11. How It Works:
The Biological Mechanisms that Generate Demographic Diversity
© 2024 Virginia J. Vitzthum, CC BY 4.0 https://doi.org/10.11647/OBP.0251.11
Tinbergen (1963) proposed that a complete understanding of any behaviour requires knowledge of its function, evolutionary history, developmental history and mechanism of operation. This chapter is largely concerned with gaining some insight into the nature of the biological mechanisms generating variation in human fertility, and, consequently, demographic diversity within and across populations. My inquiry is informed by life history theory, an analytical framework within evolutionary theory for studying maturation, reproduction and aging and the associated behavioural and physiological mechanisms underlying the allocation of resources to these processes. Different allocation patterns are referred to as life history strategies (LHSs) and are subject to natural selection. Biological mechanisms can be usefully conceptualized as a set of suitably timed strategic responses to signals. I discuss this and other ideas about the mechanisms that underlie the implementation of LHSs, and introduce the concepts of “ecomarker” and “the physiological fallacy”.
Drawing on empirical studies and theoretical models, I examine some intriguing features of human reproductive physiology that are directly relevant to demographic research in both low- and high-fertility populations. Several points, some contrary to common assumptions, emerge from this inquiry. For example: (1) the marked within- and between-population variation in many features of female reproductive functioning challenges the widespread assumption that there is a universal “normal” human biology. (2) The most likely outcome of a human conception is early loss. This unseen natural selection in the production of offspring may hamper investigations of hypothesized associations of post-natal reproductive success with resources or with offspring quality, even in low fertility populations. (3) Competition between incompatible but essential functions shapes the timing and operation of various mechanisms. Some biological, psychological and behavioural functions cannot readily co-occur. Of necessity, successful LHSs must juggle such incompatibilities regardless of the abundance of energy and other resources, therefore some reproductive mechanisms may not depend upon (or be responsive to) energy availability. (4) Biomedically, the absence of ovulation is typically considered a pathology (and in some cases it may be). However, from a life history perspective, each option of ovulating/not ovulating is a fork in the reproductive road at which there is a strategic decision to continue engaging in the possibility of reproduction or to forego the current opportunity. Not ovulating in a given cycle can be the best strategy for optimizing lifetime reproductive success.
It would be instructive to know […] by what physiological mechanisms a just apportionment is made between the nutriment devoted to the gonads and that devoted to the rest of the parental organism …
— Fisher (1930)
Evolution is a tinkerer.
— Jacob (1977)
Introduction
What Must Be Known If We Are to Have a Thorough Understanding of Human Demographic Diversity?
Posed this way, the answer to the question would seem to be, “Everything!”, and the goal appears unachievable. Recognizing this, we choose instead to focus on a single feature — perhaps age at menopause or marriage practices or hormonal concentrations. This specialization is necessary and productive but risks losing sight of the bigger questions that first piqued our curiosity.
Nearly sixty years ago, Tinbergen (1963) sought to mitigate this risk within the field of ethology1 by proposing that a comprehensive and coherent understanding of behaviour arises from integrating the answers to four complementary questions. His framing proved to be an enduring guide for the thorough study of any feature of an organism (Bateson and Laland, 2013). Paraphrased for more general application, Tinbergen’s four questions are:
- What is the function of the feature? (i.e. what is its current and/or former utility?)
- How has the feature evolved over time? (i.e. what is its phylogeny?)
- How does the feature develop in an organism? (i.e. what is its ontogeny?)
- What are the mechanisms that produce the feature? (i.e. how does it work?)
This chapter is largely concerned with the physiological mechanisms generating variation in human fertility and, consequently, demographic diversity across populations.2 Like most organismal features, individual fertility is variable, but the mean and limits of this variation are characteristic of the species and subject to natural selection. Time, resources, competing demands and the physical constraints of biological processes all limit individual fertility even in the most successful members of a population and even in the most benign environment. In addition to physiological mechanisms, there are psychological, behavioural, social and cultural pathways that generate variation in human reproductive output.3 Many of these pathways impact fertility via biological mechanisms.
Reproductive mechanisms can be delineated and studied without recourse to evolutionary theory or a consideration of Tinbergen’s other questions. However, to do so would be to miss understanding why these mechanisms operate as they do. Neither is our understanding well-served by simply assuming that all features (or variants of a feature) are evolved adaptations; there is plenty of evidence to the contrary (Williams, 1966).
Fully understanding the causes of biological variation necessarily demands incorporating an evolutionary perspective (Tinbergen, 1963; Dobzhansky, 1973). Doing so, however, does not give us leave to accept any seemingly plausible story of the adaptive advantage of some trait or another (Williams, 1966; Gould and Lewontin, 1979; Caro and Borgerhoff Mulder, 1987). Without an understanding of mechanism, we risk spinning “just-so stories” (explanations relying more on our imaginations or preferences than on empirical evidence). Rather, our plausible conjectures are better seen as starting points for generating specific and testable hypotheses about how a thing works and what function it serves.
Mace (in this volume) discusses three ways to test hypotheses about function: experimentation, comparative studies of individuals within populations and comparative studies across populations or across species. These approaches are equally applicable to investigating mechanisms. Ideally, hypotheses about mechanism are addressed by demonstrating exactly how a purported causal agent is linked to an observed outcome. How questions about mechanism are not the only ones worth asking, and they are very rarely the first to be asked. But they must be answered if we are to thoroughly understand why human fertility, mortality and health vary, whether due to immediate circumstances or as a consequence of evolutionary processes or, most likely, the dynamic interaction of both.
Evolution through natural selection is often portrayed as a winnowing process that favours individuals with “the best” features for survival and reproduction, a description that gives the faulty impression that after many generations, most members of a species are nearly identical when it comes to basic functions such as the reproductive system. This faulty impression readily lends itself to the false assumption that there is not much variation in the physiological mechanisms enabling human reproduction (and thus, such mechanisms appear unlikely to be a significant cause of fertility differentials within and between populations).
To the contrary, there is substantial and compelling empirical evidence of within-species variation in biological mechanisms. Even identical genotypes can produce different phenotypes (variants in morphological, physiological, behavioural and psychological features), an outcome of developmental and epigenetic processes that facilitate individual adaptation to the environments encountered from conception through death.
Because environments change over time and space, individuals possessing a genotype that adjusts phenotype according to shifting conditions can have an evolutionary advantage over conspecifics who express only one phenotype, no matter the conditions encountered. The capacity for a genotype to express a variety of phenotypes is known as “phenotypic plasticity” and the range of possible phenotypes for a given genotype is referred to as the “norm of reaction” (Via and Lande, 1985; West-Eberhard, 1989, 2003; Stearns, 1989; Vitzthum, 1990; 2003; Scheiner, 1993). Such plasticity can also be disadvantageous (Dewitt et al., 1988), a reminder of the importance of testing specific hypotheses. Nonetheless, the evidence for phenotypic plasticity across a wide range of taxa and phenotypes supports its importance as an adaptive mechanism (West-Eberhard, 2003), and the analyses by Jones et al. (in this volume) suggests that phenotypic plasticity plays a significant role in generating human demographic diversity.
Individual adaptation is achieved through genetic, epigenetic and ontogenetic processes shaping the organism’s phenotypes. Biological evolution is a consequence of natural selection acting on these phenotypes. Many biological mechanisms, including those associated with reproduction, are flexible and exhibit a dynamic response to external conditions. This capacity can cause variation in fertility across the many physical and social environments in which humans live. Examples of this flexibility and the potential impact on demographic diversity are discussed in this chapter.
On a broader note, failing to understand the how and why of biological features can lead to pathologizing natural variation and reifying cultural constructs of what is normal or superior. This error sometimes takes the form of assuming that the average and distribution of some feature found in one’s own population is universally true of, or an appropriate norm for, the entire human species (Mead, 1947; Vitzthum, 2020). But if, like me, your native population is WEIRD (Western, educated, industrialized, rich, democratic), then it represents only 12% of the world’s current population (Henrich et al., 2010), and bears little resemblance to the conditions typical of human history during the many hundreds of thousands of years before humans invented agriculture. To help overcome this myopia, there is a fifth question worth adding to Tinbergen’s four: How do the features of these mechanisms vary within and across human communities worldwide? Techniques developed over the past forty years have allowed us to begin to address this question; some of the answers are considered throughout this chapter.
Why Is the Study of Biological Mechanisms Useful to Demographers?
The answer, in brief, is that the identification and specification of biological mechanisms expands and enriches our understanding of how demographic variation is generated. As a case in point, an ever better grasp of mechanisms has been, and will continue to be, directly relevant to improving models of the proximate determinants of fertility.
Demographers’ investigations of how fertility, mortality and migration impact population structure brought to light the marked variation in these processes across human populations. Not long ago, most explanations for this variation concerned the influence of sociocultural and economic factors, giving little attention to the biological processes involved in the production of offspring and the maintenance of a living body. This focus reflected demography’s historical roots (Sear et al., 2016; Kreagar in this volume) and the assumption that there was little variation across human populations in such biological processes. As a consequence, much has been learned about the changes in fertility associated with varying sociocultural and economic factors (Balbo et al., 2013; Uggla in this volume), but relatively little about how these factors might play out through biological mechanisms. Even so, over time and for a variety of reasons, demographers’ growing attention to biological processes and evolutionary biologists’ keen appreciation of demographers’ population data has prompted novel and productive collaborations to address this gap (Sear et al., 2016; Kreagar in this volume; Low in this volume).
Davis and Blake (1956) proposed the first formal demographic framework identifying a finite set of behavioural and biological mechanisms (“intermediate fertility variables”) through which all other possible factors (sociocultural, economic, environmental, behavioural) must act in order to influence fertility. Of their eleven direct factors, only two (“foetal mortality from involuntary causes” and “fecundity or infecundity, as affected by involuntary causes”) are biological variables, and the latter of these included the entire morphological and neuroendocrinological mechanisms of the ovarian cycle, conception and implantation. The authors lamented the absence of relevant data that would allow an assessment of the contribution to fertility of either of these two biological intermediate fertility variables.
In 1978, Bongaarts reformulated Davis and Blake’s work as a set of eight “proximate determinants of fertility” and proposed a quantitative approach for estimating the contributions of four determinants (#1–4 in Table 1) to a population’s total fertility rate. Of these four, the only biological factor is lactational infecundability. Its inclusion in Bongaarts’ analyses was a consequence of the by-then large body of data demonstrating that breastfeeding can suppress ovulation (Gioiosa, 1955; Perez, 1971; Vitzthum, 1994) and thereby contribute to inter-population variation in fertility (these data had not yet been collected at the time of Davis and Blake’s work in 1956). Bongaarts also argued that the other four proximate determinants (#5–8 in Table 1) are not important contributors to differences in fertility between populations, but allowed that the three biological factors might be significant if venereal disease were present.
Through the years since, Bongaarts’ model has been critiqued and revised (Reinis, 1992; Wood, 1994; Stover, 1998) including a recent “tune-up” (Bongaarts, 2015). His quantitative approach has demonstrable utility in addressing certain kinds of demographic questions, and his work continues to be the most widely applied demographic model of the proximate determinants of fertility. However, Bongaarts (1978, 2015) estimated the contribution to fertility of only one of the four biological proximate determinants, neglecting the others. This may explain why some analyses using this method could not adequately account for observed between-population variation in fertility (Wood, 1994). Also, at least some of the omitted biological determinants are likely to generate fertility differences between individuals, a possibility that can’t be addressed using Bongaarts’ approach.
TABLE 1. Proximate Determinants of Fertility
|
I. Exposure factors: |
|
1. Proportion married |
|
2. Contraception |
|
3. Induced abortion |
|
4. Lactational infecundability |
|
5. Frequency of intercourse |
|
6. Sterility |
|
8. Duration of the fertile period |
Source: Bongaarts (1978)
Beginning in 1988, Maxine Weinstein (a demographer), Kenneth Campbell (an endocrinologist) and James Wood (a bioanthropologist) proposed and refined a new model, “the proximate determinants of natural fertility” (Table 2) (Campbell and Wood, 1988; Wood and Weinstein, 1988; Weinstein et al., 1990; Wood, 1994). This framework can accommodate variation among populations, among individuals within a population and within particular individuals (e.g. over time). Moreover, their approach explicitly models the contributions of a comprehensive, but nonetheless small, set of behavioural and biological proximate determinants. This attention to biological mechanisms has revealed, for example, that one of the most important potential sources of inter-population variation in fertility is intra-uterine death. Although most pregnancy losses are undetected (except with a laboratory test), this needn’t mean such loss is inconsequential for population structure (see discussion below in “Vote Early, Vote Often: Early Pregnancy Loss”).
These theoretical advancements in demographic models of fertility are attributable, in part, to a burgeoning awareness, fuelled by empirical and theoretical studies alike, of the variability, complexity and flexibility of the underlying biological mechanisms that make reproduction possible.
TABLE 2. Proximate Determinants of Natural Fertility
|
I. Exposure factors: |
|
1. Age at menarche |
|
3. Age at entry into sexual union |
|
4. Age at onset of pathological sterility |
|
II. Susceptibility factors: |
|
Fecundability factors: |
|
5. Length of ovarian cycles |
|
7. Duration of the fertile period |
|
8. Frequency of insemination |
|
9. Probability of conception from a single insemination in the fertile period |
|
10. Probability of pregnancy loss |
|
11. Length of the non-susceptible period following foetal loss |
|
12. Length of gestation resulting in a live birth |
|
13. Duration of post-partum infecundability |
Source: Wood (1994)
The Take-Homes
Before delving into the details, these are the main arguments developed in this chapter regarding the biological mechanisms regulating human reproduction.
Core themes in demography (the causes of variation in fertility and mortality) map well with those of life history theory (LHT). LHT is an analytical framework within evolutionary theory for studying maturation, reproduction, ageing and the associated behavioural and physiological mechanisms underlying the allocation of resources to these processes (Promislow and Harvey, 1990; Stearns, 1992; Roff, 1992; Charnov, 1993; Vitzthum, 2008a; Hill in this volume; Low in this volume). Because there are unavoidable trade-offs in the allocation of finite resources (e.g. time, energy, nutrients) over a lifetime, different allocation patterns (referred to as ‘life history strategies’ [LHSs]) can produce variation in the quantity and quality of offspring, thus generating opportunities for natural selection and adaptation.
One useful approach for organizing an inquiry into the physiological mechanisms associated with LHSs is to conceptualize bodily functioning as a set of suitably timed strategic responses to signals. In subsequent sections, I will use empirical studies and theoretical models of human female reproductive functioning to discuss this and other ideas about these mechanisms. Rather than attempting to catalogue all of the mechanisms that contribute to the production of offspring, my aim is to gain some insight into the nature of these mechanisms — their shared properties — with an eye towards developing research questions and strategies that help us to search for the physiological keys to human demographic diversity beyond the light from the nearest lamp post. The principle take-home points from this inquiry are summarized below.
(1) Some mechanisms rely more on the detection of change in a condition than on the assessment of a static condition. Because a life history strategy (LHS) is a series of allocation decisions, its success depends both on the relative amounts of resources distributed to competing demands and on strategic timing. Strategic timing necessarily relies on the detection/recognition of reliable signals of endogenous (internal, somatic) and exogenous (external, extra-somatic) current and changing conditions. In some instances, the change in conditions may be more readily detected and hence a more salient signal than the specific state of the condition. For example, regardless of the absolute concentration of progesterone at its peak during the menstrual cycle, it is the drop in progesterone that prompts a cascade of biological changes that characterize the ending of one cycle and the beginning of the next.
(2) LHSs are significantly constrained by factors other than energy availability, therefore some mechanisms may not depend upon (or be responsive to) energy availability. Trade-offs in the allocation of finite resources are unavoidable throughout the course of an organism’s life. Because life demands energy, considerable research has rightly been devoted to ascertaining when and how energy is apportioned to somatic versus reproductive functions, and to the competing demands within each of these arenas. This emphasis on resource distribution has, however, overshadowed the limitations imposed on LHSs by constraints other than energy availability. For example, there are physical limits to the pace at which biological processes can proceed, and some essential biological, psychological and behavioural functions cannot readily co-occur. Of necessity, successful LHSs must juggle such incompatibilities regardless of the abundance of energy and other resources.
(3) There are often multiple mechanisms involved in the implementation of a given LHS; these mechanisms are connected and communicate (“cross-talk”). Of necessity we speak of “the ovary” or “the reproductive system” as if these were autonomous entities disembodied from the organism. But often an organism must execute compatible responses to a given signal across various bodily components. Such co-ordination is not necessarily reliant on identical responses to a given status of the signal (e.g. a specific hormone concentration), but may be accomplished, for example, through the presence of similar or different types and/or numbers of receptors in the target cells. These differences in signal recognition allow the same absolute concentration of a given hormone and/or the same change in that concentration to elicit distinct yet coordinated responses in these targets. A focus in human research on the absolute concentrations of hormones reflects what we are able to measure, but may not be all that should be measured to understand the mechanisms that generate demographic diversity.
(4) Physiological mechanisms may be conditioned on circumstances experienced during pre-natal and pre-adult development. Boas’ measurements in the early twentieth century of the morphology of US immigrants and their children suggested the influence of early environments on subsequent adult biology, but the mechanisms were a mystery (Boas, 1911). Some fifty years later, physiological studies of humans native to harsh environments (high altitude, the Arctic) strengthened the arguments that adult functioning depended to some degree on conditions during development (Lasker, 1969; Leslie and Little, 2003). Subsequent epidemiological studies in industrialized countries provided further evidence of these links (Barker, 1990), and theoretical and empirical advancements in epigenetics and developmental biology opened a window into the mechanisms by which the environment can alter gene expression (Kuzawa and Thayer, 2011). This current state of knowledge prompts testable hypotheses regarding the nature of the neurohormonal mechanisms that regulate physiology. In particular, a physiological response to a specific signal may be essentially constant for all members of a species or it may differ depending upon prior exposure. For example, the responsiveness of adult reproductive functioning to resource scarcity is highly variable between individuals and populations, perhaps as a consequence of differences in resource availability during development (Vitzthum, 1990, 1997, 2001).
(5) Ovulation is optional. Although necessary for conception, ovulation is not an automatic feature of a menstrual cycle. In several studies of healthy women, anywhere from 10%-40% of the sample did not ovulate in a given cycle (Vitzthum, 2009). Biomedically, absence of ovulation is typically considered a pathology (and in some cases it may be). But, from a life history perspective, each option of ovulating/not ovulating is a fork in the reproductive road at which there is a strategic decision to continue engaging in the possibility of reproduction or to forego the current opportunity. In some contexts, not ovulating in a given cycle can be the best strategy for optimizing lifetime reproductive success.
(6) Pre-natal selection of offspring may swamp post-natal differences in offspring quality and/or parental investment. The most likely outcome of a human conception is natural loss (Roberts and Lowe, 1975; Holman and Wood, 2001; Vitzthum, 2008b). At least half of these losses occur before implantation and another quarter in the subsequent five to seven weeks. It has long been assumed that the vast majority of these early pregnancy losses (EPL) are due to genetic errors in the conceptus. However, there is now evidence that a substantial portion may reflect a maternal LHS to delay reproduction if the current conditions are sufficiently inadequate for producing a live birth (Weinberg et al., 1994; Nepomnaschy et al., 2006; Vitzthum et al., 2009a). Thus, rather than being difficult for humans to conceive (as some have argued), it is now known that human fecundity (the capacity to conceive) is many times higher than human fertility (production of a live birth). This unseen pre-natal selection in the production of offspring may hamper investigations of hypothesized associations of post-natal reproductive success with resources or offspring quality.4 Much of the selection has already occurred (i.e. the differential quality and/or subsequent survival among those concepti that have survived to birth is relatively small). In truth, everyone’s children really are all above average.
Why Is Life History Theory Useful for Understanding the Mechanisms that Generate Demographic Variation?
Malthus (1798) envisioned an unflagging human reproductive system, excepting disease or damage. He was mistaken. For example, the absence of ovulation while intensively breastfeeding a young infant is not a failure of the reproductive system, but rather the adaptive response of a physiological mechanism shaped by natural selection to reduce the risk of premature investment in the next offspring (Short, 1976).
Life history theory offers plausible and, ideally, testable evolutionary explanations for when and why reproductive effort varies. Not reproducing in some contexts may be a life history strategy (LHS) that could yield a higher lifetime reproductive success than would obligate reproduction at every opportunity.
Variation in LHSs within and across populations and generations suggests there are mechanisms for the flexible implementation of LHSs. This inference prompts a cascade of interesting questions about these biological, and perhaps adaptive, mechanisms that create variation in human birth, death and the experience in between. For example:
- What sorts of mechanisms are likely to underlie implementation of LHSs?
- What signals prompt allocating resources to one of several competing demands?
- Are all allocation decisions typically transient or might some be permanent?
- How are competing signals resolved?
- How does maturation stage interact with these signals?
- What are the constraints on LHSs?
Time is arguably the greatest constraint on LHSs. Because all individuals die, time is a scarce commodity. Time cannot be stored, created, foraged, harvested or shared. Social co-operation and/or multi-tasking may or may not mitigate against the scarcity of time, depending on the circumstances (e.g. nine women cannot make a baby in one month). Mortality schedules (the population-specific risk of death for each age or stage of life) express the length of time available for maturation (growth and development) and reproducing, and the pace at which these fundamental biological processes must be accomplished (Stearns, 1992; Charnov, 1993). Natural selection favours those organisms that respond to the scarcity of time with suitably strategic timing of their allocation decisions. For example, if mortality risk is low and the average life is long, one can afford to postpone the transition to reproductive investment until later, making use of the extended pre-reproductive period to grow larger, build knowledge, acquire skills and/or develop social capital. If the risk of death is high, natural selection tends to favour an early transition from maturation to reproduction (Promislaw and Harvey, 1990; Walker et al., 2006).
The myriad allocation decisions that constitute a LHS are not consciously cognitive but rather are executed via biological mechanisms responding to signals of endogenous (internal, somatic) and exogenous (external, extra-somatic) conditions. Strategic timing of allocation decisions requires an organism to process signals that are at least roughly reliable indicators of the current and/or changing status of these conditions.
The simplest conceptualization of a mechanism involves a reliable signal that is recognized by a transponder (receiver/transmitter), which then sends a different signal that elicits an appropriate response. For example, a signal of exogenous conditions (an “ecomarker”) is processed through a sensory system and the brain, prompting a change in biology that elicits an investment of resources and time into one of perhaps several allocation options (e.g. a predator’s growl prompts downstream responses that include rises in epinephrine and cortisol and will ultimately result in flight or freeze or fight).
At different points in a mechanism, signals may be molecular, electrochemical, morphological or psychosocial; behaviours of the individual, conspecifics or other organisms; social, economic or cultural features of the group; and physical or biotic features of the environment. Signals of endogenous conditions (e.g. fat stores, rises in glucose) typically rely on molecular signalling and often involve neuroendocrine input and coordination (e.g. hypothalamic regulation of reproduction, sleep, hunger, thirst and body temperature).
An ecomarker may have a direct role in an organism’s acquisition and/or distribution of resources, or it may act only as a reliable proxy that conveys information about factors external to the organism that have more direct roles in the organism’s life history strategy. For example, in numerous organisms, the duration and intensity of daylight is an ecomarker of extra-somatic conditions that directly influences daily sleep/wake cycles, and that also influences longer-term physiological responses to seasonal variation in environmental conditions (e.g. reproductive functioning in response to predicted changes in food availability). These responses are endpoints in a mechanism that begins with absorption of light photons in the cells of the eye’s retina, which cause molecular changes that eventually signal the pineal gland to produce and release melatonin. Receptors for melatonin are found in many brain regions, the pituitary, gut, ovaries and blood vessels. Neural receptors likely regulate circadian rhythms, and other receptors likely regulate reproductive function, cardiovascular function and body temperature (Brzezinski, 1997). It is not uncommon for molecular signals to be recognized by many different cells in an organism, each of which responds according to its own function.
Some of these conditions and the accompanying allocation decisions set the course for a lifetime (e.g. early maturation cannot be reversed). Other investment decisions are temporally limited and may incur few costs. For example, in healthy humans, pregnancy loss within a few weeks of conception does not appear to impair the probability of ovulation in the subsequent cycle (Donnet et al., 1990) or increase the subsequent mean waiting time to conception (Kaandorp et al., 2014).
Many allocation decisions re-occur throughout a lifetime as organisms navigate seasonal and circadian variations in environmental challenges and orchestrate the daily scheduling of physiological processes. For example, sleep is now recognized to be more than a means of conserving metabolic energy. Rather, it is an activity during which some necessary biological processes are better undertaken, either to avoid competing for resources with processes that must occur while awake, or because of incompatibility with such daytime processes. For instance, memory consolidation is optimized during sleep (Rasch and Born, 2013), night suckling has a greater impact on suppressing ovulation than day sucking (Elias et al., 1986; Vitzthum, 1994), and aspects of immune and reproductive functioning are modulated by melatonin, released in large measure only under cover of darkness (Nelson et al., 2002).
It merits reminding that one cannot assume which allocation response, if any, is adaptive (favoured by natural selection because it increases reproductive success) simply because of the mere fact that the organism displayed that response. Variations in such responses (i.e. better and worse allocation decisions over the course of a lifetime) are fodder for natural selection and hence the means by which LHSs can evolve in population-specific environments.
The Human Female Reproductive System — A Well-Tuned Machine or a Flexibly Responsive Behaviour?
The study of human fertility is biased towards female over male biology because pregnancy duration and other biological constraints limit the number of offspring a woman can produce and thus also, the rate of population growth. Men are not as unavoidably constrained, although, for a variety of reasons, neither is their reproductive capacity unbounded (Drea, 2005; Moya et al., 2016; Vitzthum et al., 2009b; Borgerhoff Mulder, in this volume).
The hypothalamic-pituitary-ovarian (HPO) axis, comprising three endocrine glands and the hormonal communications between these, is the primary pathway orchestrating physiological changes during the ovarian (menstrual) cycle and subsequent to fertilization (if it occurs). The hypothalamus, an almond-sized portion of the brain, links the nervous system to the endocrine system via the pituitary, a two-lobed pea-sized gland lying near the hypothalamus. The release of a neurohormone (gonadotrophin releasing hormone [GnRH]) from the hypothalamus prompts the anterior lobe of the pituitary to release other hormones into the blood that then circulate to and affect the functioning of the ovaries. The anterior pituitary’s hormonal signals stimulate the development of ovarian follicles (the structure surrounding an immature egg cell) and prompt ovulation (the release of the mature egg from a single follicle). Ovulation is followed by transformation of the ruptured follicle into the corpus luteum, which produces the progesterone necessary for sustaining a pregnancy through the subsequent five or so weeks. In the absence of a conception, the corpus luteum regresses about a week or so after ovulation, progesterone begins to fall, and menstrual bleeding occurs (Figure 1).
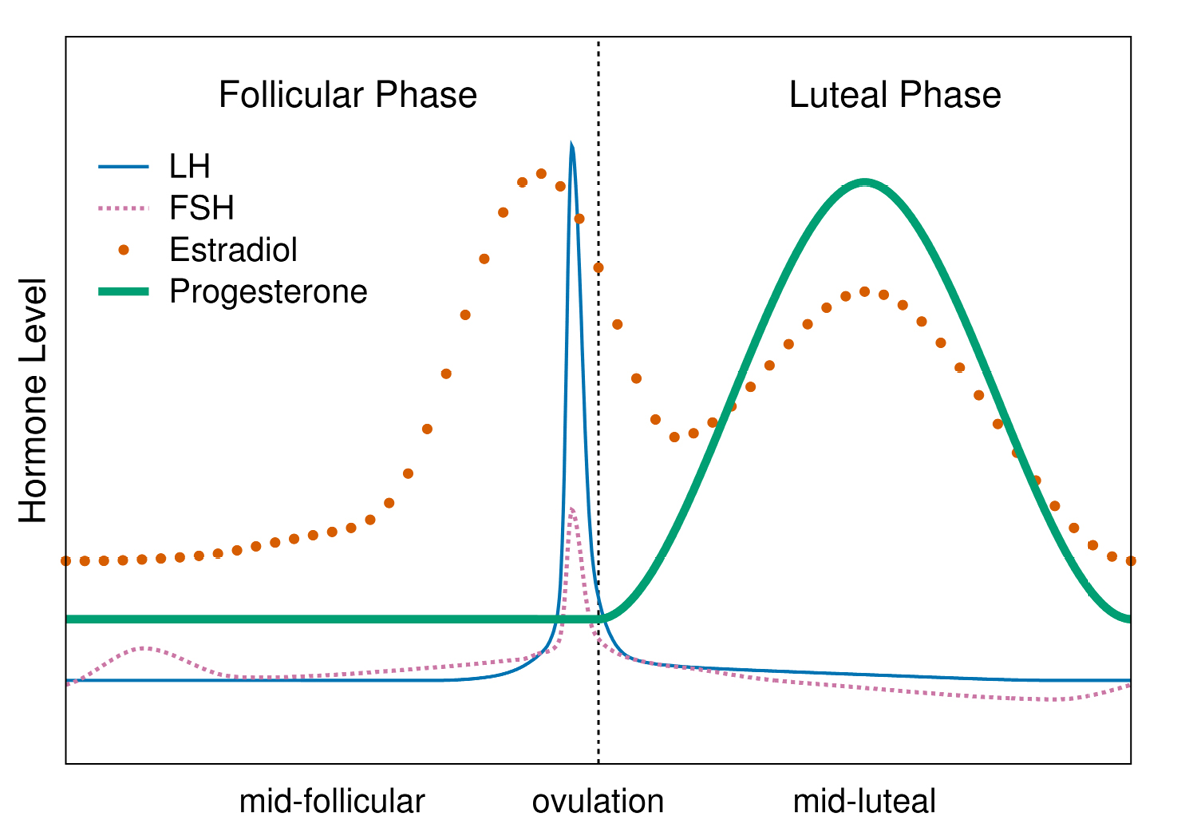
Fig. 1 Idealized depiction of hormonal changes (relative to day of ovulation) during the ovarian cycle. Follicle stimulating hormone (FSH) promotes follicle development. The estradiol peak prompts a surge in luteinizing hormone (LH), which binds to receptors on the follicle, thus initiating ovulation. The follicle transforms into the corpus luteum, which secretes progesterone. If a conception doesn’t occur, progesterone declines, culminating in menses. In an ovulatory cycle, each phase lasts from about eight to twenty-two days (phase durations are not correlated); hormone levels in ovulatory cycles are highly variable between cycles, women and populations (Vitzthum, 2009).
The more-or-less monthly appearance of menstrual bleeding in most healthy pre-menopausal women (other than those who are pregnant or breastfeeding) tends to bolster the widespread belief that the female reproductive system is unflagging in its cyclical effort to conceive. This idealized view of ovarian regularity derives in part from Descartes’ (1637) conceptualization of the body as a machine and is reflected in the work of Malthus (1798), who argued that moral restraint and early death were all that kept a population from outstripping its food supply in a few generations.
The powerful body-as-machine metaphor continues to subtly influence contemporary sciences. This impact is perhaps most evident in biomedicine. The image of a well-tuned machine, necessarily invariant in the form and coordination of its components, readily lends itself to a narrow definition of “normal” biology, and tends to perpetuate the classification of variants not meeting the criteria for “normal” as pathologies that require medical interventions. Such criteria for hormones and other biomarkers typically involve the designation of upper and/or lower thresholds outside of which the biomarker is considered abnormal. Given that medicine’s mission is to identify illness and restore the patient to health, arguably such diagnostic practices are acceptable, even desirable, regardless of the underlying misconceptions. Better to recognize all who may be sick, and a few who are aren’t, than to miss those needing treatment.
But what if the diagnostic threshold derived from a faulty assumption about normal variation has identified a treatment pool comprising more healthy people than ill: then what? If this seems far-fetched, consider the “impairment” referred to as luteal phase deficiency (LPD). First described by Jones in 1949, LPD is characterized as insufficient endogenous progesterone for the adequate development of the uterine lining, successful implantation and early pregnancy maintenance. Diagnostic criteria have included a short luteal phase (under the false belief that a normal luteal [post-ovulatory] phase is twelve to fourteen days) and low progesterone concentrations. But, in fact, the luteal phase varies considerably in cycles in healthy women (WHO, 1983), and the Practice Committee of the American Society for Reproductive Medicine (2015) has concluded, “no minimum serum progesterone concentration defines ‘fertile’ luteal function”. Examination of the uterine lining (endometrial biopsy) was thought to be the diagnostic “gold standard” for luteal phase deficiency, however, rigorous clinical trials have concluded otherwise. For example, one large double-blinded study found that about half of the mid-luteal endometrial biopsies were considered “abnormal” according to LPD diagnostic criteria in both fertile and infertile women (Coutifaris et al., 2004). In other words, natural variation had been mistakenly perceived as pathology.
Although the body is obviously not a machine, sometimes this imagery can divert us from recognizing the inherently variable nature and flexible capacities of physiological mechanisms. In his essay laying out the four questions, Tinbergen (1963) praised Konrad Lorenz for having “made us look at behaviour through the eyes of biologists”. In so far as metaphors can aid understanding, it may prove of use to flip the view and look at physiology as an ethologist might. Rather than analysing features of physiological systems (e.g hormone concentrations) as if they are species-specific morphological traits that are only modestly variable across populations, it may be more useful to think of physiological mechanisms as responsive behaviours whose range of expression reflects developmental conditions and is contingent on immediate circumstances.
This perspective is consistent with current knowledge of hormone-receptor signalling behaviours. A hormone exerts its effect by binding to a receptor and having a molecular configuration suitable for that hormone (rather like a key in a lock). The hormone-receptor complex can then signal to the cell to perform some biological response. Receptors are a large class of proteins encoded in the cell’s genes; receptors specific to a cell’s function are manufactured by that cell. Regulation of receptor manufacture is affected by several endogenous and exogenous developmental and environmental factors, depending on the specific cell and intended action.
The relative number of receptors to hormone molecules is critical in regulating the cell’s actions. Without receptors, hormones (no matter how high the concentration) cannot directly affect cell behaviour. The relationship between hormone concentration, receptor availability and biological response varies by receptor. There can be many different types of receptors for a given hormone, and a given receptor may be able to bind with different hormones.
High affinity receptors (those that form stronger molecular connections with a given hormone) can attract and bind hormones at low concentrations and trigger cell action. In other cases, hormones need to be present at high concentrations in order for enough receptors to be bound and thereby elicit a biological response. The rhythm of change may matter in some pathways (i.e. increases in the amplitude and/or frequency of pulsatile hormonal signalling, rather than a monotonic rise in hormone concentration, are necessary to prompt a response in a target cell). In some mechanisms, the presence of the hormone will prompt the cell to produce more receptors, and then, once enough hormone-receptor complexes are formed, the cell will perform its action (“upregulation” is the cell’s creation of more receptors that make the cell more sensitive to the hormone). But at other times even high hormone concentrations will not trigger the production of more receptors (e.g. insulin resistance) and the biological response is not performed. In general, when available receptors become saturated (because of hormone binding and/or receptor degradation), the cell becomes less sensitive to the presence of the hormone (a process called “downregulation”).
Physiological mechanisms are dynamic and variable, a consequence of evolution’s tinkering, using the materials and tools bestowed by previous generations to deal with the task at hand (Jacob, 1977, 1994). While some pathways may be conserved (e.g. the link from hypothalamus to pituitary to ovary), features potentially shaped by developmental environments will likely vary (e.g. individuals’ hormone concentrations and numbers of receptors). Such systems are rather like an orchestra — a composition (pathway) is typically followed, each instrument coming into play in a fairly predictable fashion, but the rhythm, volume and numbers of each kind of instrument (hormone signal) may vary as can the number of listeners (receptors) who come and go. Sometimes compositions from adjacent orchestras can be heard (cross-talk) and sometimes there’s unexpected improvisation provoked by a novel situation (new foods, environmental toxins, glucose overload).
A Delicate Balance: Strategic Trade-Offs of Incompatible Essential Functions
The primary mission of a woman’s immune system — to protect her body — is sometimes unavoidably at odds with the evolutionary imperative to reproduce (Abrams and Miller, 2011; Alvergne and Tabor, 2018). For example, because of sperm’s genetic foreignness and the health risks posed by any pathogens in deposited semen, coitus might be expected to elicit a heightened immune defence in women. Yet such a response would potentially harm the sperm required for a conception. Notably, however, sperm’s reproductive value is limited to a few days (known as “the fertile window”) leading up to and including the day of ovulation.
The key to balancing these incompatible functions is through the strategic timing of selected immune defences. Specifically, in sexually active healthy women, we would expect relatively high immune defences to protect against the risks associated with coitus, but also a transient dampening of some immune defences around the time of ovulation (the fertile window) in order to increase the chances for successful conception. In sexually abstinent healthy women, immune defences need not be as high as in sexually active women, and are not expected to change during the fertile window.
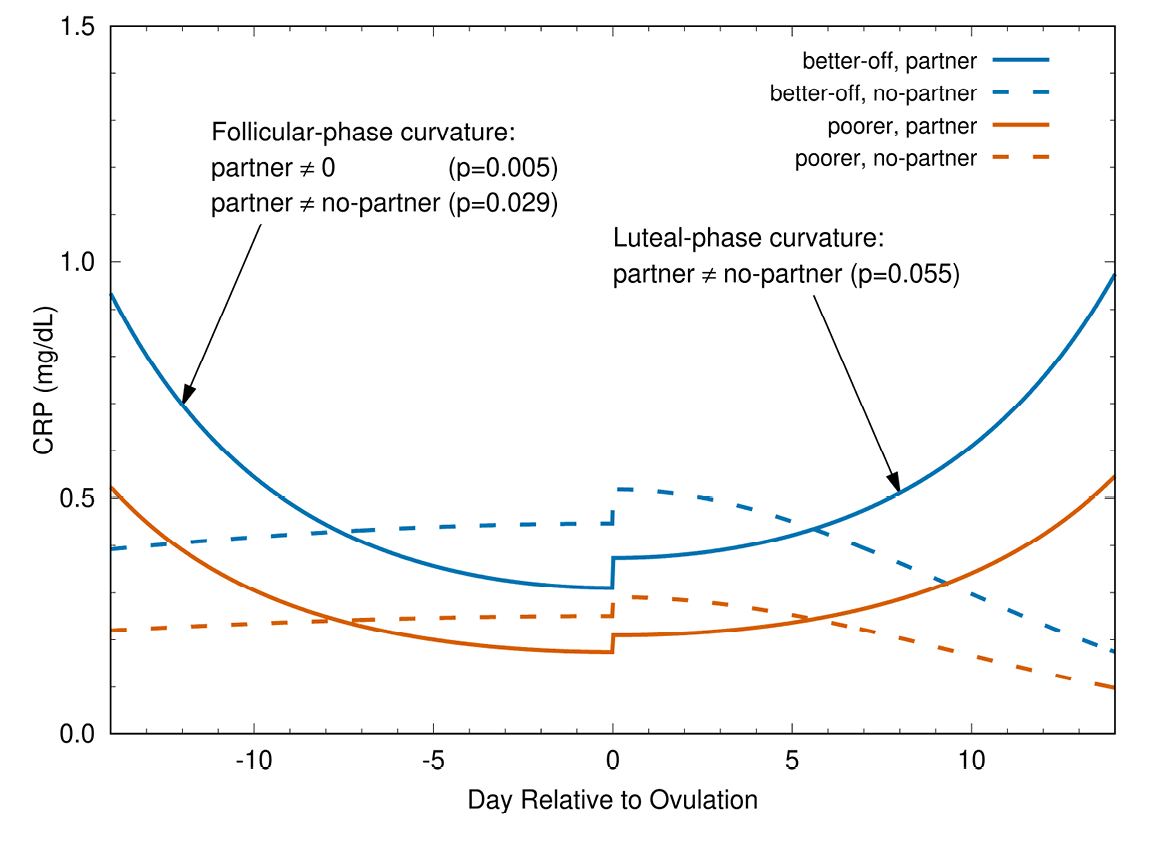
Fig. 2 Change in CRP during ovulatory menstrual cycle. Fitted models for the interaction of partnership status and socioeconomic status. CRP is significantly higher during the early follicular and late luteal phases (P = 0.029 and 0.055, respectively) in partnered (solid curves) than in unpartnered (dashed curves) women. In partnered women, CRP is lower around ovulation than at the cycle’s beginning (fitted model curvature is significant at P = 0.005). In contrast to partnered women, CRP in the ovulatory cycles of unpartnered women is more stable over time (fitted model curvature is not significantly different from 0). The small increases in CRP at ovulation are not statistically significant in these models (Lorenz et al., 2015).
These predictions have been tested and supported in studies of Bolivian women and US women. In Figure 2, the predicted patterns are observed in two Bolivian samples, one of poor women and the other of economically better-off women (Lorenz et al., 2015). The selected immune biomarker (C-reactive protein, CRP) patterns during the menstrual cycle are the same for both samples. Likewise, the pattern is similar in a sample of US women, who are wealthier than both Bolivian samples (Lorenz et al., 2017). The comparability of this pattern across samples with different energetic resources suggests that greater energy availability does not modify the need to dampen some immune defences at ovulation in sexually active women. In other words, even though this dampening may come at significant health costs to women (e.g. autoimmune diseases, sexually transmitted infections) (Beer et al., 1996; Whitacre et al., 1999; Wira and Fahey, 2008; Wira et al., 2010; Kaushic et al., 2011; Klein, 2012), it does not appear to be mitigated by greater access to energy resources.
Which Comes First — the Coitus or the Egg?
The differences in immune-reproduction co-ordination between sexually active and abstinent women are likely a consequence of seminal fluid components (e.g. cytokines) that provoke changes in the female reproductive system (Robertson and Sharkey, 2016). The presence of such components is a signal of the possibility of conception and the concomitant necessity of modifying immune responsiveness.
However, if ovulation does not occur, such shifts in immune function in response to seminal fluid are not needed and would be potentially risky for the woman’s health. Therefore, one would expect sexually active women to have a higher probability of ovulating than sexually abstinent women (i.e. ovulation is worth the risk from a shift in immunity for sexually active women, but is an unnecessary risk in the absence of sex). Consistent with this prediction, Metcalf (1983; Metcalf and MacKensie, 1980) observed lower ovulation rates in unpartnered than partnered New Zealand women, and Wilcox et al. (2004) found concurrent increasing probabilities of ovulation and coitus in a sample of US women. However, neither study could specify the direction of the causal arrow (i.e. does coitus induce ovulation or vice versa?).
Now, a recent study that included daily documentation of coitus and serial hormonal biomarkers to detect ovulation has yielded strong evidence that coitus increases the probability of ovulation in humans (Prasad et al., 2014). It is plausible (but was not tested in this study) that seminal fluid components are an essential part of the mechanism linking coitus to the physiological decision to ovulate.
This finding flips the causal arrow on the hypothesis that hormonal changes accompanying ovulation in women prompt increases in sexual attraction, desire and/or activity. Most studies of this prediction have failed to demonstrate such an association. The large majority of these studies have assumed, without biomarker confirmation, the timing and occurrence of ovulation during the study cycle. Such assumptions are untenable in light of the evidence that ovulation is not inevitable, that its timing is not restricted to a narrow mid-cycle window (reviewed in Vitzthum, 2009), and that coitus itself promotes ovulation.
These observations and arguments, however, raise other intriguing questions. If a woman is not sexually active, why bother to ovulate at all? The fact that there is still an appreciable probability of ovulation in sexually abstinent women suggests that the cost of ovulation is low. One possibility is that once the wheels are set in motion, this low-cost process chugs along unless hindered (perhaps by signals that any risk of conceiving is a poor strategy at this time). Another possibility is that, outside of breastfeeding, there has been little selection against ovulation, but neither has there been strong selection for ovulation in the absence of coitus. Since sexual abstinence was likely uncommon during human’s evolutionary history, opportunities for selecting against or for ovulation in the absence of coitus were relatively few. Even in those few instances, any resource savings in not ovulating may have been more than offset by the value of ovulating on the chance that coitus occurs.
This conjecture then raises the question, “If the cost of ovulation appears to be low, why not be an obligate ovulator regardless of coitus?” Perhaps tying ovulation to coitus is a selectively advantageous mechanism that helps to compensate for the short life of sperm, more closely linking the availability of an egg to the deposition of seminal fluid than would otherwise occur. The timing of coitus may help to explain why the duration of the pre-ovulatory (follicular) phase of the cycle is more variable than the post-ovulatory (luteal) phase. The answers to these questions await further study of the physiological mechanisms regulating ovulation, especially of the links to coitus.
Vote Early, Vote Often: Early Pregnancy Loss
The collective evidence from several studies suggests that only one in five human conceptions are born. Thus, worldwide during an average day in 2019, there were 360,000 live births, 1.8 million new conceptions, and 1.44 million naturally lost human pregnancies (the vast majority of which were unrecognized by the woman or her clinician) (Vitzthum, 2008b).
This unexpected wastage was first brought to light in 1975 by two epidemiologists who estimated pregnancy loss in England and Wales to be 78% based on the marriage rate and reasonable assumptions regarding coital frequency and other relevant factors (Roberts and Lowe, 1975). Subsequent studies, though few, have reached comparable conclusions. Boklage (1990) combined the published results of several observational studies of pregnancy loss in industrialized populations and developed a parametric model from which he estimated a total pregnancy loss of about 76%. Holman and his colleagues (Holman and Wood, 2001) mounted an impressive study that monitored nearly 500 non-contracepting married Bangladeshi women and collected urine samples, later assayed for a biomarker of implantation, from 1,561 menstrual cycles. With these data, they detected post-implantation pregnancies and losses, and estimated the total pregnancy loss from conception to birth to be about 80%.
Modelled estimates suggest that pregnancy loss is greatest between conception and implantation (about 50–60% of all concepti). But there is little direct evidence because during this early stage women are unaware that they are pregnant, and there is not yet an easy-to-collect reliable biomarker for detecting conceptions prior to implantation. Implantation, which occurs 9 ± 3 days after conception, is recognized by a rise in human chorionic gonadotropin (hCG). Over-the-counter early pregnancy tests are designed to react to the presence of this hormone in urine samples, and several studies have made use of this biomarker to estimate pregnancy loss rates. Estimates of loss occurring from implantation through the subsequent month were about 25% to 30% of implanted concepti in several studies in industrialized populations (Wilcox et al., 1999; Ellish et al., 1996; Zinaman et al., 1996; Wang et al., 2003; van Montfrans et al., 2004). In Bolivian women, 31% of implanted concepti were lost within five weeks of conception (Vitzthum et al., 2006). Based on hazard models (which produce higher and more accurate estimates), Holman and Wood (2001) estimated loss within five weeks of conception to be about 65% in 28-year-old Bangladeshi women. Among settled Turkana agriculturalists in Africa, about 70% of implanted concepti were lost by ten weeks after conception (Leslie et al., 1993). Several studies have shown that by the end of the second month of pregnancy, the risk of subsequent loss has dropped to only 10–15% (Vitzthum, 2008b). In many cultures, women decline to mention to others that they are pregnant until this stage has been reached and the risk of not going to term (i.e. not giving birth) is low.
Roughly speaking, based on the collective evidence, of one hundred conceptions, fifty-five would not successfully implant, twenty-two would be lost during the month after implantation, and three more would be lost in the subsequent months, yielding twenty live births.
Evaluating Offspring Quality
Such apparently wasted effort naturally prompts questions about the causes underlying these losses. The canonical response is that concepti are lost early in pregnancy either because their genetic defects preclude normal development or because maternal mechanisms cull poor-quality offspring unlikely to mature and contribute genes to subsequent generations. Such weeding allows a woman to redirect investments to current or future offspring (Temme, 1986; Kozlowski and Stearns, 1989; Haig, 1990, 1993, 1999). Even given abundant resources, a low-quality conceptus should be rejected quickly to avoid wasting maternal time that could be given to attempting another conception.
Perhaps the most important maternal mechanism for evaluating offspring quality depends on the embryo’s own ability to produce sufficient hCG as it begins implantation. Production of this hormone is proof of the embryo’s ability to carry out protein synthesis, the most minimal requirement of viability (Haig, 1993). In a process referred to as corpus luteum rescue, the embryo signals its presence through hCG binding to receptors on the corpus luteum, which responds by continuing progesterone production to sustain the pregnancy (recall that falling progesterone concentration results in menstruation) (Jabbour et al., 2006).
Timing as well as the volume of conceptus-produced hCG is critical in this mechanism. The rise in hCG that accompanies implantation must occur between six to twelve days after ovulation if the conception is to be sustained. The later that implantation begins, and the later that the rescued corpus luteum subsequently produces more progesterone, the more likely it is that the conceptus will be lost during the subsequent month. Failure to produce sufficient progesterone quickly enough is a consequence of the embryo’s inability to produce enough hCG, rather than any defect in the corpus luteum (Baird et al., 1991; Vitzthum et al., 2006).
In effect, through the mechanism of corpus luteum rescue, the embryo will trigger its own rescue if it can produce, at the right time, enough hCG to assure continually rising progesterone production by the corpus luteum. An unknown proportion of concepti fail this first test and menstruation renews the cycle. The maternal opportunity cost for having conceived and lost this early is very low. Early pregnancy loss does not appreciably lengthen the time to the next cycle (Vitzthum et al., 2000b), lower the probability of ovulation in the subsequent cycle (Donnet et al., 1990), or increase the subsequent mean waiting time to conception (Kaandorp et al., 2014). Furthermore, menstrual flow is not appreciably greater, which suggests that energy expenditure may not be much higher (Vitzthum et al., 2001).
For embryos that do make it past this first gateway, at least 30% and as many as 50% will be terminated before the end of the subsequent month, by which time another gatekeeping mechanism has come into play. The luteo-placental-progesterone-transition (LPPT), occurring by about five weeks since ovulation/conception and seven weeks since the first day of the last menstrual period, is a developmental period during which the production of progesterone from the placenta (an offspring structure) begins to be greater than that from the corpus luteum (a maternal structure). Because progesterone is essential for the maintenance of the pregnancy, if this shift does not occur, the pregnancy will not continue (i.e. insufficient production of placental progesterone is indicative of a poor-quality offspring).
In conceptions that do continue, the LPPT has shifted the locus of physiological control of the pregnancy from the mother to the offspring. It is in an offspring’s own interests to sustain the pregnancy and, in large measure, during the LPPT the embryo is becoming the master of its own fate. Once it has the ability to produce enough progesterone without maternal contribution, any maternal interests contrary to those of the offspring may not prevail. Consistent with this prediction, only 10–15% of those pregnancies that survive through the LPPT are subsequently lost before birth.
The LPPT is a well-documented physiological change that occurs during early pregnancy. The evolutionary explanations for the LPPT are predicated on the fact that a mother and her offspring are not genetically identical and hence the optimal degree of parental investment to give and receive are likely not to be identical (Trivers, 1974; Haig, 1993). At first consideration, the idea of parent-offspring conflict would appear to be at odds with an expectation that it is in the evolutionary interests of a mother to invest in her offspring. Life history theory, however, recognizes that there are trade-offs — what is invested in one offspring cannot be invested in another. For example, in some environments (e.g. those with high infant mortality) it may be selectively advantageous for a woman to have two smaller children rather than one larger child. Therefore, natural selection is expected to favour the maternal life history strategy that produces the number and quality of offspring that will result in the greatest lifetime reproductive success for her under the environmental conditions in which she lives, even if her LHS is not the optimal investment from the perspective of each offspring (see Strassman and Gillespie, 2002 for a notable example).
Evaluating Maternal Quality
Life history theory also predicts that maternal somatic conditions and/or external environmental circumstances that are inadequate for sustaining a pregnancy through to term may prompt rejection of a conceptus, even if that offspring is not defective (Wasser and Barash, 1983; Peacock, 1990; Vitzthum, 1990). The LPPT imposes a timing constraint on maternal decisions to terminate investment in the current conception. If it is in the mother’s evolutionary interests to do so, then termination is best effected while her own physiological mechanisms still regulate the bulk of progesterone production (i.e. before the LPPT). The timing of the LPPT reflects opposing interests. Selection on the offspring favours an early LPPT and the accompanying physiological control of pregnancy continuance. Selection on the woman favours a later LPPT so as to keep her investment options open.
Although it may initially appear paradoxical that a parent would terminate investment in a non-defective offspring, this option may be evolutionarily advantageous given the costs and risks associated with continuing a pregnancy, giving birth and breastfeeding an infant. A mother’s investment in the production of a live offspring is sufficiently high that it can, and sometimes does, cost her life (Vitzthum and Spielvogel, 2003). Before the advent of antibacterial sulfonamides in the mid-1930s, an estimated 300–900 women per 100,000 pregnancies died from pregnancy-related causes (Loudon, 2000). Currently, about 300,000 women die each year (UNFPA, 2019). Mortality is only the tip of the iceberg, with maternal morbidity affecting millions of women each year. With such high costs, natural selection on offspring is expected to be especially high early in gestation (when maternal investments, opportunity costs and risks are low) and relatively lower after birth (by which point considerable resource and opportunity costs, and much of the cumulative risk to the mother, have already been incurred).
Under the assumption that early pregnancy loss (EPL) is due almost entirely to genetic defects in the conceptus, there have not been many empirical studies of the hypothesis that maternal somatic or environmental conditions are significant contributors to EPL. Three studies have addressed these questions. The North Carolina Early Pregnancy Study (NCEPS) recruited 221 women who self-collected daily urine samples (subsequently assayed for progesterone and estrogen metabolites and hCG) while attempting to become pregnant naturally (Wilcox et al., 1999). Project REPA (Reproduction and Ecology in Provincía Aroma) collected thrice-weekly saliva (assayed for progesterone) and urine samples (tested for hCG) from 191 menstruating rural Bolivian women in a stable sexual partnership (Vitzthum et al., 2004). Twenty-four Guatemalan women self-collected thrice-weekly urine samples later assayed for several hormones (Nepomnaschy et al., 2006). Data from these studies on variability in the risk of EPL, and the environmental and hormonal mechanisms associated with these patterns, suggest life history strategies reflective of maternal factors are also at play in EPL.
In general, genetic defects are expected to occur randomly over time. Thus, if EPL were due almost entirely to genetic defects, EPL would also be expected to be randomly distributed over the course of a year. It was therefore surprising to find seasonal peaks and valleys in the distribution of EPL detected during the NCEPS (Weinberg et al., 1994). Although the pattern was clear — a peak at least four times greater than the trough occurring some time from September through December in three consecutive years — the authors were unable to explain it.
Project REPA also observed seasonal differences in EPL (Figure 3). The arduous planting and harvesting seasons had a 3.7 times greater risk of loss than the other seasons, and agropastoralists were nine times more likely to experience EPL than those engaged in some other livelihood. The authors attributed the seasonal increase in EPL to the demanding physical labour of farming, but also noted that inadequate food reserves and greater psychological or immunological stress could also be contributing.
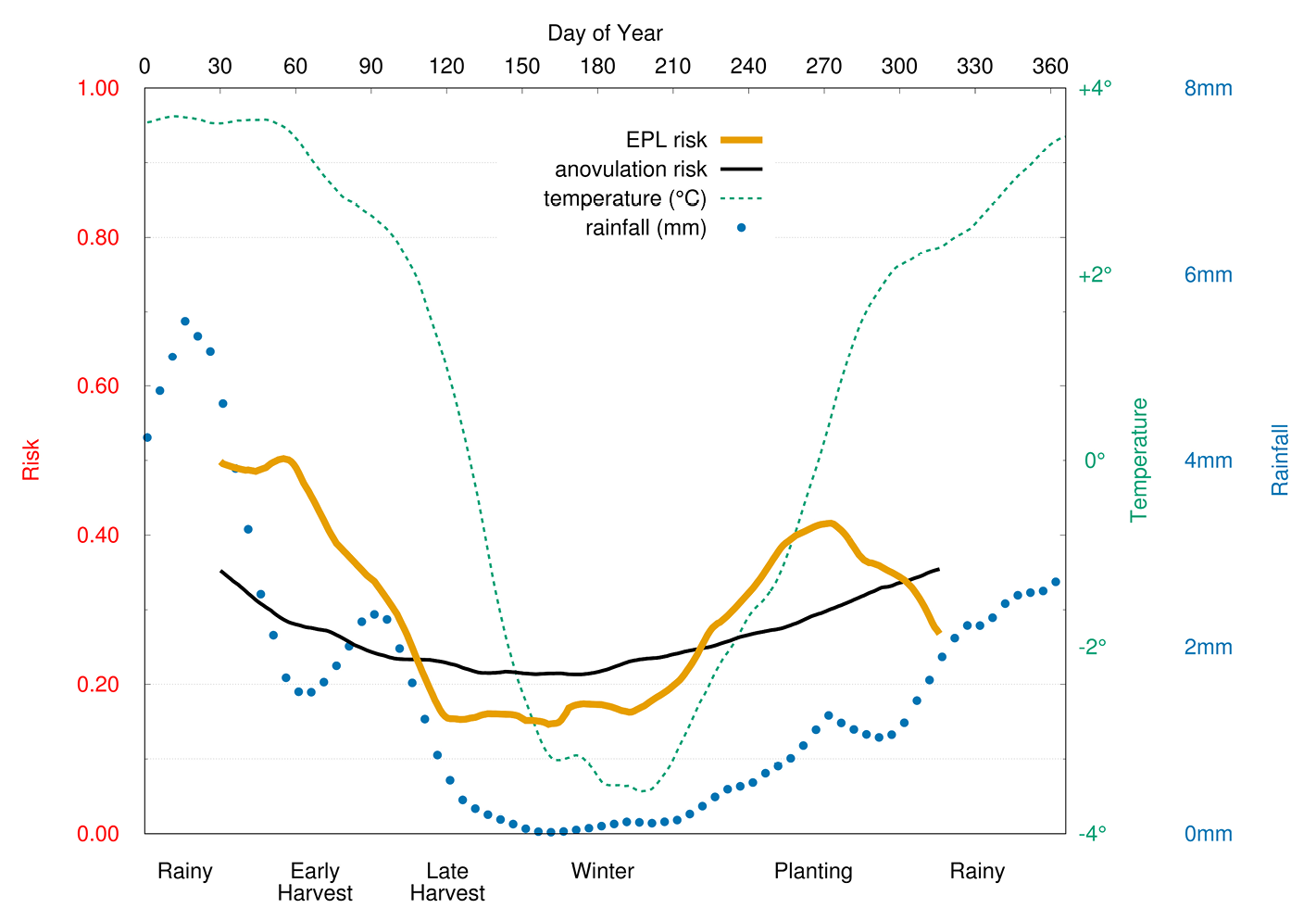
Fig. 3 Seasonal variation in anovulation and early pregnancy loss (EPL). Risk (left-hand scale) of anovulation and EPL, and daily rainfall (far right-hand scale), and minimum-temperature (near right-hand scale) as functions of time (top scale, day of year). Agricultural activities (bottom scale) are positioned relative to day of year. Risk of EPL and anovulation are elevated during the most energetically demanding periods (Vitzthum et al., 2009a).
Two proposed physiological mechanisms that might link poor maternal conditions to EPL have been tested. Reflecting the important role of ovarian steroids in preparing the uterine lining for implantation and sustaining a pregnancy, one hypothesis predicted that ovarian steroid concentrations would be lower in conception cycles that end in EPL than in conception cycles that are not lost. Data from NCEPS and Project REPA failed to support this prediction. In both studies, the ovarian steroid profiles of the successful and lost conceptions did not differ from ovulation through early pregnancy (Baird et al., 1991; Vitzthum et al., 2006) (Figure 4).
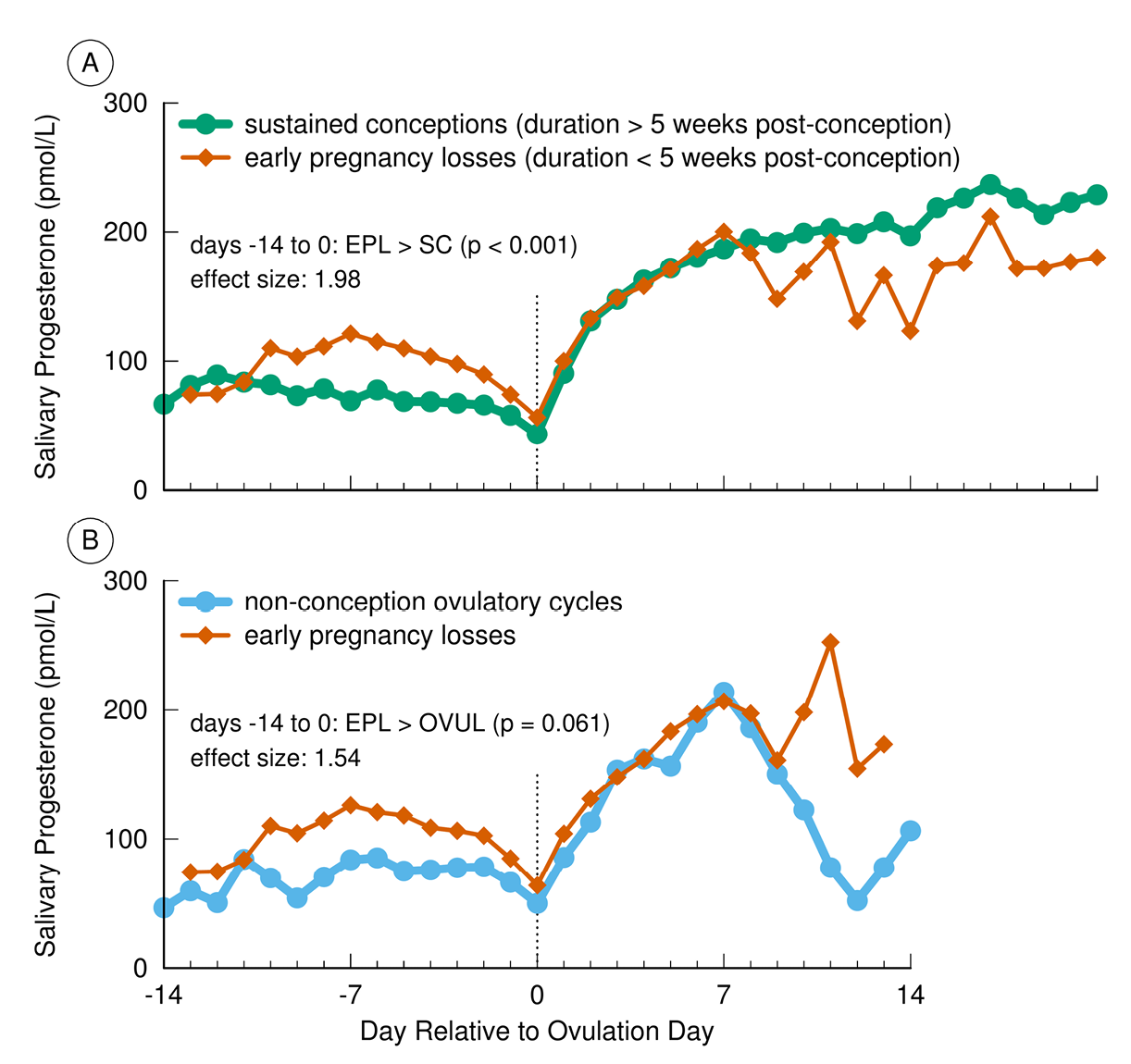
Fig. 4 Progesterone (P4) concentrations in sustained conceptions, early pregnancy losses (EPL), and non-conception cycles in Bolivian women. Post-ovulation P4 did not significantly differ between EPL and conceptions persisting for at least 5 weeks after conception (Panel A). In contrast, preovulatory P4 was significantly higher in EPL compared with sustained conceptions (Panel A) and compared to non-conception ovulatory cycles (Panel B) (Vitzthum et al., 2006).
A second plausible mechanism for maternal evaluation of conditions involves cross-talk between the HPO-axis and the hypothalamic-pituitary-adrenal (HPA) axis. During the pre-ovulatory phase of the ovarian cycle, the adrenal cortex is the main source of progesterone, typically produced at levels much lower than those of the progesterone produced by the ovaries following ovulation. However, under stressful conditions (e.g. increased physical activity, food restriction, psychosocial stress), the adrenal glands increase production of cortisol and adrenal progesterone. Elevations of these hormones early in the ovarian cycle may disrupt normal ovarian functioning including development of the follicle, ovulation, implantation and/or sustaining an implanted conceptus (Vitzthum et al., 2006).
Evidence from the Guatemalan study and from Project REPA suggests this mechanism underlies at least some EPL. In the conception cycles of the Guatemalan women, those with high cortisol concentration were 2.7 times more likely to end in EPL than those with normal cortisol concentrations. In other words, 90% of those conceptions with elevated cortisol were lost as compared to only 30% of those with normal cortisol concentrations (Nepomnaschy et al., 2006). In the conception cycles from the Bolivian women (Figure 4, Panel A), adrenal progesterone during the follicular phase was significantly higher in those pregnancies that terminated prior to the LPPT compared to those that persisted beyond this transition.
The findings from these three studies are consistent with the life history prediction that, in addition to the quality of the offspring, maternal somatic status and environmental conditions are potentially important determinants of whether or not to continue investment in a new conceptus. Although energy stores (adipose tissue) and seasonal energy availability can be major factors in maternal reproductive decisions, psychosocial, micronutrient and immunological/disease conditions may also trigger termination of reproductive investment, regardless of maternal energy adequacy.
A Pair of Paradoxes and the Physiological Fallacy
Honourable errors do not count as failures in science, but as seeds of progress …
— Gould (1998)
Beginning in the late 1970s, an intellectual dispute arose between demographers and biologists (bioanthropologists, physiologists, medical scientists, evolutionary biologists) regarding the role of energetics (caloric intake and expenditure) in human reproduction.
Biologists, on the one hand, had both good theoretical arguments and considerable data in favour of the position that energetics is a major determinant of fertility. In particular, studies of US and European women who were following calorie-restricted diets and/or regular strenuous exercise regimes (either in or outside a laboratory setting) were observed to experience disruptions in their menstrual cycles, including reductions in reproductive hormone concentrations. Furthermore, with increasing energetic severity, the disruptions could become so pronounced that ovulation and menses ceased altogether (dubbed “exercise-associated amenorrhea”). Although some observers considered these changes to be pathologies, Jerilyn Prior (1985a, 1985b, 1987) and a few others argued that these were adaptations to spare women from conception when energetically stressed, a condition that could increase maternal and offspring risks for morbidity and mortality.
Demographers, on the other hand, had a world’s worth of compelling population-level demographic data that supported the position that energetic stress (other than starvation) has only a trivial impact on human fertility. Bongaarts (1980) laid out the data and arguments in a widely influential paper in Science. Perhaps his most convincing point was that many of the very populations experiencing the most significant energetic stress were also those with the highest fertility.
Faced with a seemingly unresolvable paradox — physiological data demonstrating energetic impacts on individuals in industrialized countries yet no apparent impact on population-level fertility parameters in energetically stressed populations — the two sides, for the most part, retreated to their respective domains.
A technological development and more data, however, revived the discussion in the late 1980s. The first studies of possible differences between populations in the concentrations of reproductive steroid hormones (progesterone, estrogens) were largely motivated by an interest in finding the causes of marked population differences in the risks for breast and other cancers. In general, these studies found lower concentrations of these hormones in Asian compared to US and UK “white” populations (Dickinson et al., 1974; MacMahon et al., 1974; Trichopoulos et al., 1984; Bernstein et al., 1990; Key et al., 1990; Shimizu et al., 1990 Wang et al., 1991). There was little, if any, suggestion in the published literature from these epidemiological studies that the observed hormone differences might also cause population differences in fertility.
The first study by bioanthropologists of reproductive hormones in African populations also observed lower concentrations compared to those observed in European and US samples (van der Walt et al., 1978). However, these investigators explicitly argued that the lower concentrations were indicative of lower fecundity and were perhaps evolutionary adaptations to energetic stress. The development of salivary hormone assays (an alternative to blood-based assays) allowed other anthropologists to collect data from several energetically stressed rural populations (Democratic Republic of the Congo, Nepal, Bolivia and Poland), all of which proved to have average salivary progesterone concentrations significantly lower than the average observed in a sample of US women (Ellison et al., 1989; Panter-Brick et al., 1993; Jasienska and Ellison, 1998; Vitzthum et al., 2000a).
These additional observations generated a second paradox. Although these energetically stressed populations had relatively lower progesterone concentrations, they did not necessarily have low fertility. For example, women in the rural Bolivian population, with an average progesterone concentration only 70% that of US women, had an average of seven live births each, with some women reporting as many as thirteen offspring (Vitzthum et al., 2004).
The resolution of each of these two paradoxes was not simply a matter of figuring out who was right and who was wrong (the various studies had, in fact, been well executed by competent scientists). Rather, we needed to re-think our assumptions and interpretations of the available data with fresh eyes. This re-assessment involved taking the empirical data at face value — specifically, (1) women in industrialized populations had relatively high progesterone concentrations and experienced ovarian cycle disruption, including lower progesterone, when energetically stressed, and (2) women living in energetically stressful conditions had relatively low progesterone concentrations, but nonetheless were having lots of babies — and examining these biological patterns within an evolutionary framework. The following assessments arose from this approach (Vitzthum, 1990, 1997, 2001, 2009, 2020).
First, there is no scientific justification for assuming that hormonal data from US/European women are a normal or desirable standard against which to compare all other populations. The fact that interpopulation hormonal variation does not correspond to interpopulation differences in fertility strongly suggests that there is no species-specific “normal” progesterone concentration necessary for reproducing. Comparable to the misdirection taken in medicine by assuming that statistically defined thresholds are genuine markers of normalcy, so efforts in reproductive ecology have been led astray by assuming that higher concentrations of reproductive hormones necessarily equate with higher fecundity and fertility.
Second, acute and chronic energetic stressors are not necessarily biologically equivalent. The timing (whether pre-natal, pre-adult, or during adulthood), duration and magnitude of a stressor can all impact how an organism responds to the challenge. The disruption in ovarian function that accompanies an acute energetic demand is a temporary cessation of reproductive investment in favour of temporarily increased somatic demands. If the organism never resumes reproductive investment before dying, it is likely to be at a selective disadvantage compared to individuals who do reproduce. Therefore, if an acute temporary demand persists, the organism may become less sensitive to this demand so as to resume the normal array of bodily functions (a physiological state called “homeostasis”). Unlike acute demands, chronic energetic demands must be managed differently because these stressors are the very nature of the environment in which the organism lives and must reproduce. The organism must have a life history strategy that results in successful reproduction in these tougher conditions.
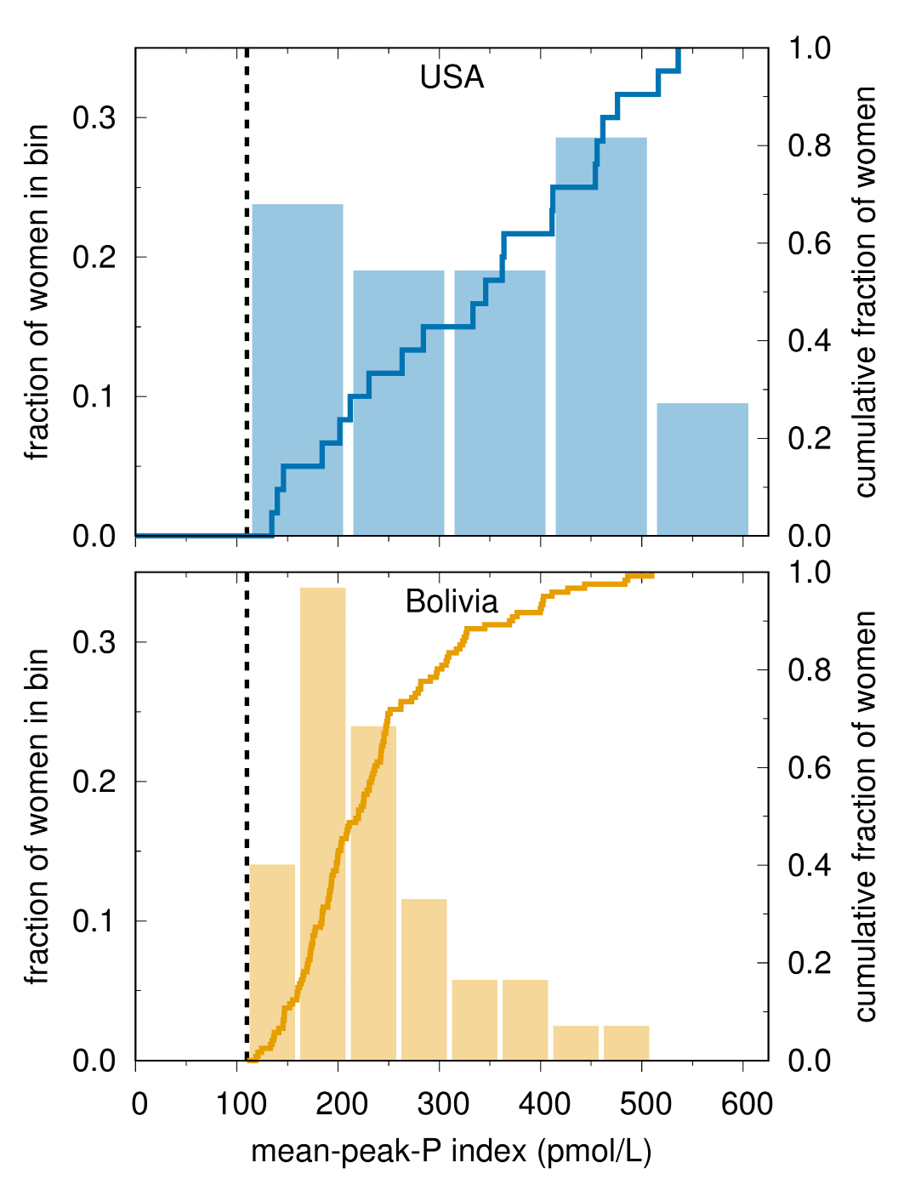
Fig. 5 Progesterone variation in ovulatory cycles. Histogram (left scale) and cumulative distribution (right scale) of a mid-luteal index of hormone concentration (mean-peak-progesterone). Progesterone concentrations differ substantially between the two populations and between women within each population (Vitzthum et al., 2004).
Third, between-population differences in reproductive hormone concentrations are not necessarily equivalent to the within-person reductions in hormone concentrations associated with an acute energetic stressor. This mistaken equivalency — a “physiological fallacy” somewhat analogous to the “ecological fallacy” (Robinson, 1950; Selvin, 1958) — ignores both the processes that generate a given hormone concentration and the units of analysis, and hence misidentifies the absolute level of the hormone as the necessarily salient signal in biological mechanisms. Rather, at least as regards the role of ovarian steroids in mechanisms that implement LHSs, the current evidence suggests that it is the temporal change in the hormone’s level that transmits information about changes in the factors that influence reproductive investments. If change is the (more) salient signal, then it is likely that there is not strong selection for specific hormone levels. Rather, there is the potential for high, yet nonetheless normal, variability in absolute hormone concentrations within and between populations (Figures 5, 6).
The prediction that marked hormonal variability is normal is supported by empirical studies demonstrating that different reproductive hormone concentrations are functionally equivalent across individuals and populations. Although progesterone concentration is significantly lower in Bolivian than in Chicago women, Bolivians successfully conceive at these lower concentrations (Figure 6) (Vitzthum et al., 2004).
In the search for mechanisms that regulate LHSs and generate demographic diversity, the largely unexamined assumption that there are necessarily species-specific “normal” concentrations of a given hormone has led us down blind alleys and obscured our understanding of how the HPO axis works. Dropping this assumption has both resolved previously inexplicable paradoxes and suggested novel models that better reflect how physiological mechanisms transform signals and implement LHSs.
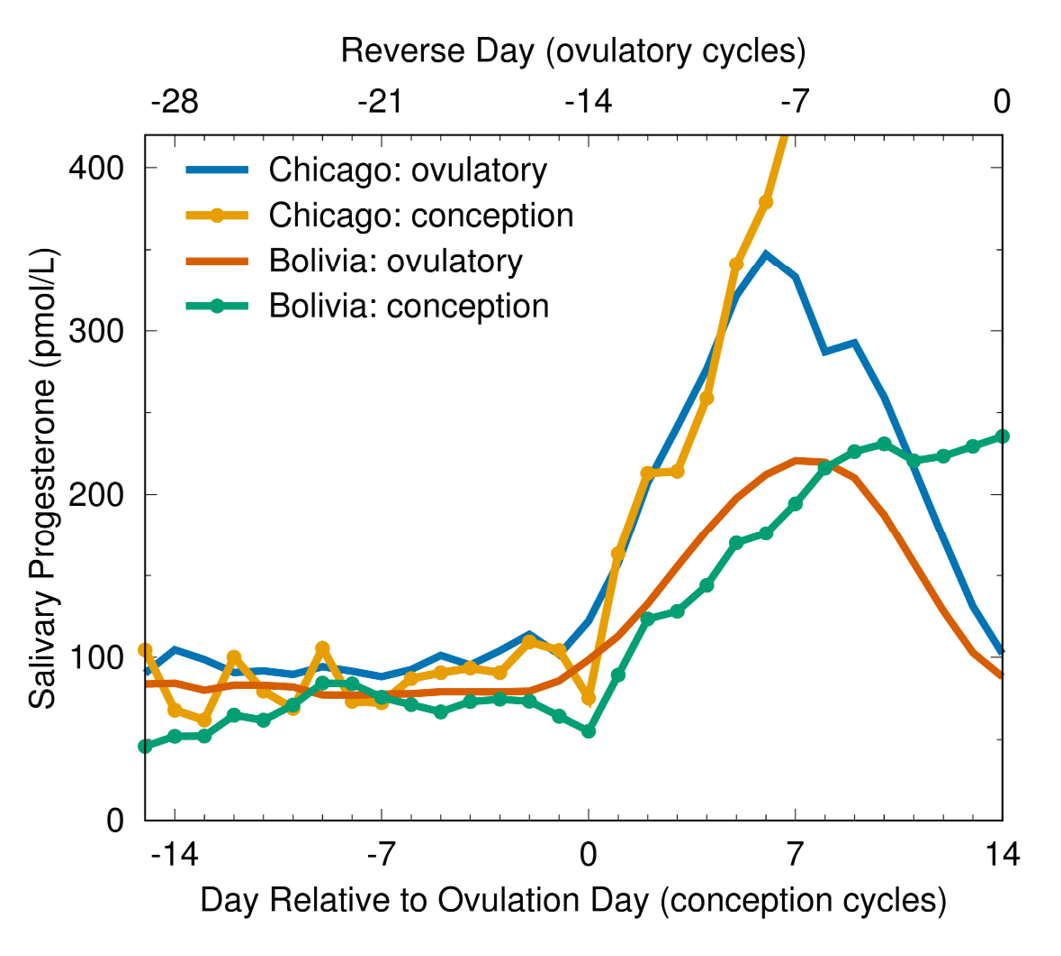
Fig. 6 Salivary progesterone profiles in conception and ovulatory non-conception cycles. Progesterone concentrations in ovulatory cycles are significantly lower in women from Bolivia than in women from Chicago throughout the ovarian cycle, and also lower during and subsequent to ovulation in conception cycles (Vitzthum et al., 2004).
Evolving Research Directions for the Study of Mechanisms
It has been nearly a century since Fisher reflected on what might be gained by knowing something of the physiological mechanisms underlying resource allocation strategies and more than half a century since Tinbergen re-emphasized the centrality of determining mechanism in our efforts to thoroughly understand a behaviour. Yet we’ve just begun to delve deeply into the unexpectedly complex details of exactly how an organism achieves successful trade-offs between survival and reproduction. This recent progress is possible because of advancements in biomarker measurements and field-friendly methods for collecting longitudinal as well as cross-sectional data in community-based studies. The expansion of complex statistical models and greater computational capacities have also improved analyses of this wealth of data. Although the investment can be high, the pay-off is often impressive.
Theoretical developments are as necessary as better technologies for discovering the origins and functioning of a specific mechanism underlying a life history strategy (LHS). For example, recognition of parent-offspring conflict regarding optimal parental investment explains how pregnancy loss can be a successful LHS in some circumstances and why there are maternal mechanisms to test offspring quality. The evolutionary insight that pregnancy is more akin to a Greek play laden with conflict than a pas-de-deux laced by harmony deepens our understanding of human biological variability and potentially prompts re-evaluations of explanatory models in related fields (e.g. medicine, reproductive technologies, demography).
Below I briefly describe two of the many research areas concerning life history mechanisms that deserve attention as we move forward.
Adaptations, Cross-talk, and Trade-offs: More Is Needed on the Mechanics of Mechanism
Whether short-term (a day, a season) or long-term, resource allocations are potentially subject to natural selection (i.e. they may be adaptive responses that have evolved to increase lifetime reproductive success in a given set of population-specific conditions). Testing whether or not a life history strategy or some specific investment decision is adaptive is a daunting task, especially in the long-lived human species. Knowledge of the specific mechanism by which a given resource allocation is accomplished facilitates the testing of adaptation hypotheses, and can enlighten efforts to address the other three of Tinbergen’s four questions.
Although it is obvious that there must be considerable coordination of actions among the various systems of an organism, for the most part investigators have understandably ignored those interactions in favour of tackling (relatively) manageable questions. It is now evident that we must begin to venture out beyond these imagined borders, and grapple with how various systems inter-communicate and thereby effect responses that are potentially adaptive for the organism.
To accomplish this goal requires figuring out the details of how a mechanism is engaged and operates. For example, at a particular point in a specific pathway, is the salient feature of a signal its absolute status (e.g. hormone concentration) and/or a change in its status (e.g. a rise or fall regardless of the signal’s baseline concentration)? What are the specific contexts and factors (nutrient or time limits? mechanical or physiological incompatibility?) that tend to prompt one response over another? By what signals and pathways is this response recognized and responded to by other organismal components?
In addition, although this chapter has focused on the physiological mechanisms by which life history strategies are implemented, resource allocation strategies may also involve somatic, behavioural or extra-somatic mechanisms. Maintaining body-fat stores, food caching (whether buried nuts or dried agricultural surplus) and building reciprocal social networks all demand time and resources diverted from immediate reproductive investment, but each of these strategies can mitigate the risk inherent in variable environments and thereby potentially increase lifetime reproductive success (Lee and Boe, in this volume). How, then, do such non-physiological mechanisms become an integral component of the organism’s LHS and thus influence the workings of physiological mechanisms and reproductive output? At least part of the answer to these questions involves learning more about the precise mechanics of the pertinent mechanisms.
Linking (Adult) Mechanisms and (Pre-adult) Ontogeny
In their tribute to Tinbergen, Bateson and Laland (2013) emphasized that consideration of “mechanism always requires specification of a point in development”. Ontogeny rightly comprises the entire developmental history of the organism up to the time at which a behaviour is occurring, and the causes of that behaviour may trace back to the organism’s conception (Tinbergen, 1963).
It has been recognized for some time that early conditions can shape an organism’s functioning during its subsequent life. This calibration of individual physiology to local environments is necessary for an organism to mature and to execute a successful LHS. We have only recently begun to identify the epigenetic processes involved in this ontogenetic preparation for the future. The conditions experienced early in life are the best predictors of those likely to be experienced throughout life, and hence the LHS shaped by that early environment is likely to be the most advantageous LHS in later life (Vitzthum, 1990, 1997, 2001, 2009).5 This process does not require any conscious decisions by an organism. Rather, among the ways in which an organism’s biology might respond to environmental signals, natural selection will favour those responses that result in a relatively greater reproductive advantage.
Thus population-specific environmental conditions experienced during an individual’s pre-adult development are likely to affect her reproductive functioning throughout adulthood. Women who developed in energetically demanding environments may be biologically acclimated to such conditions and hence are less likely to experience ovarian cycle disruption under a given energetic stressor than would a woman who developed in a more benign environment (Vitzthum, 1990, 1997, 2001, 2009). Metaphorically, women in industrialized populations are akin to hothouse flowers, cultivated under ideal conditions — small perturbations can have large negative effects until the ideal conditions are restored. In contrast, wildflowers must successfully reproduce in the typical environments in which they have grown, even if those conditions are more demanding compared to a hothouse. From the perspective of life history theory, there is nothing necessarily paradoxical about high fecundity and fertility under conditions that are energetically demanding (compared to some other environment) if these are the conditions in which the individual developed, and which are likely to persist for the rest of the individual’s reproductive life. (This argument acknowledges that there are minimum energetic requirements for somatic maintenance and pregnancy below which successful reproduction does not occur.)
The centrality of age-specific mortality risk in the evolution and execution of population-appropriate LHSs suggests there are mechanisms that convey information reflective of mortality schedules. These mechanisms likely involve epigenetic and other physiological processes that influence reproductive physiology. However, the specific endogenous or exogenous signals that generate suitable epigenetic modifications of the genetic regulation of reproductive maturation and functioning are unclear. How does reproductive physiology become attuned to the local population’s mortality schedule, or, put another way, how do mortality schedules become embodied within an individual?
For example, the timing of puberty, marking a shift of resource allocation from growth to reproduction, varies by several years within and between human populations and can change markedly in a single generation. What signal(s) prompts this transition at an age that is likely to be advantageous, given the current population-specific mortality risks? Whatever these signal(s) may prove to be, they likely have the following attributes:
- Because of the evolutionary history of extant taxa, the same signalling mechanism is likely shared across multiple phylogenetic lineages (e.g. all primates or all mammals).
- The signals must act early in life (before maturation is complete) so that the organism can grow and mature at the strategically “best” pace and age.
- The signals are proxies for mortality schedules rather than the organism having experienced death itself (by which point the signal would be of no use to the organism).
- Most (perhaps all) such signals are likely to be non-specific as to the causes of death because (given a specific time of death) the cause of death is irrelevant to one’s lifetime reproductive success.
In 1993, Chisholm proposed a life history model linking childhood experiences of stress (as reasonable proxies for mortality schedules) and later reproductive strategies. Subsequent papers (Chisholm et al., 2005; Coall and Chisholm, 2010; Sheppard and Coall, in this volume) provided additional support for the model including evidence for mediation of the physiological pathways through the HPA axis. Working from different data and premises, Geronimus (1992) proposed the “weathering hypothesis” to explain ethnic differences in the US population in the timing of fertility. She attributed this and ethnic health disparities to the negative impacts of various stressors mediated by the HPA-axis (Geronimus et al., 2006). That these different models point to the same physiological mechanism linking mortality, health and reproduction to present and past conditions suggests that more study of the HPA-axis (in particular, its cross-talk with other physiological systems and epigenetic processes) could shed light on the links between ontogeny and the responsiveness of life history strategies to mortality schedules.
Concluding Remarks
The 150th anniversary of Darwin’s publication in 1859 of On the Origin of Species by Means of Natural Selection was marked by worldwide celebrations of his far-reaching contribution. Mostly forgotten was that by 1900, for want of evidence, natural selection had relatively few supporters (Bowler, 1983). Rather, mainstream biology favoured several alternative explanations for evolution.
It is now a century since the beginnings of “The Modern Synthesis”: the integration of Darwin’s ideas about evolution and Mendel’s work on heredity. This merger of theory and mechanism by Fisher (1918), Haldane (1924), Wright (1932) and others is rightly considered the foundation of contemporary evolutionary biology (Huxley, 1942), having spawned innumerable theoretical and empirical advancements in biology and other fields. Of late there have been calls for an “extended evolutionary synthesis” and other elaborations (Pigliucci and Müller, 2010; Jablonka and Lamb, 2014; Laland et al., 2015) of the Darwinian-Mendelian model that had been catapulted by the modern synthesis. In large part, these newest developments are an outcome of investigations into the mechanisms that build an organism and manage its functioning from conception through growth and reproduction to death.
Like the modern synthesis, these extensions and the empirical evidence supporting them are worthy of demographers’ attention. Collectively they provide a roadmap to understanding the variability and plasticity of human biology and behaviour within an evolutionary framework without resorting to gene-centric reductionism or genetic determinism (which often yield unsatisfactory explanations for complex bio-behavioural phenotypes). While it is evident that humans, like any biological entity, are subject to and the result of evolutionary processes, behaviour within and between human populations is typically more than the simple expression of “a gene for” this or that phenotype (the same can be said for non-human species).
Obviously, the behaviour of an organism is never fully independent of its body. Behavioural and biological mechanisms share a fuzzy boundary across which a signal from one side of the border can prompt a cascade of responses on the other. Unpacking the interactions of biological and behavioural mechanisms is one particularly promising strategy for better understanding how extra-somatic as well as somatic factors influence reproductive functioning and generate demographic diversity.6
Consider, for example, lactational suppression of ovulation, a flexibly responsive bio-behavioural mechanism that is the very essence of being a mammal and one of the most influential determinants of variation in fertility. Breastfeeding in humans (WHO, 2009) is a coordinated behavioural repertoire of two persons: mum holds and guides the baby, who must latch on to and suckle the nipple. Mothers typically learn their part through repeated observation or active teaching from relatives and midwives. Reflexes in the infant’s central nervous system prompt suckling, itself the physical stimulus that initiates the cascade of neuroendocrinological sequelae in mum’s body that inhibits ovulation. But only to a point — mum could decide to breastfeed for years but nonetheless ovulation would return much sooner. This is not a consequence of her behaviour but of the mechanism itself, which has been shaped by natural selection to return her to a fecund state despite nursing an older infant. Mum could also decide not to breastfeed at all, but this can have negative health consequences for mum and baby alike, and for mum’s reproductive success. Leaving aside additional points regarding the somatic and extra-somatic factors that influence a mother’s decisions regarding nursing (Tully and Ball, 2011), it is evident that lactational suppression of ovulation is neither a strictly biological nor strictly behavioural mechanism, and thus its effect on fertility cannot be accurately assessed without considering both sides of the coin.
At the beginning of this chapter, I proposed adding a fifth question to Tinbergen’s four: How do the features of mechanisms vary within and across human communities worldwide? Quite a bit, as it turns out. Ages at menarche and at menopause vary by many years. Ovarian steroid concentrations in ovulatory cycles of healthy women differ severalfold without accompanying differences in fecundity or fertility. Rates of ovulation and early pregnancy loss vary seasonally and between populations.
This phenotypic variability reveals the flexibility of individual responses to local ecologies and the workings of life history strategies. Optional ovulation is a life history mechanism that prevents premature diversion of resources from a nursing infant or risky shifts in immune defences in a sexually abstinent woman, or allows foregoing the current opportunity for conception until conditions improve. Conception is not an irrevocable maternal commitment to reproduction. Gate-keeping mechanisms operating during implantation and the subsequent weeks prior to the LPPT afford opportunities for maternal termination of investment (early pregnancy loss) if the conceptus is flawed, or maternal status or extra-somatic conditions are not favourable. At these early stages, human reproduction is a “rent-to-own” contract with low exit costs.
There is practical as well as theoretical value in recognizing that physiological mechanisms have context-dependent outcomes, exemplified by the attempts to develop breastfeeding behaviour into a natural contraceptive. Much effort was invested in determining the best nursing pattern (suckling frequency and/or duration) for suppressing ovulation for the longest time post-partum (NFP, 1991). This goal proved unattainable. Researchers found that the same breastfeeding pattern did not have comparable impacts on fecundity in different women and populations, and that very different nursing patterns had similar suppressive impact (in mathematics, this is known as a “many-to-many mapping” from nursing pattern to ovulation suppression). This complex variation in signal and outcome precluded proposing a specific regime as a contraceptive. Nonetheless, if a baby’s only food was from being breastfed whenever hungry, ovulation was suppressed for an extended period, though exactly how long was not predictable with the available data. Although frustrating for health care providers, these findings are consistent with life history theory (Vitzthum, 1994).
Numerous examples of phenotypic plasticity within and across populations challenge notions of a universal “normal” human biology. While there are barriers to good health in all human populations, it should not be assumed that all deviations from such norms (especially those established with data from WEIRD populations (Heinrich et al., 2010)) are necessarily pathologies requiring fixing. Furthermore, we should not necessarily expect there to be a universal “best” adaptation in all populations to an identical challenge to reproduction or survival. For example, Beall (2006) has documented three quantitatively different phenotypes in response to low barometric pressure among indigenous high-altitude populations in the Andes, Tibet and Ethiopia. These phenotypic variants likely reflect the different biocultural histories of the populations and may also be moderated by other features of the local ecologies.
Cross-population studies have also prompted interesting questions regarding ecomarkers (signals of extra-somatic conditions). In some instances, the change in an ecomarker may be more readily detected and hence a more salient signal than the specific state of the condition. In either case, the utility of the ecomarker will likely differ across contexts (for example, directional changes in photoperiod would be a reliable ecomarker of changing seasons at high latitudes but not near the Equator).
The physiological mechanisms that underlie LHSs operate in ways that vary both within and between populations, and this variation can have profound demographic consequences. These mechanisms have evolved to respond to the changing availability of resources and the finite time during which an organism must develop and reproduce. Some essential biological, psychological and behavioural functions cannot co-occur; successful LHSs must juggle such incompatibilities regardless of the abundance of energy and/or other resources.
It turns out that evolutionary processes are more numerous and nuanced than a mere culling of the less fit and, as a consequence, reproductive functioning is more like a flexibly responsive behaviour than a well-tuned machine. The details of “how it works” are, as yet, only partially understood. Clearly there is much work left to be done and exciting revelations await.
Acknowledgements
I thank the editors for their invitation to contribute to this volume and for their admirable patience. Rebecca Sear’s guidance throughout is especially appreciated. The thoughtful comments and suggestions by Emily Chester, Abigail Page, Rebecca Sear and an anonymous reviewer strengthened the chapter; I’m grateful for their time and effort. Uncountable hours of lively debate with Jonathan Thornburg are woven throughout this work. I thank the US National Science Foundation (award 1319663), the Fulbright-NSF Arctic Research Program, and Indiana University, Bloomington, for their financial support during the preparation of this chapter.
References7
Abrams, E. T., and E. M. Miller. 2011. ‘The Roles of the Immune System in Women’s Reproduction: Evolutionary Constraints and Life History Trade‐offs’, Yearbk Phys Anthropol 146.S53: pp. 134–54, https://doi.org/10.1002/ajpa.21621
Alvergne, A., and V. H. Tabor. 2018. ‘Is Female Health Cyclical? Evolutionary Perspectives on Menstruation’, TREE 33.6: pp. 399–414, https://doi.org/10.1016/j.tree.2018.03.006
Baird, D. D., C. R. Weinberg, A. J. Wilcox, D. R. McConnaughey, P. I. Musey, and D. C. Collins. 1991. ‘Hormonal Profiles of Natural Conception Cycles Ending in Early, Unrecognized Pregnancy Loss’, J Clin Endocrinol Metab, 72.4: pp. 793–800, https://doi.org/10.1210/jcem-72-4-793
Balbo, N., F. C. Billari, and M. Mills. 2013. ‘Fertility in Advanced Societies: A Review of Research’, Eur J Popul, 29.1: pp. 1–38, https://doi.org/10.1007/s10680-012-9277-y
Barker, D. J. 1990. ‘The Fetal and Infant Origins of Adult Disease’, BMJ, 301.6761: p. 1111 https://doi.org/10.1136/bmj.301.6761.1111
Bateson, P., and K. N. Laland, 2013. ‘Tinbergen’s Four Questions: An Appreciation and an Update’, Trends Ecol Evol, 28.12: pp. 712–8, https://doi.org/10.1016/j.tree.2013.09.013
Beall, C. M. 2006. ‘Andean, Tibetan, and Ethiopian Patterns of Adaptation to High-altitude Hypoxia’, Integr Comp Biol, 46.1: pp. 18–24, https://doi.org/10.1093/icb/icj004
Beer, A. E., J. Y. Kwak, and J. E. Ruiz. 1996. ‘Immunophenotypic Profiles of Peripheral Blood Lymphocytes in Women with Recurrent Pregnancy Losses and in Infertile Women with Multiple Failed In Vitro Fertilization Cycles’, Am J Reprod Immunol, 35.4: pp. 376–82, https://doi.org/10.1111/j.1600-0897.1996.tb00497.x
Bernstein, L., J. M. Yuan, R. K. Ross, M. C. Pike, R. Hanisch, R. Lobo, F. Stanczyk, Y. T. Gao, and B. E. Henderson. 1990. ‘Serum Hormone Levels in Pre-Menopausal Chinese Women in Shanghai and White Women in Los Angeles: Results from Two Breast Cancer Case-Control Studies’, Cancer Causes Control, 1.1: pp. 51–8, https://doi.org/10.1007/BF00053183
Biomarkers Definitions Working Group. 2001. ‘Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework’, Clin Pharmacol Ther, 69.3: pp. 89–95, https://doi.org/10.1067/mcp.2001.113989
Blurton-Jones, N. ‘Controversies and Unfinished Business in Hadza Demography andEvolutionary Ecology’, in this volume.
Boas, F. 1911. Changes in Bodily Form of Descendants of Immigrants (Washington DC/New York: US GPO/Columbia University Press).
Boklage, C. E. 1990. ‘Survival Probabilities of Human Conceptions from Fertilization to Term’, Int J Fertil, 35.2: pp. 75–94. https://pubmed.ncbi.nlm.nih.gov/1970983/
Bongaarts, J. 1978. ‘A Framework for Analyzing the Proximate Determinants of Fertility’, Pop Develop Review, 4.1: pp. 105–32, https://doi.org/10.2307/1972149
—. 1980. ‘Does Malnutrition Affect Fecundity? A Summary of Evidence’, Science, 208.4444: pp. 564–9, https://doi.org/10.1126/science.7367878
—. 2015. ‘Modeling the Fertility Impact of the Proximate Determinants: Time for a Tune-up’, Demographic Res, 33: pp. 535–60, https://doi.org/10.4054/demres.2015.33.19
Borgerhoff Mulder, M. In this volume. ‘Bateman’s Principles and the Study of Evolutionary Demography’, in Human evolutionary demography, ed. by O. Burger, R. Lee and R. Sear.
Bowler, P. J. 1983. The Eclipse of Darwinism: Anti-Darwinian Evolution Theories in the Decades around 1900 (Baltimore: Johns Hopkins University Press).
Brzezinski, A. 1997. ‘Melatonin in Humans’, N Engl J Med, 336.3: pp. 186–95, https://doi.org/10.1056/nejm199701163360306
Campbell, K. L., and J. W. Wood. 1988. ‘Fertility in Traditional Societies’, in Natural Human Fertility: social and biological determinants, ed. by P. Diggory, M. Potts, and S. Teper (London: Macmillan), pp. 39–69, https://doi.org/10.1007/978-1-349-09961-0_4
Caro, T. M., and M. Borgerhoff Mulder. 1987. ‘The Problem of Adaptation in the Study of Human Behavior’, Ethol Sociobiol, 8.1: pp. 61–72, https://doi.org/10.1016/0162-3095(87)90058-6
Charnov, E. L. 1993. Life History Invariants (Oxford: Oxford University Press).
Chisholm, J. S. 1993. ‘Death, Hope, and Sex: Life-History Theory and the Development of Reproductive Strategies’, Current Anthropology, 34.1: pp. 1–24, https://doi.org/10.1086/204131
Chisholm, J. S., J. A. Quinlivan, R. W. Petersen, D. A. Coall. 2005. ‘Early Stress Predicts Age at Menarche and First Birth, Adult Attachment, and Expected Lifespan’, Human Nature, 16.3: pp. 233–65, https://doi.org/10.1007/s12110-005-1009-0
Coall, D. A., and J. S. Chisholm. 2010. ‘Reproductive Development and Parental Investment During Pregnancy: Moderating Influence of Mother’s Early Environment’, Am J Hum Biol, 22.2: pp. 143–53, https://doi.org/10.1002/ajhb.20965
Coutifaris, C., E. R. Myers, D. S. Guzick, M. P. Diamond, S. A. Carson, R. S. Legro, P. G. McGovern, W. D. Schlaff, B. R. Carr, M. P. Steinkampf, S. Silva, D. L. Vogel, P. C. Leppert, and NICHD National Cooperative Reproductive Medicine Network. 2004. ‘Histological Dating of Timed Endometrial Biopsy Tissue is Not Related to Fertility Status’, Fertil Steril, 82.5: pp. 1264–72, https://doi.org/10.1016/j.fertnstert.2004.03.069
Davis, K., and J. Blake. 1956. ‘Social Structure and Fertility: An Analytic Framework’, Econ Develop Cult Change 4.3: pp. 211–35, https://doi.org/10.1086/449714
DeLong, J. P. In this volume. ‘Pathways of Density Dependence and Natural Selection in Modern Humans’, in Human evolutionary demography, ed. by O. Burger, R. Lee, and R. Sear.
Descartes, R. 1637. Discourse on the Method of Rightly Conducting One’s Reason and Seeking the Truth in the Sciences (London: Thomas Newcombe), MDCXLIX, https://www.gutenberg.org/files/25830/25830-h/25830-h.htm
DeWitt, T. J., A. Sih, and D. S. Wilson. 1998. ‘Costs and Limits of Phenotypic Plasticity’, TREE, 13.2: pp. 77–81, https://doi.org/10.1016/S0169-5347(97)01274-3
Dickinson, L. E., B. MacMahon, P. Cole, and J. B. Brown. 1974. ‘Estrogen Profiles of Oriental and Caucasian Women in Hawaii’, N Engl J Med, 291.23: pp. 1211–3, https://doi.org/10.1056/nejm197412052912302
Dillion, L., A. Chernenoko, M. Dribe, S. Engelhardt, A. Gagnon, H. A. Hanson, H. Meeks, L. Quaranta, K. R. Smith, and H. Vézina. ‘Did Grandmothers Enhance Reproductive Success in Historic Populations?: Testing Evolutionary Theories on Historical Demographic Data in Scandinavia and North America’, in this volume.
Dobzhansky, T. 1973. ‘Nothing in Biology Makes Sense Except in the Light of Evolution’, Am Biol Teach, 35.3: pp. 125–9, https://doi.org/10.2307/4444260
Donnet, M. L., P. W. Howie, M. Marnie, W. Cooper, M. Lewis. 1990. ‘Return of Ovarian Function Following Spontaneous Abortion’, Clin Endocrinol, 33.1: pp. 13–20, https://doi.org/10.1111/j.1365-2265.1990.tb00460.x
Drea, C. M. 2005. ‘Bateman Revisited: The Reproductive Tactics of Female Primates’, Integr Comp Biol, 45.5: pp. 915–23, https://doi.org/10.1093/icb/45.5.915
Elias, M. F., J. Teas, J. Johnston, and C. Bora. 1986. ‘Nursing Practices and Lactational Amenorrhea’, J Biosoc Sci, 18.1: pp. 1–10, https://doi.org/10.1017/S0021932000006441
Ellish, N. J., K. Saboda, J. O’Connor, P. C. Nasca, E. J. Stanek, and C. Boyle. 1996. ‘A Prospective Study of Early Pregnancy Loss’, Hum Reprod, 11.2: pp. 406–12, https://doi.org/10.1093/HUMREP/11.2.406
Ellison, P. T., N. R. Peacock, and C. Lager. 1989. ‘Ecology and Ovarian Function Among Lese Women of the Ituri Forest, Zaire’, Am J Phys Anthropol, 78.4: pp. 519–26, https://doi.org/10.1002/ajpa.1330780407
Fawcett, T. W., S. Hamblin, L-A Giraldeau. 2012. ‘Exposing the Behavioral Gambit: The Evolution of Learning and Decision Rules’, Behav Ecol, 24.1: pp. 2–11, https://doi.org/10.1093/beheco/ars085
Fisher, R. A. 1918. ‘The Correlation Between Relatives on the Supposition of Mendelian Inheritance’, Trans Roy Soc Edinburgh, 52.2: pp. 399–433, https://doi.org/10.1017/S0080456800012163
Fisher, R. A. 1930. The Genetical Theory of Natural Selection (Oxford: Clarendon), p. 272.
Geronimus, A. T. 1992. ‘The Weathering Hypothesis and the Health of African-American Women and Infants: Evidence and Speculations’, Ethn Dis, 2.3: pp. 207–21. https://www.jstor.org/stable/45403051
Geronimus, A. T., M. Hicken, D. Keene, and J. Bound. 2006. ‘“Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States’, AJPH, 96.5: pp. 826–33, https://doi.org/10.2105/ajph.2004.060749
Gigerenzer, G., P. M. Todd, and the ABC Research Group. 1999. Simple Heuristics that Make Us Smart (New York: Oxford University Press).
Hutchinson, J. M. C., and G. Gigerenzer. 2005. ‘Simple Heuristics and Rules of Thumb: Where Psychologists and Behavioural Biologists Might Meet’, Behavioural Processes, 69.2: pp. 97–124, https://doi.org/10.1016/j.beproc.2005.02.019
Gioiosa, R. 1955. ‘Incidence of Pregnancy During Lactation in 500 Cases’, Am J Obstet Gynecol, 70.1: pp. 162–74, https://doi.org/10.1016/0002-9378(55)90300-8
Gould, S. J. 1998. Leonardo’s Mountain of Clams and the Diet of Worms (Cambridge, MA and London: Harvard University Press), https://doi.org/10.4159/harvard.9780674063365
Gould, S. J., and R. C. Lewontin. 1979. ‘The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme’, Proc R Soc London B, 2051161: pp. 581–98, https://doi.org/10.1098/rspb.1979.0086
Haig, D. 1990. ‘Brood Reduction and Optimal Parental Investment when Offspring Differ in Quality’, Am Nat, 136.4: pp. 550–66, https://doi.org/10.1086/285113
—. 1993. ‘Genetic Conflicts in Human Pregnancy’, Q Rev Biol, 68.4: pp. 495–532, https://doi.org/10.1086/418300
—. 1999. ‘Genetic Conflicts of Pregnancy and Childhood’, in Evolution in Health and Disease, ed. by S. C. Stearns (Oxford: Oxford University Press), pp. 77–90.
Haldane, J. B. S. 1924. ‘A Mathematical Theory of Natural and Artificial Selection’, Trans Camb Philosoph Soc, 23.5: pp. 19–41. https://doi.org/10.1017/s0305004100011750
Henrich, J., S. J. Heine, and A. Norenzayan. 2010. ‘The Weirdest People in the World?’, Behav Brain Sci, 33.2–3: pp. 61–135, https://doi.org/10.1017/S0140525X0999152X
Hill, K. ‘Anthropological and evolutionary demography’, in this volume.
Holman, D. J., and J. W. Wood. 2001. ‘Pregnancy loss and fecundability in women’, in Reproductive Ecology and Human Evolution, ed. by P. T. Ellison (Hawthorne: Aldine de Gruyter), pp. 15–38.
Huxley, J. 1942. Evolution: The Modern Synthesis (London: Allen and Unwin).
Jabbour, H. N., R. W. Kelly, H. M. Fraser, and H. O. D. Critchley. 2006. ‘Endocrine Regulation of Menstruation’, Endocr Rev, 27.1: pp. 17–46, https://doi.org/10.1210/er.2004-0021
Jacob, F. 1977. ‘Evolution and Tinkering’, Science, 196.4295: pp. 1161–6, https://doi.org/10.1126/science.860134
—. 1994. The Possible and the Actual (Jessie and John Danz Lectures) (Seattle: University of Washington Press).
Jablonka, E., and M. J. Lamb. 2014. Evolution in Four Dimensions, 2nd edn (Cambridge, Massachusetts: MIT Press).
Jasienska, G., and P. T. Ellison. 1998. ‘Physical Work Causes Suppression of Ovarian Function in Women’, Proc Biol Sci, 265.1408: pp. 1847–51, https://doi.org/10.1098/rspb.1998.0511
Jones, G. E. S. 1949. ‘Some Newer Aspects of Management of Infertility’, JAMA, 141.16: pp. 1123–9, https://doi.org/10.1001/jama.1949.02910160013004
Jones, O. R., T. H. G. Ezard, C. Dooley, K. Healy, D. J. Hodgson, M. Mueller, S. Townley, and R. Salguero-Gomez. ‘My family and Other Animals: Human Demography Under an Evolutionary Lens’, in this volume.
Kaandorp, S. P., T. E. van Mens, S. Middeldorp, B. A. Hutten, M. H. Hof, J. A. van der Post, F. van der Veen, and M. Goddijn. 2014. ‘Time to Conception and Time to Live Birth in Women with Unexplained Recurrent Miscarriage’, Hum Reprod, 29.6: pp. 1146–52, https://doi.org/10.1093/humrep/deu052
Kaushic, C., K. L. Roth, V. Anipindi, F. Xiu. 2011. ‘Increased Prevalence of Sexually Transmitted Viral Infections in Women: The Role of Female Sex Hormones in Regulating Susceptibility and Immune Responses’, J Reprod Immunol, 88.2: pp. 204–9, https://doi.org/10.1016/j.jri.2010.12.004
Key, T. J., J. Chen, D. Y. Wang, M. C. Pike, and J. Boreham. 1990. ‘Sex Hormones in Women in Rural China and in Britain’, Br J Cancer, 62.4: pp. 631–6, https://doi.org/10.1038/bjc.1990.344
Klein, S. L. 2012. ‘Immune Cells Have Sex and So Should Journal Articles’, Endocrinology, 153.6: pp. 2544–50, https://doi.org/10.1210/en.2011-2120
Kozlowski, J., and S. C. Stearns. 1989. ‘Hypotheses for the Production of Excess Zygotes: Models of Bet-Hedging and Selective Abortion’, Evolution, 43.7: pp. 1369–77, https://doi.org/10.2307/2409453
Kreager, P. ‘Evolution in the History of Population Thought’, in this volume.
Kuzawa, C. W., and Z. M. Thayer. 2011. ‘Timescales of Human Adaptation: The Role of Epigenetic Processes’, Epigenomics, 3.2: pp. 221–34, https://doi.org/10.2217/epi.11.11
Laland K. N. T. Uller, M. W. Feldman, K. Sterelny, G. B. Müller, A. Moczek, E. Jablonka, J. Odling-Smee. 2015. ‘The Extended Evolutionary Synthesis: Its Structure, Assumptions and Predictions’, Proc R Soc B, 282.1813: p. 20151019, https://doi.org/10.1098/rspb.2015.1019
Lasker, G. W. 1969. ‘Human Biological Adaptability’, Science, 166.3912: pp. 1480–6, https://doi.org/10.1126/science.166.3912.1480
Lee, R., and C. Boe. ‘Sociality, food sharing, and the evolution of life histories’, in this volume.
Leslie, P. W., and M. A., Little. 2003. ‘Human Biology and Ecology: Variation in Nature and the Nature of Variation’, Am Anthropol, 105.1: pp. 28–37, https://doi.org/10.1525/aa.2003.105.1.28
Leslie, P. W., K. L. Campbell, and M. A. Little. 1993. ‘Pregnancy Loss in Nomadic and Settled Women in Turkana, Kenya: A Prospective Study’, Hum Biol, 65.2: pp. 237–54. https://www.jstor.org/stable/41464498
Lorenz, T. K., C. M. Worthman, V. J. Vitzthum. 2015. ‘Links Among Inflammation, Sexual Activity and Ovulation: Evolutionary Trade-offs and Clinical Implications’, Evol Med Public Health, 2015.1: pp. 304–24, https://doi.org/10.1093/emph/eov029
Lorenz, T. K., G. E. Demas, and J. R. Heiman. 2017. ‘Partnered Sexual Activity Moderates Menstrual Cycle-Related Changes in inflammation Markers in Healthy Women: An Exploratory Observational Study’, Fertil Steril 107.3: pp. 763–73, https://doi.org/10.1016/j.fertnstert.2016.11.010
Loudon, I. 2000. ‘Maternal Mortality in the Past and its Relevance to Developing Countries Today’, Am J Clin Nutr, 72.1: pp. 241S-6S, https://doi.org/10.1093/ajcn/72.1.241S
Low, B. (year). ‘A Biologist’s Perspective on Evolutionary Demography’, in Human Evolutionary Demography, ed. by O. Burger, R. Lee and R. Sear.
Mace, R. (year). ‘Why Do We Do What We Do?’, in Human Evolutionary Demography, ed. by O. Burger, R. Lee and R. Sear.
MacMahon, B., P. Cole, J. B. Brown, K. Aoki, T. M. Lin, R. W. Morgan, and N. C. Woo. 1974. ‘Urine Oestrogen Profiles of Asian and North American Women’, Int J Cancer, 14.2: pp. 161–7, https://doi.org/10.1002/ijc.2910140204
Malthus, T. R. 1798. An Essay on the Principle of Population (London: J. Johnson), https://www.gutenberg.org/files/4239/4239-h/4239-h.htm
McNamara, J. M., and A. I. Houston. 2009. ‘Integrating Function and Mechanism’, TREE, 24.12: pp. 670–5, https://doi.org/10.1016/j.tree.2009.05.011
Mead, M. 1947. ‘The Concept of Culture and the Psychosomatic Approach’, Psychiatry, 10.1: pp. 57–76, https://doi.org/10.1080/00332747.1947.11022625
Metcalf, M. 1983. ‘Incidence of Ovulation from the Menarche to the Menopause: Observations of 622 New Zealand Women’, N Z Med J, 96.738: pp. 645–8. https://pubmed.ncbi.nlm.nih.gov/6576257/
Metcalf, M. G., and J. A. Mackenzie. 1980. ‘Incidence of Ovulation in Young Women’, J Biosoc Sci, 12.3: pp. 345–52, https://doi.org/10.1017/S002193200001289X
Moya, C., K. Snopkowski, and R. Sear. 2016. ‘What do Men Want? Re-examining Whether Men Benefit from Higher Fertility than is Optimal for Women’, Phil Trans R Soc B, 371.1692: p. 20150149, https://doi.org/10.1098/rstb.2015.0149
NFP. 1991. ‘Conference Proceedings: Natural Family Planning: Current Knowledge and New Strategies for the 1990s’, Am J Obstet Gynecol, 165.6.2.
Nelson, R. J., G. E. Demas, S. L. Klein, and L. J. Kriegsfeld. 2002. Seasonal Patterns of Stress, Immune Function, and Disease (Cambridge: Cambridge University Press), https://doi.org/10.1017/CBO9780511546341
Nepomnaschy, P. A., K. Welch, D. McConnell, B. S. Low, B. I. Strassmann, and B. G. England. 2006. ‘Cortisol Levels and Very Early Pregnancy Loss in Humans’, Proc Natl Acad Sci (USA), 103.10: pp. 3938–42, https://doi.org/10.1073/pnas.0511183103
Panter-Brick, C., D. S. Lotstein, and P. T. Ellison. 1993. ‘Seasonality of Reproductive Function and Weight Loss in Rural Nepali Women’, Hum Reprod, 8.5: pp. 684–90, https://doi.org/10.1093/oxfordjournals.humrep.a138120
Peacock, N. 1990. ‘Comparative and Cross-Cultural Approaches to the Study of Human Female Reproductive Failure’, in Primate Life History and Evolution, ed. by J. DeRousseau (New York: Wiley-Liss), pp. 195–220.
Perez, A., P. Vela, R. Potter, and G. S. Masnick. 1971. ‘Timing and Sequence of Resuming Ovulation and Menstruation after Childbirth’, ‘Pop Stud’, 25.3: pp. 491- 503, https://doi.org/10.1080/00324728.1971.10405820
Pigliucci, M., and G. B. Müller (eds.). 2010. Evolution — The Extended Synthesis (Cambridge, Massachusetts: MIT Press).
Practice Committee of the American Society for Reproductive Medicine. 2015. ‘Current Clinical Irrelevance of Luteal Phase Deficiency: A Committee Opinion’, Fertil Steril, 103.4: pp. e27-e32, https://doi.org/10.1016/j.fertnstert.2014.12.128
Prasad, A., S. L. Mumford, G. M. Buck Louis, K. A. Ahrens, L. A. Sjaarda, K. C. Schliep, N. J. Perkins, K. A. Kissell, J. Wactawski-Wende, and E. F. Schisterman. 2014. ‘Sexual Activity, Endogenous Reproductive Hormones and Ovulation in Premenopausal Women’, Horm Behav, 66.2: pp. 330–8, https://doi.org/10.1016/j.yhbeh.2014.06.012
Prior, J. C. 1985a. ‘Luteal Phase Defects and Anovulation: Adaptive Alterations Occurring with Conditioning Exercise’, Sem Repro Endocrinol, 3.1: pp. 27–33, https://doi.org/10.1055/s-2007-1022601
—. 1985b. ‘Hormonal Mechanisms of Reproductive Function and Hypothalamic Adaptation to Endurance Training’, in The Menstrual Cycle and Physical Activity, ed. by J. Puhl (Champaign, Illinois: Human Kinetics), pp. 63–75.
—, 1987. ‘Physical Exercise and the Neuroendocrine Control of Reproduction’, Baillieres Clin Endocrinol Metab, 1.2: pp. 299–317, https://doi.org/10.1016/S0950-351X(87)80065-4
Promislow, D. E. L., and P. H. Harvey. 1990. ‘Living Fast and Dying Young: A Comparative Analysis of Life-History Variation Among Mammals’, J Zoology, 220.3: pp. 417–37, https://doi.org/10.1111/j.1469-7998.1990.tb04316.x
Rasch, B., and J. Born. 2013. ‘About Sleep’s Role in Memory’, Physiolog Reviews, 93.2: pp. 681–766, https://doi.org/10.1152/physrev.00032.2012
Reinis, Kia I. 1992. ‘The Impact of the Proximate Determinants of Fertility: Evaluating Bongaarts’ and Hobcraft’s and Little’s Methods of Estimation’, Populat Stud, 46.2: pp. 309–26, https://doi.org/10.1080/0032472031000146256
Roberts, C. J., and C. R. Lowe. 1975. ‘Where Have All the Conceptions Gone?’, Lancet, 305.7905: pp. 498–9, https://doi.org/10.1016/S0140-6736(75)92837-8
Robertson, S. A., and D. J. Sharkey. 2016. ‘Seminal Fluid and Fertility in Women’, Fert Steril, 106.3: pp. 511–9, https://doi.org/10.1016/j.fertnstert.2016.07.1101
Robinson, W. S. 1950. ‘Ecological Correlations and the Behavior of Individuals’, Am Sociolog Rev, 15.3: pp. 351–7, https://doi.org/10.2307/2087176
Roff, D. 1992. The Evolution of Life Histories: Theory and Analysis (New York: Chapman Hall).
Scheiner, S. M. 1993. ‘Genetics and Evolution of Phenotypic Plasticity’, Ann Rev Ecol Systemat, 24: pp. 35–68, https://doi.org/10.1146/annurev.es.24.110193.000343
Sear, R., and R. Mace. 2008. ‘Who Keeps Children Alive? A Review of the Effects of Kin on Child Survival’, Evol Hum Behav, 29.1: pp. 1–18, https://doi.org/10.1016/j.evolhumbehav.2007.10.001
Sear, R., D. W. Lawson, H. Kaplan, and M. K. Shenk. 2016. ‘Understanding Variation in Human Fertility: What Can We Learn from Evolutionary Demography?’, Phil Trans R Soc B, 371.1692: p. 20150144, https://doi.org/10.1098/rstb.2015.0144
Selvin, H. C. 1958. ‘Durkheim’s Suicide and Problems of Empirical Research’, Am J Sociol, 63.6: pp. 607–19. https://doi.org/10.1086/222356
Sheppard P., and D. Coall. ‘What Has Childhood Done for Us? The Role of Ontogeny in Understanding Human Demographic Behaviour’, in this volume.
Shimizu, H., R. K. Ross, L. Bernstein, M. C. Pike, and B. E. Henderson. 1990. ‘Serum Oestrogen Levels in Postmenopausal Women: Comparison of American Whites and Japanese in Japan’, Br J Cancer, 62.3: pp. 451–3, https://doi.org/10.1038/bjc.1990.316
Short, R. V. 1976. ‘Definition of the Problem – The Evolution of Human Reproduction’, Proc R Soc Lond B Biol Sci, 195.1118: pp. 3–24, https://doi.org/10.1098/rspb.1976.0095
Stearns, S. C. 1976. ‘Life-History Tactics: A Review of the Ideas’, The Quarterly Review of Biology, 51.1: pp. 3–47, https://doi.org/10.1086/409052
—. 1989. ‘The Evolutionary Significance of Phenotypic Plasticity’, BioScience, 39.7: p. 436, https://doi.org/10.2307/1311135
—. 1992. The Evolution of Life Histories (Oxford: Oxford University Press).
Stover, J. 1998. ‘Revising the Proximate Determinants of Fertility Framework: What Have We Learned in the Past 20 Years?’, Stud Family Planning, 29.3: pp. 255–67, https://doi.org/10.2307/172272
Strassmann, B. I., and B. Gillespie. 2002. ‘Life-history Theory, Fertility and Reproductive Success in Humans’, Proc R Soc Lond B, 269.1491: pp. 553–62, https://doi.org/10.1098/rspb.2001.1912
Temme, D. H. 1986. ‘Seed Size Variability: A Consequence of Variable Genetic Quality Among Offspring?’, Evolution, 40.2: pp. 414–7, https://doi.org/10.2307/2408819
Tinbergen, N. 1963. ‘On Aims and Methods of Ethology’, Zeitschr Tierpsychol, 20.4: pp. 410–33, https://doi.org/10.1111/j.1439-0310.1963.tb01161.x
Trichopoulos, D., S. Yen, J. Brown, P. Cole, and B. MacMahon. 1984. ‘The Effect of Westernization on Urine Estrogens, Frequency of Ovulation, and Breast Cancer Risk. A Study of Ethnic Chinese Women in the Orient and the USA’, Cancer, 53.1: pp. 187–92, https://doi.org/10.1002/1097-0142(19840101)53:1<187::AID-CNCR2820530133>3.0.CO;2-N
Trivers, R. L. 1974. ‘Parent-Offspring Conflict’, Am Zool, 14.1: pp. 249–64, https://doi.org/10.1093/icb/14.1.249
Tuljapurkar, S. ‘The Challenges of Evolutionary Biodemography and the Example of Menopause’, in this volume.
Tully, K. P., and H. L. Ball. 2013. ‘Trade-offs Underlying Maternal Breastfeeding Decisions: A Conceptual Model’, Maternal Child Nutrit, 9.1: pp. 90–8, https://doi.org/10.1111/j.1740-8709.2011.00378.x
Uggla, C. ‘Contextual Effects on Fertility and Mortality: Complementary Contributions from Demography and Evolutionary Life History Theory’, in this volume.
UNFPA. 2019. United Nations Population Fund: Trends in Maternal Mortality: 2000 to 2017 Executive Summary, https://www.unfpa.org/resources/trends-maternal-mortality-2000-2017-executive-summary
van der Walt, L. A., E. N. Wilmsen, and T. Jenkins. 1978. ‘Unusual Sex Hormone Patterns Among Desert-Dwelling Hunter-Gatherers’, J Clin Endocrinol Metab, 46.4: pp. 658–63, https://doi.org/10.1210/jcem-46-4-658
van Montfrans, J. M., M. H. A. van Hooff, J. A. Huirne, S. J. Tanahatoe, S. Sadrezadeh, F. Martens, J. M. G. van Vugt, and C. B. Lambalk. 2004. ‘Basal FSH Concentrations as a Marker of Ovarian Ageing are not Related to Pregnancy Outcome in a General Population of Women over 30 Years’, Hum Reprod, 19.2: pp. 430–4, https://doi.org/10.1093/humrep/deh088
Via, S., and R. Lande. 1985. ‘Genotype-Environment Interaction and the Evolution of Phenotypic Plasticity’, Evolution, 39.3: pp. 505–22, https://doi.org/10.2307/2408649
Vitzthum, V. J. 1990. An Adaptational Model of Ovarian Function, Res. Rep. No. 90–200 (Ann Arbor: Population Studies Center, University of Michigan).
—. 1994. ‘The Comparative Study of Breastfeeding Structure and its Relation to Human Reproductive Ecology’, Yearb Phys Anthropol, 37.S19: pp. 307–49, https://doi.org/10.1002/ajpa.1330370611
—. 1997. ‘Flexibility and Paradox: the Nature of Adaptation in Human Reproduction’, in The Evolving Female: a Life History Perspective, ed. By M. E. Morbeck, A. Galloway, A. Zihlman (Princeton: Princeton University Press), pp. 242–58.
—. 2001. ‘Why Not So Great is Still Good Enough: Flexible Responsiveness in Human Reproductive Functioning’, in Reproductive Ecology and Human Evolution, ed. By P. T. Ellison (New York: Aldine de Gruyter), pp. 179–202.
—. 2003. ‘A Number No Greater than the Sum of Its Parts: The Use and Abuse of Heritability’, Hum Biol, 75.4: pp. 539–58, https://doi.org/10.1353/hub.2003.0064
—. 2008a. ‘Evolutionary Models of Women’s Reproductive Functioning’, Annual Rev Anthropol, 37.1: pp. 53–73, https://doi.org/10.1146/annurev.anthro.37.081407.085112
—. 2008b. ‘Evolution and Endocrinology: the Regulation of Pregnancy Outcomes’, in Medicine and Evolution: Current Applications, Future Prospects, ed. by S. Elton and P. O’Higgins (Boca Raton: CRC Press), pp. 99–126, https://doi.org/10.1201/9781420051377
—. 2009. ‘The Ecology and Evolutionary Endocrinology of Reproduction in the Human Female’, Yearb Phys Anthropol, 140.S49: pp. 95–136, https://doi.org/10.1002/ajpa.21195
—. 2020. ‘Lunar Fantasies: Normative Assumptions versus Normal Variation in Human Ovarian Cycling’, Plenary session, Meetings, Human Biology Association, Los Angeles.
Vitzthum V. J., P. T. Ellison, S. Sukalich, E. Caceres, and H. Spielvogel. 2000a. ‘Does Hypoxia Impair Ovarian Function in Bolivian Women Indigenous to High Altitude?’, High Alt Med Biol, 1.1: pp. 39–49, https://doi.org/10.1089/152702900320676
Vitzthum, V. J., H. Spielvogel, E. Caceres, and J. Gaines. 2000b. ‘Menstrual Patterns and Fecundity Among Non-lactating and Lactating Cycling Women in Rural Highland Bolivia: Implications for Contraceptive Choice’, Contraception, 62.4: pp. 181–7, https://doi.org/10.1016/s0010-7824(00)00164-5
Vitzthum, V. J., H. Spielvogel, E. Caceres, and A. Miller. 2001. ‘Vaginal Bleeding Patterns Among Rural Highland Bolivian Women: Relationship to Fecundity and Fetal Loss’, Contraception, 64.5: pp. 319–25, https://doi.org/10.1016/s0010-7824(01)00260-8
Vitzthum, V. J., and H. Spielvogel. 2003. ‘Epidemiological Transitions, Reproductive Health, and the Flexible Response Model’, Econ Hum Biol 1.2: pp. 223–42, https://doi.org/10.1016/S1570-677X(03)00037-6
Vitzthum, V. J., H. Spielvogel, J. Thornburg. 2004. ‘Interpopulational Differences in Progesterone Levels During Conception and Implantation in Humans’, PNAS, 101.6: pp. 1443–8, https://doi.org/10.1073/pnas.0302640101
Vitzthum, V. J., H. Spielvogel, J. Thornburg, and B. West. 2006. ‘A Prospective Study of Early Pregnancy Loss in Humans’, Fertil Steril, 86.2: pp. 373–9, https://doi.org/10.1016/j.fertnstert.2006.01.021
Vitzthum, V. J., J. Thornburg, and H. Spielvogel. 2009a. ‘Seasonal Modulation of Reproductive Effort During Early Pregnancy in Humans’, Am J Hum Biol, 21.4: pp. 548–58, https://doi.org/10.1002/ajhb.20936
Vitzthum, V. J., C. M. Worthman, C. M. Beall, J. Thornburg, E. Vargas, M. Villena, R. Soria, E. Caceres, and H. Spielvogel. 2009b. ‘Seasonal and Circadian Variation in Salivary Testosterone in Rural Bolivian Men’, Am J Hum Biol, 21.6: pp. 762–8, https://doi.org/10.1002/ajhb.20927
Walker, R., M. Gurven, K. Hill, A. Migliano, N. Chagnon, R. De Souza, G. Djurovic, R. Hames, A. M. Hurtado, H. Kaplan, K. Kramer, W. J. Oliver, C. Valeggia, and T. Yamauchi. 2006. ‘Growth Rates and Life Histories in Twenty-Two Small-Scale Societies’, AJHB, 18.3: pp. 295–311, https://doi.org/10.1002/ajhb.20510
Wang, D. Y., T. J. Key, M. C. Pike, J. Boreham, and J. Chen. 1991. ‘Serum Hormone Levels in British and Rural Chinese Females’, Breast Cancer Res Treat, 18.1: pp. S41-S45, https://doi.org/10.1007/bf02633526
Wang, X., C. Chen, L. Wang, D. Chen, W. Guang, and J. French. 2003. ‘Conception, Early Pregnancy Loss, and Time to Clinical Pregnancy: A Population-Based Prospective Study’, Fertil Steril, 79.3: pp. 577–84, https://doi.org/10.1016/S0015-0282(02)04694-0
Wasser, S. K., and D. P. Barash. 1983. ‘Reproductive Suppression among Female Mammals: Implications for Biomedicine and Sexual Selection Theory’, Quart Rev Biol 58.4: pp. 513–38, https://doi.org/10.1086/413545
Weinberg, C. R., E. Moledor, D. D. Baird, and A. J. Wilcox. 1994. ‘Is There a Seasonal Pattern in Risk of Early Pregnancy Loss?’, Epidemiology, 5.5: pp. 484–9, https://www.jstor.org/stable/3702202
Weinstein, M., J. W. Wood, M. A. Soto, and D. D. Greenfield. 1990. ‘Components of Age-specific Fecundability’, Population Studies, 44.3: pp. 447–67, https://doi.org/10.1080/0032472031000144846
West-Eberhard, M. J. 1989. ‘Phenotypic Plasticity and the Origins of Diversity’, Annu Rev Ecol Syst, 20: pp. 249–78, https://doi.org/10.1146/annurev.es.20.110189.001341
—. 2003. Developmental Plasticity and Evolution (Oxford: Oxford University Press), https://doi.org/10.1093/oso/9780195122343.001.0001
Whitacre, C. C., S. C. Reingold, and P. A. O’Looney. 1999. ‘A Gender Gap in Autoimmunity’, Science, 283.5406: pp. 1277–8, https://doi.org/10.1126/science.283.5406.1277
WHO (World Health Organization). 1983. ‘A Prospective Multicenter Trial of the Ovulation Method of Natural Family Planning. III. Characteristics of the Menstrual Cycle and of the Fertile Phase’, Fertil Steril, 40.6: pp. 773–8, https://doi.org/10.1016/s0015-0282(16)47478-9
—. 2009. Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals (Geneva: WHO), https://www.ncbi.nlm.nih.gov/books/NBK148970/
Wilcox, A. J., D. D. Baird, and C. R. Weinberg. 1999. ‘Time of Implantation of the Conceptus and Loss of Pregnancy’, N Engl J Med, 340: pp. 1796–9, https://doi.org/10.1056/nejm199906103402304
Wilcox, A. J., D. D. Baird, D. B. Dunson, D. R. McConnaughey, J. S. Kesner, and C. R. Weinberg. 2004. ‘On the Frequency of Intercourse Around Ovulation: Evidence for Biological Influences’, Hum Reprod, 19.7: pp. 1539–43, https://doi.org/10.1093/humrep/deh305
Williams, G. C. 1966. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought (Princeton: Princeton University Press).
Wira, C. R., and J. V. Fahey. 2008. ‘A New Strategy to Understand How HIV Infects Women: Identification of a Window of Vulnerability During the Menstrual Cycle’, AIDS, 22.15: pp. 1909–17, https://doi.org/10.1097/qad.0b013e3283060ea4
Wira, C. R., J. V. Fahey, M. Ghosh, M. V. Patel, D. K. Hickey, D. O. Ochiel. 2010. ‘Sex Hormone Regulation of Innate Immunity in the Female Reproductive Tract: The Role of Epithelial Cells in Balancing Reproductive Potential with Protection Against Sexually Transmitted Pathogens’, Am J Reprod Immunol, 63.6: pp. 544–65, https://doi.org/10.1111/j.1600-0897.2010.00842.x
Wood, J. W. 1994. Dynamics of Human Reproduction: Biology, Biometry, Demography (New York: Aldine de Gruyter).
Wood, J. W., and M. Weinstein. 1988. ‘A Model of Age-Specific Fecundability’, Popul Stud (Camb), 42.1: pp. 85–113, https://doi.org/10.1080/0032472031000143136
Worthman, C. M., and E. J. Costello. 2009. ‘Tracking Biocultural Pathways in Population Health: The Value of Biomarkers’, Ann Hum Biol, 36.3: pp. 281–97, https://doi.org/10.1080/03014460902832934
Wright, S. 1932. ‘The Roles of Mutation, Inbreeding, Crossbreeding and Selection in Evolution’, in Proceedings of the Sixth International Congress of Genetics (1), ed. by D. F. Jones (Ithaca, New York: Genetics Society of America), pp. 356–66.
Zinaman, M. J., E. D. Clegg, C. C. Brown, J. O’Connor, and S. G. Selevan. 1996. ‘Estimates of Human Fertility and Pregnancy Loss’, Fertil Steril, 65.3: pp. 503–9, https://doi.org/10.1016/s0015-0282(16)58144-8
Glossary
biomarker: “A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working Group 2001); examples include hormone concentrations, birth weight, blood pressure and many others; biomarker choice and interpretation depends on many factors (Worthman and Costello 2009)
C-reactive protein (CRP): an acute-phase protein produced by the liver in response to signals from immune agents. Low circulating concentrations of CRP reflect a broad array of processes related to ongoing baseline somatic maintenance, but rise abruptly within about two hours of an acute insult
conspecific: members of the same species
corpus luteum: the transformed follicle that had enclosed the egg cell prior to ovulation
cross-talk: biomolecular communication between two signalling pathways
downregulation: in the context of hormones and their targets, the cell becomes less sensitive to the presence of a hormone because available receptors have become saturated due to hormone binding and/or receptor degradation (also see upregulation)
early pregnancy loss (EPL): natural termination within the first few weeks of conception
ecomarker: a reliable signal of extra-somatic conditions
endocrine system: comprises the internal organs that release hormones directly into the circulatory system in order to affect the functioning of more distant target organs
epigenetic: refers to structural changes of the chromosome that alter the expression of the genotype (and thus modify phenotype) without changing the DNA sequence
ethology: the study of behaviour (usually refers to non-human animal behaviour)
extra-somatic: external to the body (e.g. social, cultural, economic, physical and biotic factors and the behaviour of others)
fecundity: refers to the capacity to conceive
fertility: refers to the production of a live birth
follicle: the structure surrounding an egg cell prior to ovulation
follicular phase: the pre-ovulatory segment of the ovarian cycle
genotype: the genetic sequences in an individual that contribute to a phenotype
gonadotrophin releasing hormone (GnRH): is synthesized and released by the hypothalamus; stimulates the synthesis and release of gonadotrophins (LH and FSH) from the anterior pituitary
human chorionic gonadotropin (hCG): produced by the conceptus; binds to receptors on the corpus luteum which, as a consequence, maintains its production of progesterone essential to sustaining the pregnancy
hypothalamic-pituitary-adrenal (HPA) axis: the physiological system comprising the hypothalamus, pituitary and adrenal gland; plays a central role in responding to stressors
hypothalamic-pituitary-ovarian (HPO) axis: the physiological system comprising the hypothalamus, pituitary and ovary; plays a central role in the regulation of reproduction
life history strategy (LHS): a pattern of maturation, reproduction and resource allocation that is subject to natural selection
life history theory (LHT): an evolutionary framework for studying maturation, reproduction and aging, and the associated mechanisms underlying resource allocation of to these processes
luteal phase: the post-ovulatory segment of the ovarian cycle
luteal phase deficiency (LPD): a disorder that is said to be caused by insufficient progesterone during the luteal phase but that lacks definitive diagnostic criteria
luteo-placental-progesterone-transition (LPPT): a shift in progesterone synthesis during pregnancy from the corpus luteum to the placenta
norm of reaction: the range of possible phenotypes for a given genotype
ontogeny: the development of an organism from conception until death
ovulation: the release of the mature egg from a single follicle
phenotype: a morphological, physiological, behavioural or psychological feature of an organism; a phenotype may be determined only by genes, or solely by environmental factors, or by the interaction of genes and environmental factors
phenotypic plasticity: capacity for a genotype to express a variety of phenotypes
phylogeny: the evolutionary relationships among current and extinct species
somatic: referring to the body (also see extra-somatic)
upregulation: in the context of hormones and their targets, the cell’s creation of more receptors so that the cell is more sensitive to the hormone (also see downregulation)
1 A glossary of terms is provided at the end of this chapter.
2 For additional discussions of evolutionary demography using Tinbergen’s framework see the following chapters in this volume: Mace on function, Jones et al. on phylogeny, and Sheppard and Coall on ontogeny.
3 For examples see the following chapters in this volume: Blurton-Jones, Borgerhoff Mulder, Dillion et al., Lee and Boe, and Tuljapurkar.
4 In an evolutionary context, higher quality offspring simply means those offspring having attributes (for example, larger size) that usually confer a relatively greater likelihood of survival.
5 Similar arguments have been made independently regarding the psychological mechanisms underpinning animal behaviors that are flexibly responsive to environmental cues (Gigerenzer et al., 1999; Hutchinson and Gigerenzer, 2005; McNamara and Houston, 2009; Fawcett et al., 2012).
6 Recall that extra-somatic refers to social, cultural, economic, physical and biotic factors, and the behaviours of others.
7 Note this chapter has been posted on the Open Science Framework website since 10/04/2020, after it was accepted for publication, so the references will reflect when the chapter was written and not the OBP publication date.
