeholders and on-the-ground impact. At times, many residents in the concerned area are not even aware that they live in a TFCA, or how this can change their lives.
Political and financial challenges at the local, national and regional levels may hinder the establishment of TFCAs. National inter-agency competition, disagreements within and between local communities, and conflict between international agencies, NGOs, and supporting donors may all have negative consequences. Facilitated cross-border movement of people and goods can cause security challenges and other risks, such as disease transfer, spread of invasive species, increased human-wildlife conflict, and increased illegal wildlife traffic and other criminal activities. In establishing a TFCA, it is thus essential to consult and engage all key stakeholders, and especially local communities, beginning in the planning phase, as well as to prioritise investment in on-the-ground impact-generating activities, to achieve conservation, social and development goals.
My experience in developing the Mayombe Transfrontier Initiative, between Angola, Republic of the Congo, DRC, and Gabon was most revealing (Ron, 2011a). In 2000, we initiated conservation efforts in the Angolan component of the Mayombe forest. From the start, it became clear that the striking difference in the level of degradation between the countries that share the Mayombe forest (Figure 2.B) could not be sustainable. Moreover, uncontrolled logging for timber and poaching of primates, elephants, parrots, pangolins, and other threatened species were driven, to a large extent, by illegal cross-border wildlife traffickers. It was evident that cooperation between the four countries that shared the forest was essential (Ron, 2003), so we solicited financial support from several international organisations. Initial support focused on high-level meetings and negotiations (Ijang et al. 2012). Unfortunately, local stakeholders perceived these mediation attempts as unbalanced. Finally, through governmental leadership, a Memorandum of Understanding was signed between the first three countries in 2009, with Gabon joining in 2013. A study was implemented through extensive consultation with stakeholders, and a strategic plan focusing on the most needed on-the-ground activities was adopted (Ron, 2011b). While conservation efforts have progressed at the national level, the same originally identified threats are still prominent throughout the TFCA, so it is now critical that substantial funding be allocated to the strategy’s actual on-the-ground implementation.

Figure 2.B The Mayombe Forest; the tree line in the photo marks the border between Angola (top) and the Republic of the Congo. Efforts are currently underway to protect forest and surrounding area as a TFCA. Photograph by Tamar Ron, CC BY 4.0.
So, what is the conclusion? Go transfrontier? The answer is yes—but not in every case—and very carefully. Perspective must be kept through long term planning, while keeping the focus on local-level priorities.
Box 2.3 Privately Owned Lands for African Conservation
ARC Centre of Excellence in Coral Reef Studies,
James Cook University,
Townsville, Australia.
gscumming@gmail.com
With rates of species loss increasing and natural communities under pressure worldwide from human demands, the creation and maintenance of protected areas continues to be a vitally important conservation strategy. At the 2014 World Parks Congress in Sydney, Australia, there was widespread recognition of the need to increase the total amount of land and ocean under protection. However, this cannot be achieved by governments simply setting aside more land. Protected areas are ultimately created by people for people, and if they are to be successful, they must be created and managed in a way that is socially acceptable and sustainable.
Committing more land to biodiversity conservation means achieving a consensus between political, economic, societal, and ecological forces. This is particularly important in heavily populated landscapes, especially in Africa where local communities still bear the scars of a history of colonialism and top-down decision-making. One possible solution is to provide incentives that encourage private landowners to engage voluntarily in conservation. The area of land in private nature reserves in South Africa (both individually- and community-owned) is already estimated to be nearly twice the extent of government-owned protected areas (de Vos et al., 2019). The dynamics of privately protected areas and their overall contributions to biodiversity are, however, largely undocumented and poorly understood.
The number of privately protected areas in South Africa has increased rapidly since the end of apartheid in 1994 (de Vos et al., 2019). This increase can be partly attributed to increased tourism in South Africa and partly to the removal of perverse subsidies that kept marginal agricultural land in production (see also Section 4.5.3). Unlike statutory reserves, privately protected areas receive little or no financial support from the government and must ensure their own survival by generating revenue. They can be economically self-sufficient only if they can generate enough income from tourism. Two models appear to be particularly effective: either offering a high-cost, high-investment Big Five game viewing experience (i.e. staying in a comfortable bungalow, being guided by knowledgeable individuals), or providing a cheaper, lower-investment experience that focuses on affordable accommodation with access to hiking trails, striking scenery, and outdoor recreational opportunities (Clements et al., 2016). These models may be particularly effective in areas adjacent to national parks. For example, Shamwari Private Game Reserve, one of the more successful upper-end privately protected areas (Figure 2.C), is adjacent to Addo Elephant National Park in the Eastern Cape.

Figure 2.C A group of tourists watching two young giraffes (Giraffa camelopardalis, VU) play-fighting on Shamwari Private Game Reserve, South Africa. Shamwari successfully linked luxurious accommodation with wildlife safari activities to tap into the profitable conservation industry on private lands. Photograph by Iky’s Photographic, https://commons.wikimedia.org/wiki/File:Shamwari_Private_Game_Reserve.jpg, CC BY-SA 4.0.
The conservation value of private lands, and particularly those that stock large herbivores, has been questioned in South Africa because of concerns about economic influences on their management. For example, tourist demand for wildlife viewing experiences can drive the overstocking of large animals, such as elephants, in small Southern African protected areas, even though higher densities of elephants do not necessarily provide a better tourism experience (Maciejewski and Kerley, 2014). Overstocking of large mammals can also lead to the conversion of woodlands to thickets, decreasing both conservation and tourism value (Cumming et al., 1997). Conversely, many private lands in the Western and Eastern Cape of South Africa have high conservation potential; many private lands in the Cape sit lower in the landscape than parks, which, in water-scarce South Africa, have been focused on mountainous water catchment areas, and many harbours threatened lowland vegetation (Winter et al., 2007). Lowland ecosystems with their richer soils are under higher pressure from agriculture and settlement, meaning that well-managed private areas may make a disproportionately large contribution to the conservation of globally rare and endemic fynbos plants and animals (e.g. proteas, heathers, reptiles, and birds). Several governmentally supported programmes, such as the stewardship programme of the South African National Biodiversity Institute (SANBI), have been created to foster biodiversity conservation on private lands by providing information and encouraging good management practices (Rouget et al., 2014).
The owners and managers of privately protected areas could potentially interact with one another, and with the leadership of provincial and national parks, on a wide range of issues. But the managers of private lands are often poorly connected in these networks and may not benefit from knowledge sharing in the same way as managers of established reserves (Maciejewski and Cumming, 2015). In addition, many privately protected areas are not profitable, with the result that financial demands may push managers to make short-term decisions that attract revenue (e.g. overstocking large herbivores or suppressing wildfires) but have harmful long-term ecological consequences. Possible measures to ensure that private conservation efforts are both sustainable and effective include governmental interventions through tax breaks and support, and improved integration of private lands with national and provincial parks and their managers. Private conservation has considerable promise as a strategy for Africa, but its full potential will only be realised if it is achieved equitably with secure land tenure and supportive governments.
Through these different conservation partnerships models (see also Chapter 13), Africans have surpassed expectations in how rapidly they have expanded their conservation areas network. Illustrating the progress, Cameroon has augmented its existing protected areas system with nine new national parks between 2000 and 2015, with an additional nine in the proposal phase (UNEP-WCMC, 2019). The new parks include Takamanda National Park, which connects with Nigeria’s Cross River National Park to form one of West Africa’s largest continuous formally protected areas; it also plays a critical role in protecting the world’s last remaining Cross River gorillas (Gorilla gorilla diehli, CR), of which fewer than 300 remain. As of mid-2019, protected areas covered over 38% of Tanzania’s land area (more than 361,000 km2, an area larger than Germany or Côte d’Ivoire [UNEP-WCMC, 2019])!
Sub-Saharan Africa’s marine protected areas (MPA, Section 13.4.1) are similarly also expanding. For example, in 2017, Gabon declared 26% of its territorial waters protected, offering a haven to at least 20 species of whales and dolphins, and 20 species of sharks and rays (Parker, 2017). More recently, the Seychelles created two new MPAs that cover an area of 210,000 km2—an area the size of Great Britain. The South African government, in collaboration with World Wide Fund For Nature (WWF), has taken the addition step by creating a forum (http://mpaforum.org.za) to improve MPA governance, and a website (https://www.marineprotectedareas.org.za) to teach the public more about South Africa’s rapidly expanding MPA system.
Many of Africa’s protected areas are nothing more than paper parks, areas that are protected on paper but not in reality.
It is important, however, to keep in mind that protecting a certain area of land and water should not in itself be the only goal in conservation. Even when a country has numerous protected areas, certain unique ecosystems may remain unprotected. Being safeguarded in name is not enough, protected areas must also be maintained and managed to achieve meaningful conservation success. Too many protected areas are nothing more than paper parks, areas that are protected on paper but not in reality. Two of the most important causes of protected area failure are lack of buy-in from local people, and lack of investment, financially or otherwise, from local and national governments (Watson et al., 2014; McClanahan et al., 2016; Gill et al., 2017).
Fortunately, African conservation biologists regularly employ a can-do attitude, shown in a long history of resourcefulness in the face of resource constraints. For example, conservationists from all over the region have established, and are partnering with, non-profit NGOs to facilitate a variety of innovative mechanisms to advance biodiversity conservation (see also Section 15.3). One notable example is the African Parks Network; as of mid-2019, African Parks, in partnership with its host governments, are managing 15 national parks in nine countries, covering 10.5 million hectares. Through this collaboration, which includes extensive community engagement and law enforcement, several once-declining parks are now seeing their wildlife prospering. For example, lions were reintroduced to Rwanda in 2016 after a 20-year absence, elephant strongholds in Chad and the DRC are been secured, and populations of threatened large mammals on Zambia’s Liuwa Plains have increased by 50% to over 100% in just a few years (African Parks, 2016). Not only do recovering wildlife populations here and elsewhere attract more tourists, they also provide opportunities to attract new people to conservation, through environmental education (Figure 2.7), public health services, and other community upliftment programmes that improve the well-being of local peoples (see Box 1.2). These benefits then provide additional positive feedback towards wildlife conservation, for example by encouraging an increasing number of poachers to transition into new fulfilling lives as conservation professionals (Cooney et al., 2017).

Figure 2.7 Environmental education plays an important role in teaching people about the importance of their natural heritage and conservation. Here a group of school children releases a ringed yellow-fronted tinkerbird (Pogoniulus chrysoconus, LC) in Wondo Genet, Ethiopia, as part of a project that combines citizen science with long-term wildlife monitoring. Photograph by Çağan Şekercioğlu, CC BY 4.0.
By seeing and being exposed to all the social and economic benefits biodiversity conservation efforts offer, many local communities have been inspired to take the lead in protecting wildlife on their own lands. For example, community efforts have successfully safeguarded Mali’s savannah elephants (Canney and Ganamé, 2015) and Rwanda’s mountain gorillas (Gorilla beringei beringei, EN) (Kalpers et al., 2003) through periods of conflict. Locally managed forest reserves now protect more than 36,000 km2 of land in Tanzania (Roe et al., 2009), while conservation efforts on community conserved areas in Kenya have renewed hope for the future of the world’s rarest antelope, the hirola (Beatragus hunter, CR) (King et al., 2016). These examples have set a positive, enterprising tone that has enabled conservation to play in increasingly prominent role in multiple economies through the creation of job opportunities while also improving Africans’ overall quality of life.
2.4 Ongoing Conservation Challenges
Despite many examples of progress, conservation challenges and conflicts persist across Africa. As a result, the region lags in several aspects with regards to safeguarding our natural heritage (Table 2.1). The causes are many and vary by region. Below is a discussion of some of the more prominent impediments to effective conservation action in Africa.
2.4.1 Persistent poverty
Poverty can drive desperate people to illegal actions, even though they understand the detriment these actions may have on society at large and their own futures.
There is a direct link between poverty and conservation failure (Oldekop et al., 2016; Hauenstein et al., 2019). This is a problem particularly in Africa, where millions of people live in extreme poverty that is difficult to escape. Faced with hard choices to ensure there is food on the table, poverty can drive desperate people to illegally collect natural products from protected areas, even though they likely understand the detriment these actions may have on society at large and their own futures. Other vulnerable peoples that live close to the land, such as traditional hunter-gatherers and pastoralists, are increasingly pushed into wildlife sanctuaries by mining, deforestation, agricultural expansion, and development that encroach on their traditional lands. Lacking the resources to defend their land and/or support to transition to new lifestyles, these marginalised communities are often left desolate, with few if any legal options to support their livelihoods.
Table 2.1 A comparison between the number of species and number of threatened species for several major groups of animals and plants present in Sub-Saharan Africa.
|
Group |
Species assessed by IUCNa |
Species threatened with extinction |
Data deficient species |
|
|
Numberb |
Percentage |
|||
|
Vertebrate animals |
10,463 |
1,464 |
14 |
|
|
Mammals |
1,226 |
203 |
17 |
196 |
|
Primates |
97 |
39 |
40 |
1 |
|
Carnivores |
85 |
13 |
15 |
3 |
|
Bats |
248 |
23 |
9 |
55 |
|
Birds |
2,265 |
233 |
10 |
18 |
|
Birds of preyc |
141 |
32 |
23 |
0 |
|
Vultures |
10 |
7 |
70 |
0 |
|
Amphibians |
840 |
213 |
25 |
152 |
|
Reptiles |
736 |
109 |
15 |
123 |
|
Ray-finned fishes |
5,650 |
637 |
11 |
846 |
|
Cichlids |
1,026 |
232 |
23 |
146 |
|
Arthropods |
2,368 |
637 |
27 |
334 |
|
Arachnids |
186 |
142 |
76 |
2 |
|
Insects |
1,796 |
396 |
22 |
246 |
|
Ants |
8 |
6 |
100 |
0 |
|
Butterflies |
305 |
72 |
24 |
32 |
|
Dragonflies |
737 |
70 |
10 |
67 |
|
Plants |
4,916 |
2,165 |
44 |
294 |
|
Cycads |
68 |
48 |
71 |
0 |
|
Ferns |
115 |
47 |
41 |
3 |
Source: IUCN, 2019, current as of April-2019
a Low species richness generally reflects inadequate data because only a few species were evaluated. For example, 100% of ants are listed as threatened, but only eight species have been evaluated; there are more ant species in many African towns and villages.
b Categories included: Extinct in the Wild, Critically Endangered, Endangered, Vulnerable
c Includes raptors, falcons, and owls
Further complicating matters, many well-intentioned citizens and organisations from western countries continue to have overly simplistic views of Africa. By imposing their outsider views on rare species management in Africa, these groups exacerbate the impacts of poverty, by cutting off funding sources of well-functioning conservation programmes. A good example comes from regulating trophy hunting of rare animals. Some African mammals, such as lions and elephants, are globally rare, but locally common in well-managed private game reserves and community conserved areas. Due to their global rarity, land managers of such well-managed populations can earn large fees from foreign hunters targeting these sought-after trophy species; the money earned supports local communities by boosting the local economy and conservation efforts (Lindsey et al., 2007; IUCN/PACO, 2009; Cooney et al., 2017). Unfortunately, the hunting of rare species remains controversial because many people dislike seeing charismatic animals killed. Consequently, campaigns from western countries (e.g. Hance, 2018) have significantly impeding the African trophy hunting industry, with no exemption for effective self-supporting land managers. By limiting and threatening the benefits regulated trophy hunting can bring to well-managed conservation areas and poor communities (Mbaiwa, 2018), there is fear that these campaigns will achieve the opposite of their intended purposes, by removing the incentive to protect those rare and/or charismatic species. Conservation requires all parties involved to weigh the benefits as well as unintended consequences of wildlife trade—i.e. overharvesting and black markets (Lenzen et al., 2012; Hsiang and Sekar, 2016), land grabbing (see Section 5.2), corruption, and terrorism (Christy and Stirton, 2015) and adapt as and when needed. Section 14.3 provides some solutions on how to link conservation with development.
2.4.2 Obstructive mindsets
Colonial Africa has provided many examples showing that conservation activities implemented in an authoritarian manner are bound to fail. Yet, authoritarian mindsets continue to impede conservation efforts throughout the region. Work from Guinea-Bissau has shown that authoritarian conservation actions that disempower or displace local communities are more likely to worsen than overcome conservation challenges in post-colonial Africa (Cross, 2015). Conservation in Africa is as much about people as it is about wildlife; this book provides many examples to show how human welfare and conservation are tied to one another.
At the same time, integrating diverging cultural beliefs about the natural environment into conservation practices also remains an obstacle (Dickman et al., 2015). Many Africans continue to fixate on cultural justifications (“We have been hunting for many generations”, Figure 2.8) without acknowledging that human population growth, more sophisticated weapons, and increased levels of consumption are putting unsustainable pressure on natural landscapes. Others believe that the destruction of nature is simply not possible because their ancestors will intervene before this happens, effectively removing individual or community responsibility from conservation management and planning. Breaking down such barriers is hard, frustrating, and takes a long time to achieve. It requires an interdisciplinary approach (Section 1.1) bringing together aspects of conservation science and the social sciences to find common ground. Despite the challenges to putting effective conservation into practice, it is important to remember that fortress conservation models—telling people how they should act, with little to no local input—are more likely to produce enduring counter-productive results.

Figure 2.8 A group of hunters carry a western lowland gorilla (Gorilla gorilla gorilla, CR) that was shot while raiding crops in southern Cameroon. While retaliatory killings is the traditional method for dealing with problem animals (but see Section 14.4), killing rare species such as gorillas is generally forbidden by customary and statutory laws. Photograph by Edmond Dounias/CIFOR, CC BY 4.0.
2.4.3 Weak governance/institutional structures
Africa’s natural environment and its people often fall victim to weak governance and institutional structures. It is well-known that weak policies, failing governments, and civil conflict hamper conservation efforts and drive biodiversity declines (Nackoney et al., 2014; Brito et al., 2018; Daskin and Pringle, 2018). But even in well-functioning countries, government officials turning a blind eye (either willingly, or because they lack capacity) may enable corporations to cut corners for increased profits at the cost of the environment. Corruption and greed also fuel land grabbing (Section 5.2), black markets (Hauenstein et al., 2019), and unwarranted protected area degazettement (Section 13.7.3). There is broad interest to challenge these behaviours which benefit only a handful of people at the cost of thousands of others (Box 2.4). Fixing these issues will rely on strengthening institutional capacity on multiple levels (Amano et al., 2018).
Weak policies, failing governments, and civil conflict hamper conservation efforts and drive biodiversity declines.
Box 2.4 Malawi: No Longer a Weak Link in the Elephant Ivory Trafficking Chain?
Lilongwe Wildlife Trust,
Lilongwe, Malawi.
http://www.lilongwewildlife.org
International efforts to combat illegal wildlife trade—now the fourth largest transnational crime in the world (Nellemann et al., 2016)—have intensified in recent years, but Malawi has escaped public scrutiny due to its small size and relatively small wildlife numbers. Despite these factors, Malawi’s wildlife populations have been decimated by poaching in the last few decades. For example, Kasungu National Park’s wildlife was so abundant in the 1980s that animals were translocated to the Kruger National Park in South Africa. Back then, elephants numbered as high as 2,000. Today, there are no more than 60.
Southern Africa’s principle transit hub for wildlife trafficking
In 2016, CITES identified Malawi as a “country of primary concern”, and Southern Africa’s principle transit hub for ivory trafficking. Malawi’s own Illegal Wildlife Trade Review (Waterland et al., 2015), published a year earlier, had come to similar conclusions, uncovering evidence of large-scale international trafficking of bushmeat, carnivore pelts, tortoises, pangolins, orchids, ivory, and rhino horn. The revelations served a wake-up call for urgent action to protect not just Malawi’s own wildlife but also wild populations throughout Southern Africa.
Central to region’s poaching hotspots
Why is Malawi such a significant link in the trafficking chain? The first clue is geography. Malawi is surrounded by Africa’s biggest elephant poaching hotspots. Selous Game Reserve in Tanzania reportedly lost 25,000 elephants between 2009 and 2013, while 1,000 elephants were killed in Mozambique’s Niassa Province in 2011, alone (Booth and Dunham, 2016). Poaching in Zambia’s Luangwa Valley is well above the CITES average (Nyirenda et al., 2015). Wasser et al. (2015) found that all the study samples of ivory seized from consignments weighing more than half a tonne between 2006 and 2014 originated from ecosystems immediately bordering Malawi.
Malawi has already been implicated in some of the largest ivory seizures in the world. The biggest impoundment ever—at 7.5 tonnes, equivalent to over 1,500 elephants—was made in Singapore in 2002 and had been shipped from Malawi’s capital, Lilongwe (Wasser et al., 2007, 2015). In 2013, 2.6 tonnes of ivory were confiscated from a container within Malawi’s borders at Mzuzu. Fifty cases were recorded between 2010 and 2014, including numerous smaller examples of ivory trafficking. With an estimated 10% interception rate, the true scale of ivory trafficking was evidently much larger than previously thought (Waterland et al., 2015).
Risk-reward ratio in favour of criminals
Malawi’s weak wildlife legislation was another significant factor. Coupled with under-resourced law enforcement and high levels of corruption, this offered an attractive risk-to-reward ratio for wildlife criminals. The individuals convicted of trafficking in the 2013 Mzuzu case escaped with a fine of just US $5,000 for a 2.6-tonne haul. This paled in comparison to the penalties handed out in other countries. For example, during the same period, a Zambian man was sent to prison for five years for trafficking 12.5 kg of ivory, a South African man received 10 years and a US $392,000 fine for trafficking one tonne of ivory and, in Kenya, a man was fined US $233,000 for trafficking a single tusk weighing 3.4 kg.
While sentencing in the Mzuzu case was hampered to some extent by limitations of the law, it was also indicative of the fact that, historically, trafficking was not treated as a serious crime in Malawi. Most wildlife prosecutions had taken place in lower courts and have been prosecuted by lower-ranked officials. The average fine for ivory trafficking was found to be just US $40 between 2011 and 2014. This is an extremely low amount given the potential profits from the trade of ivory and, thus, provides virtually no deterrent to traffickers. Awareness, motivation, and cooperation within and between departments like the police and border forces were found to be severely lacking. Government resources to combat wildlife crime are also limited, with many other causes competing for funding and attention.
Management of wildlife crime data also made life easier for wildlife criminals. Take the case of a Chinese national who was arrested and prosecuted for an ivory trafficking offence under one name, deported under a second name, and reported by the INTERPOL country office to INTERPOL headquarters under a third. This shows the ease with which criminals are circumventing the weak systems currently in place.
Turning the Tide
Today, however, things are changing. Recommendations from the 2015 Illegal Wildlife Trade Review were swiftly executed, strengthening the process from investigations and arrest right through to prosecution and sentencing. As a result, in just four years, over 1.5 tonnes of ivory were confiscated, average monthly arrests for wildlife crime jumped from 0.7 to 9.5, and custodial sentence rates rose to over 90%, with judgments passed of up to 18 years. Remember that, in comparison, no-one convicted of a wildlife crime between 2010 and 2015 had been put behind bars and the average fine was just $40.
Other initiatives included improving protected area management, launching the country’s first wildlife crime investigations unit, and establishing an Inter-Agency Committee to Combat Wildlife Crime to improve cooperation and information sharing. Critical amendments to wildlife legislation were also passed in record time, and technical expertise from partners was harnessed to maximise impact. Lilongwe Wildlife Trust is currently the only NGO sanctioned to prosecute wildlife crime cases in partnership with an African government. In short, Malawi has strengthened each stage of the enforcement chain.
These successes came about largely as a result of a collaborative, innovative, and holistic approach that moved beyond traditional wildlife conservation to incorporate practices used in combatting serious organised crime.
Support from the very top
Strengthened legislation and enforcement will continue to be a critical deterrent, but advocacy has also been a critical tool for securing high-level political will and turning it into action. The President of Malawi, himself, His Excellency Peter Mutharika, backed the nation’s “Stop Wildlife Crime” campaign (Figure 2.D) and the Malawi Parliamentary Conservation Caucus continues to raise awareness through the media, essentially holding stakeholders such as the police or judiciary to account by highlighting both successes and questionable outcomes.

Figure 2.D Campaigners taking to the streets in support of Malawi’s “Stop Wildlife Crime” campaign, which the President of Malawi also supports. Photograph by Lilongwe Wildlife Trust, CC BY 4.0.
Focus on trafficking
Poaching has been a major focus of conservation efforts elsewhere in Africa, and local poachers can still expect to feel the full weight of the Malawian law. However, bringing traffickers to justice is proving a more effective use of limited resources. After all, it is members of organised international crime syndicates that ultimately exploit local communities, incite corruption, threaten our national security, and provide the routes to overseas markets.
What’s next for Malawi?
Sustaining Malawi’s astounding turnaround will be no easy feat. But with continued determination, as well as local and international cooperation and support, we believe that these criminal networks can be disrupted enough to halt the impending extinction of one of Africa’s most iconic species.
The same tenacity and high-level commitment we have witnessed in the last five years must now be applied to other conservation challenges, as attention is being turned to the protection of Malawi’s wider biodiversity. In 2018, a further 216 species of animals, plants and trees were placed under legal protection, and lessons from combatting wildlife crime can now be applied to other illegal or unsustainable practices, such as trades in timber, charcoal, and fish.
When it comes to pioneering conservation, Malawi is one to watch. Let’s hope that there are more achievements to celebrate in another five years’ time.
2.4.4 Skills shortages
Scientific advances depend on increased or updated knowledge. That is also true for conservation biology—effective conservation depends on local experts who can design and implement monitoring and research projects, apply adaptive management (Section 10.2.3) when needed, act as managers and advocates for conservation activities, and increase awareness of the importance of the environment (Laurance, 2013). It is thus of great concern that conservation in Africa continues to face an enduring skills shortage (Wilson et al., 2016). Illustrating the problem, a recent review found that, over the past three decades, only 129 of the scientific articles focussed on West African birds were produced in international journals by local authors. This productivity contrasts strongly with Europe, where 12,380 ornithological articles were produced over the same time (Cresswell, 2018). Another review, covering all of Africa, found that less than 30% of the continent’s birds received attention in international journals (Beale, 2018). While high-impact publications are not the only metric to estimate conservation success, they provide an accurate accounting of persistent knowledge gaps, as well as skills shortages further down the hierarchy, from researchers and teachers to rangers and other fieldworkers down to citizen scientists.
There are many reasons for these skills shortages. Some of the most prominent foundational issues include a fragmented communication network that limits skills transfer, financial and other resources limitations, a shortage of quality training institutes, and overburdened teachers at existing educational facilities. Fortunately, many of these shortfalls are currently being addressed. For example, new people are being involved in conservation activities through citizen science projects (see Box 15.3), innovative funding mechanisms are being developed (Section 15.3), legal and organisational structures are being adapted to foster increased collaboration (Section 15.4) and freely-accessible resources such as this textbook are being made available.. It is important to continue to build on this progress by supporting such initiatives, and continuously highlighting to others the importance of nature to their own well-being.
2.4.5 Competing interests
Like stock market investments, the benefits to be gained from conservation may take years to materialise.
Because of competing interests (for land, natural resources, etc.), there is always a risk that a wealthy business will threaten a conservation initiative with competing offers that typically include promises of jobs and development (Koohafkan et al., 2011). Local peoples, especially those in poverty, may find it hard to turn down such attractive counteroffers, even if they recognise that those offers rarely live up to the promises made. Conservation biologists should carefully consider what such offers on the table might look like and factor in how their conservation programmes compete and bring better results for all.
People concerned with the environment have worked hard to better highlight that conservation has the potential to be profitable and to spur sustainable development. These activities have seen the emergence of fields such as environmental economics, and methods to put a market value on ecosystem services (Section 4.5). Unfortunately, some conservation biologists have fallen into a trap of (over)emphasising the economic benefits that conservation can bring, without a realistic representation of the upfront investment required or the length of time required for a tangible return on investment. Like stock market investments, the benefits to be gained from conservation may take years to materialise, sometimes with very little to show for it in the meantime. Given that all investments require either expendable capital or credit, willing stakeholders with neither are essentially being asked to maintain a more restrictive livelihood over an unsustainable (and often undisclosed or unknown) period of time. It is crucial for conservation biologists to set realistic expectations and to offer a balanced approach that provides interim funding/credit options. Such options could perhaps include microloans, village savings and loan associations (http://www.care.org/vsla), or community conservation banks (https://sema.fzs.org/en/conservation-banks) such as those established by Frankfurt Zoological Society (FZS) in Tanzania. It is also important to incorporate benefits beyond immediate financial gain when starting or expanding conservation programmes. Conservation actions should also aim to provide concrete benefits, whether financial or otherwise, to local communities from an early stage. In that way, if a project comes to a premature end, one can still point to the progress made, which will make it easier to engage with that community when future opportunities arise.
2.5 Conclusion
Because of the many challenges that conservation projects continue to face, the list of Sub-Saharan African species and ecosystems that are threatened with extinction and destruction continues to grow every year. In a recent assessment, BirdLife International identified 51 Important Bird and Biodiversity Areas (IBA) in Sub-Saharan Africa—many of them national parks—in danger of ecosystem degradation (BirdLife International, 2019). A United Nations (UN) assessment similarly found that the outlook of 12 natural World Heritage Sites situated in Sub-Saharan African “in danger” (http://whc.unesco.org/en/danger). These are substantial and challenging problems that will keep conservation biologists very busy in the future. These problems need to be faced head-on to ensure that future generations will also be able to enjoy the natural treasures and resources the region has to offer.
2.6 Summary
- Sub-Saharan Africa supports extremely diverse ecological communities across its eight terrestrial biomes (which include forests, savannahs, woodlands, grasslands, scrublands, deserts, and mangroves) as well as multiple freshwater and marine ecosystems. The region’s complex climate, geology, and history have contributed to the development of its exceptional biodiversity.
- Conservation in Africa has gone through major changes over the past few centuries including traditional relationships with nature; exploitation of wildlife and natural resources by European settlers in the 17th and 18th centuries; western practices of setting aside land shielded from human influences; and more recently integrated conservation and development practices.
- Africa’s conservation biologists and the broader public have shown tremendous fortitude and initiative to overcome the various challenges facing biodiversity over the last few decades. This includes greatly expanding the protected areas network, passing laws protecting the environment, and establishing productive partnerships.
- By reaping the benefits from conservation activities in and around protected areas, many private individuals and local communities have been inspired to take the lead in protecting biodiversity on their own lands.
- Historical legacies, poverty, greed, weak governance, consumptive needs by an increasing human population, and competing interests remain challenges to conservation in Africa. Many of these challenges lead to threats to the future persistence of many species and ecosystems, including environmental degradation and overharvesting.
2.7 Topics for Discussion
- The human population of Sub-Saharan Africa is predicted to increase dramatically in coming decades. How do you think this growth will affect the region’s biodiversity? Do you think that the increase in human population will increase consumptive needs?
- What are the main international and national organisations contributing to conservation in your region? What projects are they working on? What are the most important goals of those projects? What do you think are the biggest challenges facing those projects?
- Conservation in Africa has gone through several stages through history. Can you summarise each of these stages in two or three sentences? What do you think are the strengths and weaknesses of each stage?
2.8 Suggested Readings
Abrams, R.W., E.D. Anwana, A. Ormsby, et al. 2009. Integrating top-down with bottom-up conservation policy in Africa. Conservation Biology 23: 799–804. https://doi.org/10.1111/j.1523-1739.2009.01285.x Africa requires locally-adapted conservation policies.
African Parks. 2019. Unlocking the value of protected areas. African Parks Annual Report 2018 (Johannesburg: African Parks). https://www.africanparks.org/unlocking-value-protected-areas An overview of activities undertaken by a successful conservation NGO.
Balmford, A., J.L. Moore, T. Brooks, et al. 2011. Conservation conflicts across Africa. Science 291: 2616–19. https://doi.org/10.1126/science.291.5513.2616 Many of the challenges to conservation in Africa are rooted in population growth.
Beale, C.M., S. van Rensberg, W.J. Bond, et al. 2013. Ten lessons for the conservation of African savannah ecosystems. Biological Conservation 167: 224–32. https://doi.org/10.1016/j.biocon.2013.08.025 Learning from past efforts to guide future actions.
Cooney, R., D. Roe, H. Dublin, et al. 2017. From poachers to protectors: Engaging local communities in solutions to illegal wildlife trade. Conservation Letters 10: 367–74. https://doi.org/10.1111/conl.12294 Guidelines for engaging the local community in conservation activities.
Cross, H. 2015. Displacement, disempowerment and corruption: Challenges at the interface of fisheries, management and conservation in the Bijagós Archipelago, Guinea-Bissau. Oryx 50: 693–701. https://doi.org/10.1017/S003060531500040X Authoritative top-down strategies will likely worsen rather than solve conservation conflicts.
Hauenstein, S., M. Kshatriya, J. Blanc, et al. 2019. African elephant poaching rates correlate with local poverty, national corruption and global ivory price. Nature Communications 10: 2242. https://doi.org/10.1038/s41467-019-09993-2 Linking poverty, corruption, and wildlife declines.
Kalpers, J., E.A. Williamson, M.M. Robbins, et al. 2003. Gorillas in the crossfire: Population dynamics of the Virunga mountain gorillas over the past three decades. Oryx 37: 326–37. https://doi.org/10.1017/S0030605303000589 African conservationists have had to deal with a very dynamic, and sometimes dangerous environment.
Prendergast, D.K., and W.M. Adams. 2003. Colonial wildlife conservation and the origins of the Society for the Preservation of the Wild Fauna of the Empire (1903–1914). Oryx 37: 251–60. https://doi.org/10.1017/S0030605303000425 Consider how conservation biologists today face similar challenges to those a century ago.
Bibliography
Abrams, R.W., E.D. Anwana, A. Ormsby, et al. 2009. Integrating top-down with bottom-up conservation policy in Africa. Conservation Biology 23: 799–804. https://doi.org/10.1111/j.1523-1739.2009.01285.x
African Parks. 2016. African Parks annual report 2015: Conservation at scale (Johannesburg: African Parks). https://www.africanparks.org/sites/default/files/uploads/resources/2017-05/APN_AnnualReport_2015.pdf
Amano, T., T. Székely, B. Sandel, et al. 2018. Successful conservation of global waterbird populations depends on effective governance. Nature 553: 199–202. https://doi.org/10.1038/nature25139
Balmford, A., J.L. Moore, T. Brooks, et al. 2011. Conservation conflicts across Africa. Science 291: 2616–19. https://doi.org/10.1126/science.291.5513.2616
Beale, C.M. 2018. Trends and themes in African ornithology. Ostrich 89: 99–108. https://doi.org/10.2989/00306525.2017.1407834
BirdLife International. 2019. IBA’s in Danger-Site Summary. http://datazone.birdlife.org/site/ibaidsites
Booth, V.R., and K.M. Dunham. 2016. Elephant poaching in Niassa Reserve, Mozambique: Population impact revealed by combined survey trends for live elephants and carcasses. Oryx 50: 94–103. https://doi.org/10.1017/S0030605314000568
Borrini-Feyerabend, G., M. Pimbert, M.T. Farvar, et al. 2004. Sharing power. Learning by doing in co-management of natural resources throughout the world (Cenesta: IIED and IUCN/CEESP/CMWG). http://pubs.iied.org/G01089
Brito, J.C., S.M. Durant, N. Pettorelli, et al., 2018. Armed conflicts and wildlife decline: Challenges and recommendations for effective conservation policy in the Sahara‐Sahel. Conservation Letters 11: e12446. https://doi.org/10.1111/conl.12446
Brugiere, D., and R. Kormos. 2009. Review of the protected area network in Guinea, West Africa, and recommendations for new sites for biodiversity conservation. Biodiversity and Conservation 18: 847–68. https://doi.org/10.1007/s10531-008-9508-z
Buffenstein, R., and S. Yahav. 1991. Is the naked mole-rat Heterocephalus glaber an endothermic yet poikilothermic? Journal of Thermal Biology 16: 227–32. https://doi.org/10.1016/0306-4565(91)90030-6
Burgess, N., J.D. Hales, E. Underwood, et al. 2004. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment (Washington: Island Press).
Canney, S., and N. Ganamé. 2015. The Mali elephant project, Mali. In: Conservation, Crime and Communities: Case Studies of Efforts to Engage Local Communities in Tackling Illegal Wildlife Trade, ed. by D. Roe (London: IIED). http://pubs.iied.org/14648IIED/
Chinsembu, K.C. 2015. Plants as antimalarial agents in Sub-Saharan Africa. Acta Tropica 152: 32–48. https://doi.org/10.1016/j.actatropica.2015.08.009
Christy, B., and B. Stirton. 2015. How killing elephants finances terror in Africa. National Geographic. http://on.natgeo.com/1I5N2aO.
Cioc, M. 2009. The Game of Conservation: International Treaties to Protect the World’s Migratory Animals (Columbus: Ohio University Press).
Clements, H., J. Baum, and G.S. Cumming. 2016. Money and motives: An organizational ecology perspective on private land conservation. Biological Conservation 197: 108–15. https://doi.org/10.1016/j.biocon.2016.03.002
Cooney, R., C. Freese, H. Dublin, et al. 2017. The baby and the bathwater: Trophy hunting, conservation and rural livelihoods. Unasylva 249: 3–16. http://www.fao.org/3/i6855en/I6855EN.pdf
Cooney, R., D. Roe, H. Dublin, et al. 2017. From poachers to protectors: Engaging local communities in solutions to illegal wildlife trade. Conservation Letters 10: 368–74. https://doi.org/10.1111/conl.12294
Cresswell, W. 2018. The continuing lack of ornithological research capacity in almost all of West Africa. Ostrich 89: 123–29. https://doi.org/10.2989/00306525.2017.1388301
Cross, H. 2015. Displacement, disempowerment and corruption: challenges at the interface of fisheries, management and conservation in the Bijagós Archipelago, Guinea-Bissau. Oryx 50: 693–701. https://doi.org/10.1017/S003060531500040X
Cumming, D.H.M., M.B. Fenton, I.L. Rautenbach, et al. 1997. Elephants, woodlands and biodiversity in southern Africa. South African Journal of Science 93: 231–36
Daskin, J.H., and R.M. Pringle. 2018. Warfare and wildlife declines in Africa’s protected areas. Nature 553: 328–32. https://doi.org/10.1038/nature25194
Davis A.P., T.W. Gole, S. Baena, et al. 2012. The impact of climate change on indigenous Arabica coffee (Coffea arabica): Predicting future trends and identifying priorities. PLoS ONE 7: e47981. https://doi.org/10.1371/journal.pone.0047981
de Vos, A., H.S. Clements, D. Biggs, et al. 2019. The dynamics of proclaimed privately protected areas in South Africa over 83 years. Conservation Letters 12: e12644. https://doi.org/10.1111/conl.12644
Dickman, A., P.J. Johnson, F. van Kesteren, et al. 2015. The moral basis for conservation: How is it affected by culture? Frontiers in Ecology and the Environment 13: 325–31. https://doi.org/10.1890/140056
Dunning, J.B. Jr. 2008. CRC Handbook of Avian Body Masses (Boca Raton: CRC Press).
Foley, J.A., M.T. Coe, M. Scheffer, et al. 2003. Regime shifts in the Sahara and Sahel: Interactions between ecological and climatic systems in Northern Africa. Ecosystems 6: 524–32. https://doi.org/10.1007/s10021-002-0227-0
Geisler, C., and R. de Sousa. 2001. From refuge to refugee: the African case. Public Administration and Development 21: 159–70. https://doi.org/10.1002/pad.158
Gill, D.A., M.B. Mascia, G.N. Ahmadia, et al. 2017. Capacity shortfalls hinder the performance of marine protected areas globally. Nature 543: 665–69. https://doi.org/10.1038/nature21708
Gorenflo, L.J., S. Romaine, R.A. Mittermeier, et al. 2012. Co-occurrence of linguistic and biological diversity in biodiversity hotspots and high biodiversity wilderness areas. Proceedings of the National Academy of Sciences 109: 8032–37. https://doi.org/10.1073/pnas.1117511109
Hance, J. 2018. Trump’s elephant, lion trophy hunting policy hit with double lawsuits. Mongabay https://news.mongabay.com/2018/03/trumps-elephant-lion-trophy-hunting-policy-hit-with-double-lawsuits.
Hauenstein, S., M. Kshatriya, J. Blanc, et al. 2019. African elephant poaching rates correlate with local poverty, national corruption and global ivory price. Nature Communications 10: 2242. https://doi.org/10.1038/s41467-019-09993-2
Hsiang, S., and N. Sekar. 2016. Does legalization reduce black market activity? Evidence from a global ivory experiment and elephant poaching data. NBER Working Paper 22314 (Cambridge: National Bureau of Economic Research). https://doi.org/10.3386/w22314
Ijang, T.P., N. Cleto, N.W. Ewane, et al. 2012. Transboundary dialogue and cooperation: First lessons from igniting negotiations on joint management of the Mayombe Forest in the Congo Basin. International Journal of Agriculture and Forestry 2: 121–31. https://doi.org/10.5923/j.ijaf.20120203.08
IUCN. 2004. An introduction to the African Convention on the Conservation of Nature and Natural Resources (Gland: IUCN). https://portals.iucn.org/library/efiles/documents/EPLP-056-rev.pdf
IUCN. 2019. The IUCN Red List of Threatened Species. http://www.iucnredlist.org
IUCN/PACO. 2009. Big game hunting in West Africa. What is its contribution to conservation? (Ouagadougou: IUCN/PACO). https://portals.iucn.org/library/sites/library/files/documents/2009-074-En.pdf
Jarvis, J.U.M., M.J. O’Riain, N.C. Bennett, et al. 1994. Mammalian eusociality: A family affair. Trends in Ecology and Evolution 9: 47–51. https://doi.org/10.1016/0169-5347(94)90267-4
Juhé-Beaulaton, D., and B. Roussel. 2002. Les sites religieux vodun, des patrimoines en permanente evolution. In: Patrimonialiser la nature tropicale: Dynamiques locales, enjeux internationaux, ed. by M.-C. Cormier-Salem et al. (Paris: IRD). http://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers09-03/010028405.pdf
Kalpers, J., E.A. Williamson, M.M. Robbins, et al. 2003. Gorillas in the crossfire: Population dynamics of the Virunga mountain gorillas over the past three decades. Oryx 37: 326–37. https://doi.org/10.1017/S0030605303000589
King, J., A. Wandera, M.I. Sheikh, et al. 2016. Status of Hirola in Ishaqbini Community Conservancy (Masalani: Ishaqbini Hirola Community Conservancy; Northern Rangelands Trust). https://nrt-kenya.squarespace.com/s/Hirola-sanctuary-status-report-May2016-mjwy.pdf
Klopper, R.R., L. Gautier, C. Chatelain, et al. 2007. Floristics of the angiosperm flora of Sub-Saharan Africa: An analysis of the African Plant Checklist and Database. Taxon 56: 201–08.
Koohafkan, P., M. Salman, and C. Casarotto. 2011. Investments in land and water. SOLAW Background Thematic Report TR17 (London: FAO). http://www.fao.org/fileadmin/templates/solaw/files/thematic_reports/TR_17_web.pdf
Kümpel, N.F., A. Quinn, and S. Grange. 2015. The distribution and population status of the elusive okapi, Okapia johnstoni. African Journal of Ecology 53: 242–45. https://doi.org/10.1111/aje.12221
Laine, A. 1990. Mythe et Réalités: Les Enfants nés Pour Mourir en Afrique de l’Ouest. Episteme 1: 87–97.
Laurance W.F. 2013. Does research help to safeguard protected areas? Trends in Ecology and Evolution 28: 261–66. https://doi.org/10.1016/j.tree.2013.01.017
Lenzen, M., D. Moran, K. Kanemoto, et al. 2012. International trade drives biodiversity threats in developing nations. Nature 486: 109–12. https://doi.org/10.1038/nature11145
Lewis, M.P., G.F. Simons, and C.D. Fennig. 2014. Ethnologue: Languages of the World (Dallas: SIL International). https://www.ethnologue.com
Lindsey, P.A., P.A. Roulet, and S.S. Romanach. 2007. Economic and conservation significance of the trophy hunting industry in sub-Saharan Africa. Biological Conservation 134: 455–69. https://doi.org/10.1016/j.biocon.2006.09.005
Lotz, C.N., J.A. Caddick, M. Forner, et al. 2013. Beyond just species: Is Africa the most taxonomically diverse bird continent? South African Journal of Science 109(5/6): 1–4. https://doi.org/10.1590/sajs.2013/20120002
Maciejewski, K., and G. Cumming. 2015. The relevance of socioeconomic interactions for the resilience of protected area networks. Ecosphere 6: 1–14. https://doi.org/10.1890/ES15-00022.1
Maciejewski, K., and G.I. Kerley. 2014. Elevated elephant density does not improve ecotourism opportunities: Convergence in social and ecological objectives. Ecological Applications 24: 920–26. https://doi.org/10.1890/13-0935.1
MacKenzie, J.M. 1997. The Empire of Nature: Hunting, Conservation and British Imperialism (Manchester: Manchester University Press).
Mbaiwa, J.E. 2018. Effects of the safari hunting tourism ban on rural livelihoods and wildlife conservation in Northern Botswana. South African Geographical Journal 100: 41–61. https://doi.org/10.1080/03736245.2017.1299639
McClanahan, T.R., and P.S. Rankin. 2016. Geography of conservation spending, biodiversity, and culture. Conservation Biology 30: 1089–101. https://doi.org/10.1111/cobi.12720
Miller, J.M., S. Hallager, S.L. Monfort, et al. 2011. Phylogeographic analysis of nuclear and mtDNA supports subspecies designations in the ostrich (Struthio camelus). Conservation Genetics 12: 423–31. https://doi.org/10.1007/s10592-010-0149-x
Miller, S.E., and L.M. Rogo. 2011. Challenges and opportunities in understanding and utilisation of African insect diversity. Cimbebasia 17: 197–218
Mittermeier, R.A., P. Robles-Gil, M. Hoffman, et al. 2004. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions (Chicago: University of Chicago Press).
Nackoney, J., G. Molinario, P. Potapov, et al. 2014. Impacts of civil conflict on primary forest habitat in northern Democratic Republic of the Congo, 1990–2010. Biological Conservation 170: 321–28. https://doi.org/10.1016/j.biocon.2013.12.033.
Nellemann, C., R. Henriksen, A. Kreilhuber, et al. 2016. The rise of environmental crime—A growing threat to natural resources, peace, development and security (Cambridge: UNEP). http://wedocs.unep.org/handle/20.500.11822/7662
Nyirenda, V.R., P.A. Lindsey, E. Phiri, et al. 2015. Trends in the illegal killing of African elephants (Loxodonta africana) in the Luangwa and Zambezi Ecosystems of Zambia. Environment and Natural Resources Research 5: 24–36. https://doi.org/10.5539/enrr.v5n2p24
Oldekop, J.A., G. Holmes, W.E. Harris, et al. 2016. A global assessment of the social and conservation outcomes of protected areas. Conservation Biology 30: 133–41. https://doi.org/10.1111/cobi.12568
Olson, D.M., E. Dinerstein, E.D. Wikramanayake, et al. 2001. Terrestrial ecoregions of the world: A new map of life on Earth. BioScience 51: 933–38. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Parker, L. 2017. New ocean reserve, largest in Africa, protects whales and turtles. National Geographic. http://on.natgeo.com/2samx3a
Prendergast, D.K., and W.M. Adams. 2003. Colonial wildlife conservation and the origins of the Society for the Preservation of the Wild Fauna of the Empire (1903–1914). Oryx 37: 251–60. https://doi.org/10.1017/S0030605303000425
Rebelo, A.G. 1992. Red Data Book species in the Cape Floristic Region: Threats, priorities and target species. Transactions of the Royal Society of South Africa 48: 55–86. https://doi.org/10.1080/00359199209520256
Roe D., F. Nelson, and C. Sandbrook. 2009. Community management of natural resources in Africa: Impacts, experiences and future directions. Natural Resource Issues 18 (London: IIED). http://pubs.iied.org/pdfs/17503IIED.pdf
Ron, T. 2003. The conservation of the Maiombe Forest, Cabinda, Angola, within the framework of a transfrontier conservation initiative. The World Parks Congress, September 2003, Durban, South Africa.
Ron, T. 2007. Southern Africa Development Community (SADC) proposed framework for transfrontier conservation areas (TFCAs). Issues and options report. Approved by the SADC Integrated Committee of Ministers (ICM). http://www.tbpa.net/docs/SDC_SADC%20final%20report%20draft_Tamar%20Ron.pdf
Ron, T. 2011a. Potential for designating protected areas for conservation and for identifying conservation corridors as part of the planning process of the Mayombe Forest Ecosystems Transfrontier Conservation Area. Report for the Governments of Angola, Congo, and DRC, UNEP and IUCN.
Ron, T. 2011b. Towards a transboundary protected area complex in the Mayombe Forest Ecosystems. Five years strategic plan and roadmap. With inputs from Angola, Congo, DRC, UNEP and IUCN. Adopted by the Mayombe Transfrontier Initiative’s Governments on March 2013.
Rouget, M., M. Barnett, R.M. Cowling, et al. 2014. Conserving the Cape Floristic Region. In: Fynbos: Ecology, Evolution, and Conservation of a Megadiverse Region, ed. by N. Allsopp et al. (Oxford: Oxford University Press).
Roussel, B. 1994. Des dieux à l’homme. Les plantes des vaudous. Hommes et Plantes 7–8: 46–49.
Sinclair, I., and P. Ryan. 2011. Birds of Africa South of the Sahara. Penguin Random House, Johannesburg, South Africa.
Sokpon, N., A. Ametepe, and V. Agbo. 1998. Forêts sacrées et conservation de la biodiversité au Bénin: cas du pays Adja au Sud-Ouest du Bénin, Annales des Sciences Agronomiques 1: 47–64.
Spalding, M.D., H.E. Fox, G.R. Allen, et al. 2007. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 57: 573–83. https://doi.org/10.1641/B570707
UNEP-WCMC. 2019. World Database on Protected Areas. http://www.protectedplanet.net
Vasilijevic, M., K. Zunckel, M. McKinney, et al. 2015. Transboundary Conservation: A systematic and integrated approach. Best Practice Protected Area Guidelines, Series 23 (Gland: IUCN). https://doi.org/10.2305/IUCN.CH.2015.PAG.23.en
Wasser, S.K., C. Mailand, R. Booth, et al. 2007. Using DNA to track the largest ivory seizure since the 1989 trade ban. Proceedings of the National Academy of Sciences 104: 4228–33. https://doi.org/10.1073/pnas.0609714104
Wasser, S.K., L. Brown, C. Mailand, et al. 2015. Genetic assignment of large seizures of elephant ivory reveals Africa’s major poaching hotspots. Science 349: 84–87. https://doi.org/10.1126/science.aaa2457
Waterland, S, J. Vaughan, E. Lyman, et al. 2015. Illegal wildlife trade review of Malawi (Lilongwe: DNPW). https://www.lilongwewildlife.org/wp-content/uploads/IWT-Review-Malawi.pdf
Watson, J.E., N. Dudley, D.B. Segan, et al. 2014. The performance and potential of protected areas. Nature 515: 67–73. https://doi.org/10.1038/nature13947
Watterson, G.G. 1963. Conservation of nature and natural resources in modern African states. IUCN Publications New Series 1 (Morges: IUCN). https://portals.iucn.org/library/sites/library/files/documents/NS-001.pdf
WHC (World Heritage Committee). 2007. Nomination of natural, mixed and cultural properties to the World Heritage List - Virunga National Park. Decision 31COM 8B.74 (Christchurch: UNESCO. https://whc.unesco.org/en/decisions/1377
Wilson, K.A., N.A. Auerbach, K. Sam, et al. 2016. Conservation research is not happening where it is most needed. PLoS Biology 14: e1002413. https://doi.org/10.1371/journal.pbio.1002413
Winter, S.J., H. Prozesky, and K.J Esler. 2007. A case study of landholder attitudes and behaviour toward the conservation of renosterveld, a critically endangered vegetation type in Cape Floral Kingdom, South Africa. Environmental Management 40: 46–61. https://doi.org/10.1007/s00267-006-0086-0
World Bank. 2017. Global economic prospects, June 2017: A fragile recovery (Washington: World Bank). https://openknowledge.worldbank.org/bitstream/handle/10986/26800/9781464810244.pdf
World Bank. 2019. World Bank Open Data: Sub-Saharan Africa. http://data.worldbank.org/region/sub-saharan-africa
WWF/TNC. 2013. Freshwater Ecoregions of the World. http://www.feow.org
Zunckel, K. 2014. Southern African Development Community transfrontier conservation guidelines: The establishment and development of TFCA initiatives between SADC Member States (Gabarone: SADC TFCA).