13. Genetics and Reproductive Behaviour: A Review
© 2024 Melinda C. Mills and Felix C. Tropf, CC BY 4.0 https://doi.org/10.11647/OBP.0251.13
Fertility and reproduction have been core topics across multiple disciplines, including the study of reproductive behaviour outcomes such as tempo (timing) and quantum (number) of fertility, but also fecundity, infertility and reproductive development. The aim of this chapter is to provide a comprehensive and introductory overview of the central theoretical and empirical approaches to the study of the genetics of human reproductive behaviour and review key findings. We start with a brief definition of fertility and reproduction, followed by an overview of interdisciplinary approaches and findings. We then explore why it may be useful to adopt a biodemographic and genetic approach to reproduction, the central empirical methods that have been used, core findings to date, and conclude with a discussion and reflection on future directions of research.
Introduction
Fertility and reproduction have been central topics in the disciplines of (evolutionary) demography, sociology, anthropology, biology, medical sciences, and genetics. Broad interest likewise stretches across human, plant and animal studies. This chapter focuses on human reproductive choice, which includes the study of outcomes such as the timing and number of births. These are often also related to development traits such as the onset of menarche or menopause, the onset of sexual behaviour, and infertility related diseases. Although the majority of research on this topic within the social sciences has focused on social science and environmental explanations (Balbo and others, 2013), there is a growing body of research that adopts a biodemographic or sociogenomic approach (Mills and Tropf, 2016). Although reproductive behaviour has been largely linked to choice and decision-making — thus regarded as highly socially-determined — a growing amount of evidence highlights the importance of biological and genetic factors, which have been shown to be intertwined with social determinants and behavioural aspects of reproduction.
The aim of this chapter is to provide an interdisciplinary overview of the burgeoning genetics of reproductive behaviour literature, take stock of the central findings, and suggest promising areas of research in the future. We review work primarily in the areas of demography, sociology, and genetics, but with some attention to related disciplines and research in evolutionary biology and anthropology, reproductive medicine, psychology, and behavioural and molecular genetics. This is an introductory chapter aimed at providing an overview. For more specific reviews about research on the genetic association between fertility and psychological traits or on leveraging results from genetic discovery studies for evolutionary research (both topics we touch upon in this chapter), see also Kim and Lee (2019) and Guo and others (2018).
The current chapter provides an overview of this research to date starting with a brief definition of fertility and reproduction, the link with natural selection, stark differentiation of this research from historical eugenics and a brief overview of socio-environmental explanations. We then turn to a summary of the central behavioural and molecular empirical approaches, together with core findings. This is followed by a reflection regarding the differences of genetic effects in relation to certain country or birth cohort contexts and between the sexes. We then conclude with a discussion and reflection.
Defining Fertility and Reproduction
The terms fertility and reproduction take on different meanings in demography and sociology, reproductive medicine, and genetics. In demography, fertility refers to the actual bearing of live births. Demographers and sociologists often discuss two interrelated aspects of fertility, namely the “quantum” or actual number of children individuals have over a certain period, and the “tempo” or timing of when they have these children (Bongaarts and Feeney, 2000). Tempo is obviously highly related to quantum since the delaying of first births may result in a lower quantum or number of children. For this reason, we often use fertility and reproductive behaviour interchangeably throughout the chapter. Especially when reviewing the literature, we focus on the number of children ever born (NEB) as a measure of fertility quantum and on age at first birth (AFB) as a measure of fertility tempo.
In reproductive medicine, “fertility” is used in a different manner and related to the ability of individuals and couples to conceive. Infertility denotes the ability/inability of couples, women or men, to conceive and have children given unprotected intercourse (Joffe, 2010), while in demography this is signified by the terms (in)fecundity or sterility. In biological research, the focus is often on lifetime reproductive success (LRS) (Byars and others, 2010) or the number of offspring (Zietsch and others, 2014), which is what demographers refer to as “quantum” or the number of children ever born. In evolutionary research, fertility quantum is often used as a surrogate measure of “fitness”. If the number of surviving (and reproducing) children of an individual is computed relative to those of their peers of the same birth cohort, this might indicate relative reproductive (dis-)advantages for individuals and has been use as a proximate measure for relative fitness (Kirk and others, 2001; Stearns and others, 2010). This in turn is used to measure how far the fertility quantum leads to relatively higher chances of successfully transmitting genes to the next generation. This link to fitness means that fertility has vital consequences for the study of natural selection and evolution, while fertility quantum remains a largely imperfect proxy for fitness as discussed in more detail elsewhere (Mcgraw and Caswell, 1996; Jones and Bird, 2014).
Fertility, Natural Selection, and Evolution
Improvements in hygiene and the reduction in prenatal, infant and child mortality in industrialized societies means that the number of children ever born (i.e. quantum) has emerged as a readily available proximate measure for lifetime reproductive success (LRS) relating (imperfectly) — see also Mcgraw and Caswell, 1996; Jones and Bird, 2014) — to fitness (Stearns and others, 2010). This refers to Fisher’s (1930) fundamental theorem of natural selection (Fisher, 1930), which states that because fertility is highly correlated with fitness that its heritability at equilibrium should be, in theory, close to zero. As we demonstrate shortly, however, a series of studies have produced evidence that this is not the case.
Non-zero heritability of fertility indicates ongoing natural selection. If specific genetic variants are associated with higher reproductive success, they are passed on to the next generation more often than others and we expect them to become more frequent in future generations. A couple of studies therefore explored whether genes, which are associated with number of children ever born, are also associated with other traits. If genes that increase height, for example, are also associated with having more children, we expect future generations to be (genetically) taller than current ones (Stulp and others, 2015). A number of studies therefore used both twin data and, more recently, molecular genetic data to “live-track” ongoing human evolution (Milot and others, 2011; Kirk and others, 2001; F C Tropf and others, 2015b; N. Barban and others, 2016; Sanjak and others, 2017).
As we have argued previously, there are several reasons to remain cautious about predictions of the actual evolutionary change that we can expect from previous findings of ongoing natural selection in humans (Courtiol and others, 2016). Firstly, the relationships between genotypes and phenotypes remain poorly understood. Secondly, comparable phenotypic information is often not present across multiple generations. Thirdly, much of natural selection on contemporary human populations is driven by cultural and environmental factors that themselves change very rapidly. Only selection sustained in one direction over many generations produces significant genetic change. Fourthly, while it is imperative to measure physiological changes across generations, their relevance for population characteristics such as average number of children, education, body-size or heart rate are (most likely) negligible in the short term compared to cultural changes.
As discussed more extensively in other chapters in this volume, natural selection in humans is often studied using several key traits or phenotypes, such as height, which can be measured with or without the use of genetics. As reviewed elsewhere (Courtiol and others, 2016), this ranges from the simplest design of a twin or family model that measures how much variation is attributed to genetic differences between relatives to the use of actual whole-genome data.
Is Adopting a Genetic Approach to Fertility Related to Eugenics?
There has been a reticence to adopt a genetic or biological approach to fertility, particularly in some disciplines and quarters, due to the assumption that it may be linked to eugenics. It is essential to clarify that the research described in this chapter and conducted by these researchers is not related to eugenics and we actively oppose this link. As we have previously noted elsewhere (Mills and Tropf, 2016), there is a dark history of eugenic policies that emerged in the 1880s and that were linked to atrocities in recent history. Eugenics focused on so-called “improvements” that could be made to humanity via supposedly scientific methods that were misguided and incorrect and involved selective breeding. The aim of the eugenics movement was “to affect reproductive practice through the application of theories of heredity” (Levine and Bashford, 2010, p. 3). The aim was to prevent life (sterilization, contraception, abortion), make life “fitter” (training, rearing of children, public health) and promote pronatalist goals, but also, at its most extreme, to end life (so-called euthanasia of the disabled) (Levine and Bashford, 2010). The eugenic approach has been widely, and rightly, condemned by all serious scientific audiences. It is essential to note that the type of research described in this chapter and within the mainstream of contemporary peer-reviewed research in behavioural and molecular genetics has no eugenic goals or ties. Considering this grave history of linking eugenics with fertility, however, we continue to find it essential to explicitly acknowledge this point and to be vigilant in order to prevent similar abuses in the future.
Socio-environmental Predictors of Reproductive Behaviour
Fertility and reproduction, as discussed in this chapter, remain largely behavioural outcomes, related not only to genetics and biology, but also influenced by individual and partner-level choices and preferences, and institutional environments. For this reason, although we focus on the “genetics” of fertility, we acknowledge that it is one piece of the puzzle and that socio-environmental predictors will be the strongest predictors in many cases. Various reviews have examined the core factors that predict fertility outcomes (Balbo and others, 2013; Mills and others, 2011), which we briefly summarize here. Factors influencing fertility are generally divided into three theoretical levels of micro- or individual factors, meso-level, which includes the family level, for example, and macro- or societal-level factors.
The core micro-level factors that impact fertility have been identified as partnership formation, including instability and quality of partnerships, multiple partnering and re-partnering and the emergence of different types of partnerships such as cohabitation (Billari and Kohler, 2002; Mills and Blossfeld, 2005). Partnering often impacts the timing, postponement and ultimately the number of children. Education is also a prominent predictor, usually based on Becker’s classic theory of human capital (Becker and Becker, 2009). Education levels, particularly for women, are likewise seen as key in fertility decision-making, linked to opportunity costs, impact of enrolment and role conflict, as well as the field of education chosen, with most studies examining how higher education results in fertility postponement (Tropf and Mandemakers, 2017). Economic and employment uncertainty are also key, building on Easterlin’s theory of economic deprivation (Easterlin, 1976), which posits that in historical periods of general economic uncertainty and rising unemployment, individuals will forgo partnering and fertility. It relates to the “affordability clause” to have children (Rindfuss, Ronald R., Vandenheuvel, 1990), with multiple empirical studies demonstrating how economic, employment and temporal uncertainty results in family formation postponement (Mills and others, 2005).
Perhaps the most relevant for this current review is the body of literature on the intergenerational transmission of demographic behaviour and, in particular, fertility. Empirically speaking, this work often compares the similarities of particular fertility-related events (menopause, age at first child, number of children) across successive generations. They then mostly observe a moderately positive correlation between parents and their offspring. The bulk of this research has focused on number of children (Murphy and Wang, 2001) and the tempo of fertility — mainly the intergenerational transmission of teenage motherhood (Kahn and Anderson, 1992). Others have examined how parents transmit value, preferences, attitudes and contraceptive knowledge (Rijken and Liefbroer, 2009). This relates literature that examines the socioeconomic status of the family of origin, often finding a negative relationship between the father’s education or the mother’s levels of employment (Balbo and others, 2013).
Meso-level factors have also been shown as important predictors, including social networks and interaction which involves social learning (to gain knowledge, for example about contraceptives or what it is like to be a parent) and social influence (how peer groups impact attitudes and behaviour) (Balbo and Barban, 2014). Social capital and access to resources such as goods, money, ability to help or power have also been shown as important predictors (Balbo and Mills, 2011). The gendered division of labour at the household level has likewise been shown as an important factor regulating fertility (Mills and others, 2008).
Macro-level societal factors are also core predictors of reproductive trends. This includes the focus on economic period effects such as the commonly observed pro-cyclical relationship between economic growth, recessions and fertility (Sobotka, Tomáš, Skirbekk, V., Philipov, 2011). This is often strongly related to research on employment trends and the impact of employment. Social policy measures and welfare regimes, including labour-market, family and market constellations, tax and housing have also been extensively evaluated, but with mixed results in relation to their direct or causal impact on postponement and number of children (Mills and others, 2011). Larger value and attitude changes in addition to the widespread “contraceptive revolution” characterized by the second demographic transition has also been touched upon as an important explanation for the postponement and foregoing of children (Lesthaeghe, 1995).
Biodemographic and Genetic Approaches to Fertility
Although we continue to acknowledge the strong impact of socio-environmental factors on reproductive behaviour, a growing number of researchers argued for some time that fertility may be influenced by an individual’s genetic architecture and beyond, such as proteins, hormones, neurons, gametes and other factors (Udry, 1996; Wachter and Bulatao, 2003; Wachter, 2008; Freese, 2008). The importance of biological factors underlying fertility was recognized by early demographers in their recognition of key “proximate determinants” of fertility, including fecundability, contraceptive use, exposure to intercourse or sterility (Bongaarts, 1978). Some of the earliest calls to integrate genetic considerations into demographic fertility research were by Udry (Udry, 1996), who was able to think beyond the existing data constraints of the period to hypothesize plausible relationships between reproductive biology, social environment and fertility. He acknowledged that not only fertility outcomes, but also behavioural choices and motivations to have children were likely guided by genetics and hormones. This was followed by a larger biodemographic focus on this topic at the start of the 2000s (Wachter and Bulatao, 2003; Rodgers and others, 1999; Rodgers and Kohler, 2012). Biodemographic and genetic approaches to fertility can be roughly divided into two types of research: behavioural genetics, which adopts a twin and family design, and molecular genetics, which uses whole-genome data, often from unrelated individuals using various methods.
Behavioural Genetics: Twin and Family Studies
A series of early twin and family studies, mostly in demography, have linked biological and genetic components to fertility behaviour (Kohler and others, 1999; Kohler and Rodgers, 2003; Rodgers and others, 1999; Kirk and others, 2001). Adopting a “twin design”, they separate the genetic (i.e. examining monozygotic twins) and shared (i.e. growing up in the same household) or non-shared environment. Monozygotic twins are genetically identical, sharing 100% of the same genetic material while dizygotic twins — just as any brother and sister — share on average around 50%. If monozygotic pairs are more similar in their fertility behaviour in comparison to dizygotic twins, this is interpreted as a reflection of genetic effects. The extent to which genes influence a certain behaviour or disease — the “heritability” — is quantified as the proportion of variance in that trait within a population, which is explained by genetic variance (Visscher and others, 2008). The simplest way to estimate heritability is to subtract the correlation in a trait between dizygotic twins from the correlation between monozygotic twins and multiply the result by two (see also Snieder and others (2010) for a very short but quite comprehensive introduction to simple twin modelling). The central premise is that genetic and biological dispositions of individuals influence fertility either directly via genetically mediated variations, or, since many aspects regulating fertility possess considerable volitional control (e.g., decision of age at first birth, fertility preferences), via underlying temperament or personality influences on fertility decisions.
Figure 1 provides an overview of key studies to date that have examined the heritability mainly of the number of children ever born (NEB) and age at first birth (AFB) across different countries and birth cohorts. We see that there is more information on women as opposed to men, which is typical in this area of research and often related to data-gathering customs. We likewise observe that the heritability for AFB women ranges between just over 0% to 35% of the observed variance within these birth cohorts (i.e. 0.002 Denmark 1931–52 to 0.35 UK 1930–39). For the NEB for women, the range is 24–43% and for men between 24–28%. A recent meta-analysis of all twin studies conducted until 2015 suggests that across all fertility traits studied, on average around 30% of the variance is associated with genetic differences in a population (Polderman and others, 2015).
Key studies include early work in Denmark (Rodgers and others, 2001) that found around a 30% heritability of number of children ever born, which was later replicated in Sweden (Zietsch and others, 2014). Others have estimated a heritability of around 26% for women in Finland (Nisén and others, 2013), the UK (Tropf and others, 2015a), and Australia (Kirk and others, 2001), whereas others have found no effect in the US (Neiss and others, 2002) nor in some birth cohorts in Denmark (Rodgers and others, 2008). The twin studies also show considerable variation between the sexes, across countries and birth cohorts, which we return to in our discussion of GWA studies (genome-wide association studies) later in this chapter.
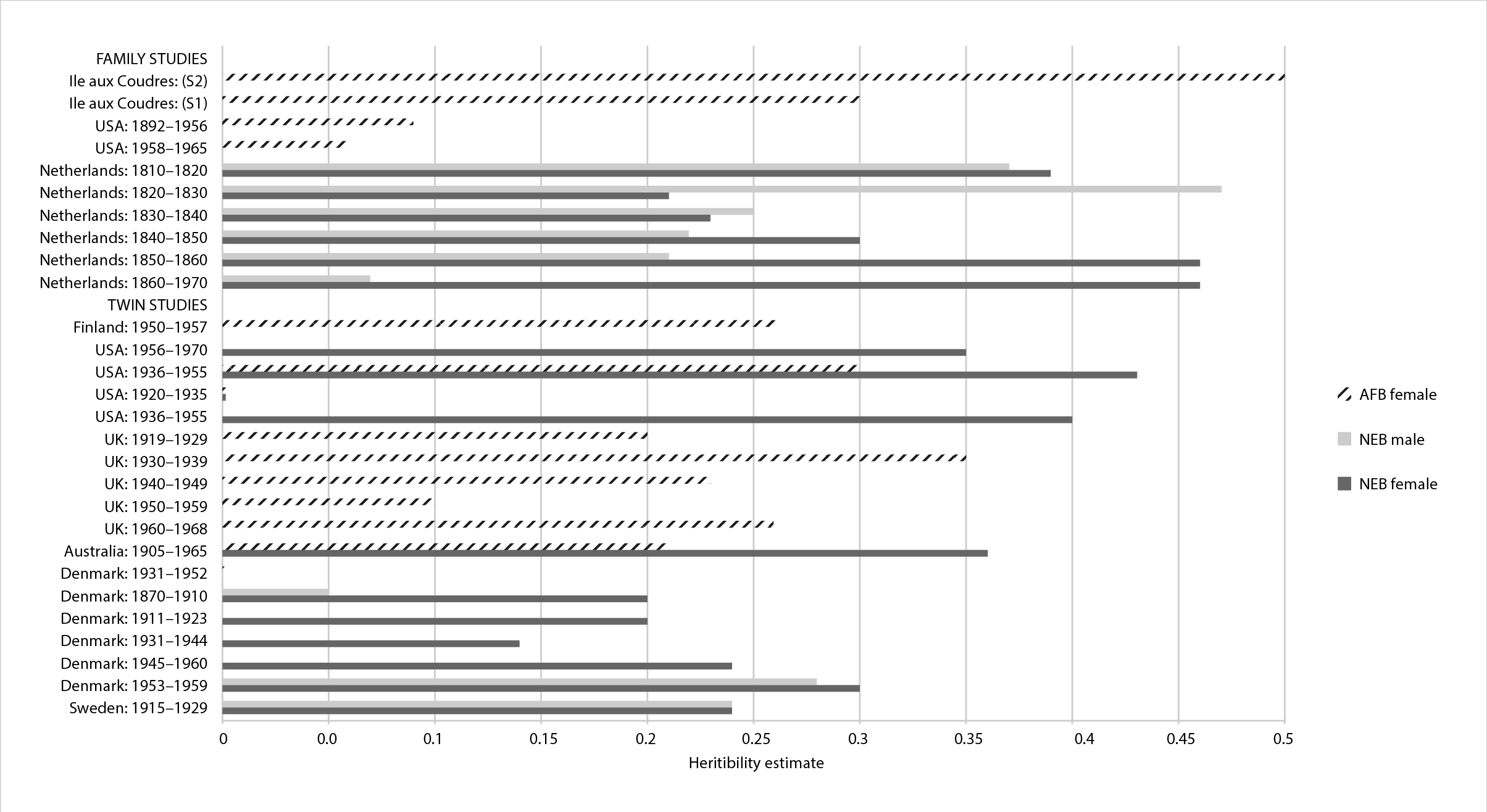
Figure 1 Summary of fertility heritability estimates by birth cohort and country by fertility trait: (AFB) age at first birth, (NEB) number of children ever born. Source: Adapted from Mills & Tropf (2015) and Barban et al. (2016), (produced by authors).
Molecular Genetic Approaches
Recent advances in methods and the widespread availability of large sociogenomic datasets has resulted in a rise of studies adopting a genetic approach. Whereas behavioural genetics focused on whether fertility has a genetic basis and if so, to what extent it is heritable — and suffer from several strong assumptions and practical limitations (Tropf and others, 2015; Nolte and others, 2019) — molecular genetics attempts to isolate where the genetic variants are located, in addition to a focus on the structure and function of DNA, and the generation of individual based genetic scores predicting fertility behaviour (Mills and others, 2018). These genetic variants are called single nucleotide polymorphisms (SNPs) and allow us to explore the data in new ways by applying novel statistical tools.
Candidate Gene Studies
An initial and early approach applying molecular genetic data was the candidate gene approach, which has an a priori hypothesis about the underlying biological pathway of a trait and directly focuses on a gene or set of markers. This was often due to the fact that certain datasets only genotyped smaller areas on the genome, offering only limited genetic markers also for small sample sizes. Although this technique is still used when the results are derived from a large GWAS (genome-wide association study), discussed shortly, previous work has been heavily criticized for producing false positive results. In this type of candidate-gene approach, genetic variants were compared with a sample of individuals (treatment group) that had the genetic marker with those who did not (control group). Although there are no direct candidate-gene studies on core fertility traits, several early studies examined sexual behaviour (Guo and others, 2008; Halpern, 2000) and contraceptive use (Daw and Guo, 2011), generally in relation to hypotheses related to risky behaviour and sensation seeking and linking it to the dopamine receptor or serotonin transporter. These studies have now been criticized for small sample sizes and lack of statistical power, false positives and biased positive results (Ioannidis, 2005).
GREML Studies
The increased availability of genome-wide molecular genetic data across the whole genotype for a larger number of individuals was coupled with new analytical techniques. A core advance is the Genomic-Relationship-Matrix based restricted maximum likelihood (GREML) method, which produces a more direct estimate of heritability using single genes across the whole genome for unrelated individuals. GREML analysis is a feature of the statistical program, which provides different types of Genome-Wide Complex Trait Analyses (Yang and others, 2011). GREML allows researchers to quantify the extent to which common genetic variants influence certain traits, such as the age at first birth and total number of children (Tropf and others, 2015b). Simply put, the GREML method calculates the genetic similarity between unrelated individuals based on their genetic material (i.e., their SNPs). This genetic similarity matrix is then related to the similarity in an outcome amongst individuals — which in our case is fertility. For example, if you share what we call your “segregating genetic material” (i.e., what makes you genetically you) at a level of 0.05% with one group and 2.5% with another, we would say that you have a higher similarity in your fertility behaviour with the second group. Parallel to twin studies, we expect closer related pairs of individuals to be more similar in their phenotypes if the phenotype has a genetic basis. Since the genetic overlap between pairs of individuals from different families is very small, large numbers of people are required for this type of analysis.
The first study to examine fertility in the form of age at first birth and number of children ever born was published in 2015 (Tropf and others, 2015), with the main results shown in Figure 2. Using Dutch and UK-based data, the main finding was that for the first time we were able to quantify the extent to which common genetic variants (SNPs) influence fertility. This study found that the differences in women’s age at first birth (AFB) and the number of children ever born (NEB) were associated with genetic differences. For the age at first birth, 15% of the observed variance was explained by genetic variation in common genes; for the number of children, it was 10% (see Figure 2).In demography and sociology, it is well established that the AFB and NEB are strongly correlated. In other words, if you have your first child later, you will have fewer children (Sobotka, 2004; Tropf and others, 2015b). The aforementioned study (Tropf and others, 2015b) also shows that the genetic effects for both outcomes overlap, which is partly explained by the association between AFB and NEB. In other words, it appears that the genes related to the time that women have their first child appear also to influence the number of children they ultimately have. The study thus partly explains why women who have children earlier also have a higher number of children.
This study, and similar ones that followed (Beauchamp, 2016; Kong, 2017), also contribute to the controversial debate about whether humans still evolve via natural selection. If particular genes are related to higher reproductive success (i.e. having more children), these genes will be passed on with a higher frequency to future generations. As discussed previously, NEB is seen as a proxy for “fitness”, and additive genetic variance in NEB therefore indicates ongoing natural selection within modern populations under study. The study examined women from the UK and the Netherlands born in the twentieth century, showing that those who had a genetic predisposition for an earlier age at first birth have had a reproductive advantage across the generations. Genes associated with an earlier AFB have been passed on more frequently to the next generation, allowing the authors to conclude that natural selection acts not only in historical, but also contemporary populations (Tropf and others, 2015b).
Studies on contemporary evolution, however, raise some perplexing findings (Tropf and others, 2015; Beauchamp, 2016). If genes associated with an earlier AFB, for example, are more likely to be passed down to the next generation, why is it that younger generations in the contexts that were studied were not having their children at an even earlier age? What we see in fact, is that women are doing exactly the opposite in most industrialized nations. Since the 1970s, women are having their first child around 4–5 years later, which is now on average at age 28–29 years (Mills and others, 2011). This massive postponement in the age at first birth suggests that the socio-environmental influences considered as important by social scientists and discussed previously, such as women’s educational expansion and entry into the labour market and the widespread use of effective contraception, has had a much stronger influence on fertility trends than natural selection. However, physiological changes should not be ignored and given the fact that both genes and the socio-environment can be shown to empirically matter for fertility, there is still a need for an integrative “sociogenomic” research design that draws from both genetics and the social sciences to better understand and predict human fertility.
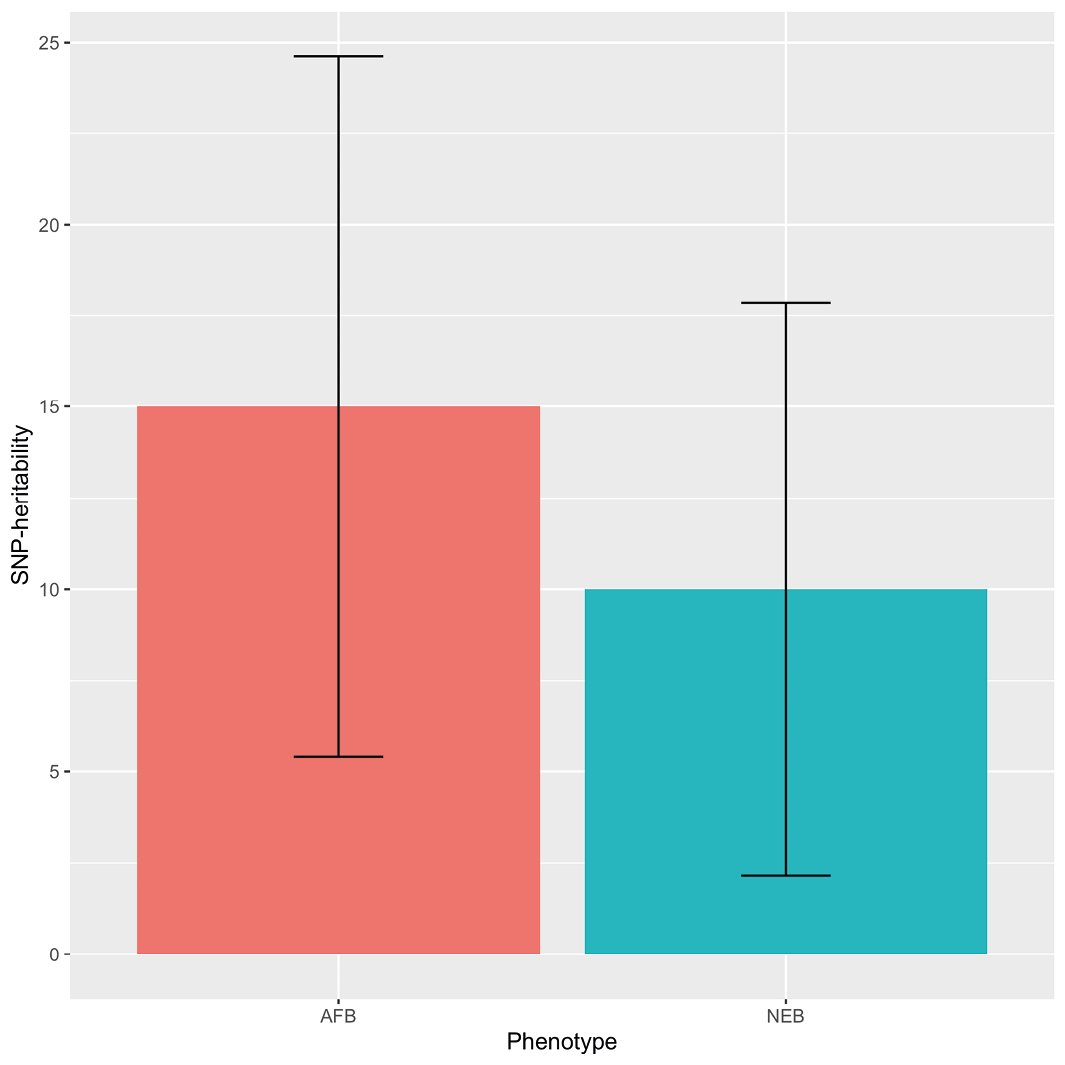
Figure 2 Estimates of the genetic variance explanation from common genes for the age at first birth (AFB) and the number of children ever born (NEB). (The genetic variance component is called heritability). Note: Adapted from Tropf et al. (2015)
GWAS (Genome-Wide Association Studies) and Polygenic Scores
GWAS
Since around 2006, Genome-Wide Association Studies (GWAS) emerged as a promising new approach to connect genetic variants to a phenotypical outcome of interest (Visscher and others, 2017). GWAS refers to hypothesis-free testing of genetic associations with outcomes of interest without any a priori assumptions about either the biological pathway or a particular location. It likewise embraces the fact that there are multiple genes (polygenic) and pathways associated with fertility that are difficult to specify in advance with our current state of knowledge. In GWA studies, we rapidly scan markers across the whole genome of many people (>100,000) to find genetic variations associated with a particular trait. GWAS are possible due to the completion of the Human Genome Project in 2003 and the International HapMap Project in 2005, which enable us to detect and measure genetic polymorphisms. As with other genetic data available until now, it is necessary to have the DNA from each participant in the study, often via a blood or saliva sample. Each person’s DNA is then placed on tiny microarray chips and scanned on automated laboratory machines. These machines quickly overview each person’s genome for strategically selected markers of genetic variation, referred to as SNPs (single nucleotide polymorphisms).
A GWAS therefore runs millions of separate regressions on the phenotype (outcome) of interest across the genome. Due to the large number of SNPs that are tested in GWASs, an association must achieve a stringent threshold of statistical significance (P < 5x10-8) in order to be considered as validated. A positive association refers to the case where there is a greater frequency of a genetic variant in individuals with that trait compared to those in the control group (i.e. absence of trait). The association identifies a genomic region and not a specific causative mutation that might be involved in the development of the trait or behaviour.
The computational GWAS approach remains promising for social science research due to the fact that it overcomes some of the mistakes inherent in candidate-gene studies in the past. But also, since complex fertility traits often evade the specification of a priori biological pathways, it remains a useful exploratory technique. It is also the only technique currently available that has the potential to discover novel genotype-phenotype associations, which could then be used in further, more reliable follow-up studies and test for indications of where researchers need to search and pursue potential biological pathways. It also allows population stratification to be controlled — to some extent — which, however, remains a key issue in avoiding bias and misinterpretation of results in this type of research (Wray and others, 2013 and Mills and others, 2022).
Previous GWAS discoveries successfully detected SNPs that are associated with reproduction. Over seventy GWASs have been published for thirty-two traits and diseases associated with reproduction (Montgomery and Zondervan, 2014). This includes identification of genes such as those related to age at menarche (Sulem and others, 2009; He and others, 2009; Elks and others, 2010), menopause (He and others, 2009; Snieder and others, 1998; Perry and others, 2013), and endometriosis (Painter and others, 2011). The first GWAS on reproductive behaviour isolates 12 loci for age at first birth (AFB) and number of children ever born (NEB) (Nicola Barban and others, 2016).. It engages in an analysis of sixty-two datasets with information from 238,064 men and women for age at first birth, and almost 330,000 men and women for the number of children. The study showed that DNA variants linked with the age at which people have their firstborn are also associated with other characteristics reflecting reproduction and sexual development, such as the age at menarche, voice-breaking in boys, and the age at which women experience menopause. Some of these genes were already known to influence infertility, while others had not yet been studied. The genes that were isolated also pointed to pathways and tissue types that were involved in human development, infertility, and sperm differentiation in men.
This was extended recently by two considerably larger studies on AFB (~540,000 individuals) and age at first sexual intercourse (~389,000) (Mills and others, 2021), as well as NEB (~717,000) and childlessness (~450,000) (Mathieson and others, Forthcomoing). Due to heavily increased sample sizes, these studies isolated almost one hundred and three hundred loci for AFB and AFS, and forty-three new loci for NEB and childlessness. A stunning finding was that — linking the contemporary findings to ancient genome data — that the FADS1/2 locus has been under natural selection for over 10,000 years and appears still to be so today.
Polygenic Scores (PGS)
Since reproduction is a complex behavioural outcome, it is not simply one candidate gene that can be used to predict outcomes. Rather, it is often a myriad of genetic loci compiled into a comprehensive polygenic score (PGS), which has been explored in detail in relation to the previous AFB and NEB GWAS (Nicola Barban and others, 2016) in another paper (Mills and others, 2018). We now summarize these results here. A polygenic score is a linear combination of the effects of genetic variants present across the whole genome and can be interpreted as a single quantitative measure of genetic predisposition. Just as a battery of multiple questions on personality types or attitudes towards immigration can make up a scale that is measured by one index, a PGS assumes that individuals fall somewhere on a continuum of genetic predisposition resulting from small individual contributions from many genetic variants.
How does a PGS calculated from the previously mentioned GWAS of AFB and NEB (Barban and others, 2016) work? To examine PGSs of AFB and avoid false positives from examining the associations in a limited dataset, results were tested and replicated across four different datasets: HRS (United States), LifeLines (Netherlands), TwinsUK, and STR (Sweden). Using OLS models this study carefully examined results from the large scale GWAS on reproductive behaviour by Barban et al. (2016) and found that the PGS for AFB explains around 1% of the variance (for women) in AFB and around 0.2% for NEB. While these numbers seem small, in some cases when the variants are combined, they can explain 9% of the probability of women remaining childless or six months of the delay in AFB per standard deviation (SD). Using a Cox model that accounts for right-censoring in the AFB, 1 SD in the AFB PGS is associated with a reduction of around 8% in the hazard ratio of reproduction for women and 3% for men. The PGS of NEB is associated with a 1 SD increase in the PGS, decreasing the probability of remaining childless by 9% in women. Importantly, with the increased sample sizes in the more recent discovery studies (Mills and others, 2021), GWAS-derived PGS explain already up to 6% of the variance in reproductive traits, which is expected to further increase with ever-growing sample sizes.
The genetic tendency to have a later AFB is also linked to an overall shifting of the reproductive period, linked with both a later onset of menarche and menopause (Mostafavi and others, 2017). As with other studies that have used PGSs from GWAS discoveries on complex behavioural traits such as educational level, a certain amount of reflection is in order. The most recent meta-GWAS, which finds seventy-four significant hits for educational, explains around 3.2% of the observed variance (Okbay and others, 2016). We therefore turn to additional reasons for this variation now.
How Do Genetic Effects Vary Across Populations or the Sexes?
Birth Cohort and Country Variation
GWA studies often combine genetic data from individuals from different countries and historical time periods in order to gain a large enough sample size. By doing this they assume that the influence of genes on individuals is universal across time and place. As the review in this chapter until now has illustrated, previous twin studies estimated AFB and NEB to be around 30% heritable with GREML estimates suggesting that genetic differences should be able to explain around 10–15% of the differences in fertility between individuals in a population. However, large GWA studies, which aimed to uncover the specific genes that are related to fertility and other complex traits, have produced much lower estimates.
A recent study (Tropf and others, 2017) demonstrated that this may be attributed to the fact that GWAS methods rely highly on data from different countries and historical periods, which potentially “hides” heritability because combining these data sets could mask large differences. In other words, if the genes that are important for fertility differ across countries, birth cohort or historical periods, it may be difficult to detect genetic variants when combining data from diverse populations. Using data from six countries (Australia, Estonia, Netherlands, Sweden, the UK and the US; overall 35,062 men and women) and several historical periods, the study found that 40% of genetic effects on education and timing of fertility (i.e. age when someone has her or his first child) are being “hidden” or “watered down” when data across populations in different countries and time periods are combined. For the number of children, this value increases up to 75%. In contrast, physical traits such as height are not impacted. The genes connected with height thus seem to be the same across populations.
Next to rare variants and insufficient sample size, GWAS discoveries might therefore be limited by heterogeneity across cohorts and birth cohorts under study (Tropf and others, 2017). Heterogeneity can arise on the phenotypic level if the phenotypic measurement differs across cohorts and birth cohorts, on the genotypic level if linkage disequilibrium differs across populations under study and by gene-environment interaction. The predictive power of the whole-genome methods increases up to fivefold when taking heterogeneity across cohorts and birth cohorts into account (Tropf and others, 2017). Given that fertility is largely environmentally determined and modified, it is likely that gene-environment interactions are important across the many cohorts included in GWAS discoveries as well as across birth cohorts. This in turn may be one reason for the comparably small predictive power of the polygenic scores. Combining data sets from vastly different countries and historical periods could be muddying the waters.
Sex Differences
Another aspect that deserves further attention in future research is sex differences in fertility and reproductive outcomes. This seems obvious since there are sex differences in biological makeup, in processes and diseases implicated in infertility and in behaviour. For women, ovulatory problems, tubal damage, endometriosis, cervix cancer and polycystic ovary syndrome are prominent causes of infertility, with sperm defects and testis cancer being central factors for men (Blundell, 2007). As we have seen from previous sections, these diseases are partly heritable. But there is also a behavioural component, since genes are implicated in different ways in relation to fertility and certain personality traits, including sociability, impulsivity and emotionality (Briley and others, 2017). These traits, which may have different effects on male and female fertility, have been shown to be heritable (Robinson and others, 2008).
Almost identical results in heritability estimates for men and women (Rodgers and others, 2001) might suggest that the same genes are important for male and female fertility. However, a study by Nisén et al. (Nisén and others, 2013), for example, shows that genes predicting childlessness in women are associated with low education among women and high education among men. Therefore, the genetic architecture of fertility might differ considerably between the sexes. Verweij and colleagues (Verweij and others, 2017) tested whether genetic loci operated differently in male and female fertility in the form of sexual dimorphism. Sexual dimorphism, or in other words, differences in secondary sex characteristics, can result in intralocus sexual conflicts, when genes that increase male fertility decrease female fertility, and vice versa. Using Swedish data, Verweij et al. (Verweij and others, 2017) estimated twin, GREML and AFB-PGS models on childlessness. They found that variation in individual differences in childlessness was explained by around 47% in the twin model and 59% (women) / 56% (men) in a GREML sibling model while the genetic correlation across sexes was significantly lower than 1. Using the PGS of AFB they also found significantly higher odds of remaining childless — however, only for women. The study concluded that partly different sets of genes influenced childlessness in men and women.
Discussion and Conclusion
The aim of this chapter was to provide an up-to-date review and comprehensive overview of research on reproductive behaviour in the area of fertility and genetics. We first emphasized that when working in an interdisciplinary area such as fertility or reproduction, it is important to be cognizant of the varying terminology used across the disciplines. Genetic research on fertility behaviour also revived interest in understanding contemporary natural selection and evolution, since reproductive success codetermines the successful transmission of genes of an individual to the next generation. The link with fertility and genetics also has a dark history in early misguided work in eugenics, which we firmly condemn and distance from the more serious scientific research reviewed here.
Genetic Approaches to Fertility
Our review examined first behavioural genetic (twin model) approaches to fertility, followed by the more recent growth in molecular genetic approaches. We summarized how twin studies have demonstrated that the age at first birth (AFB) is around 0–35% heritable compared to around a 24–43% heritability of number of children ever born (NEB).
Molecular genetic studies, which use data from the whole human genome, first started with candidate gene studies, which proved difficult to replicate. This was then followed by GREML (Genomic-Relationship-Matrix based Restricted Maximum Likelihood) techniques that allowed researchers to produce more direct estimates of the proportion of phenotypic variance explained by genetic variance across the entire genome for unrelated individuals. Studies predicated that AFB and NEB were 10% and 15% explained by genetic variance in common genes, respectively.
Describing the percentage age of variation in genetic differences, however, does not uncover the actual genes or their biological functions. For this reason, researchers have turned to GWA studies. This is hypothesis-free testing across the genome to find associations with a particular fertility trait in order to isolate key genetic loci. We summarized results of previous studies, isolating hundreds of genetic loci for AFB, age at first sexual intercourse, NEB, and childlessness that were linked with human development, infertility and sperm differentiation. We then explained how these discoveries of genetic loci from GWAS can be compiled into a single polygenic score (PGS), which predicted up to 6% of the fertility outcomes. We also learned that the PGS for AFB and NEB are relatively strong predictors of the probability to remain childless (e.g. by 9% in women). Highlighting the evidence of ongoing natural selection in humans, we wish to emphasize that the relationship between fertility tempo, quantum and fitness and the derived evolutionary consequences are often still simplified. Future research should aim to integrate in-depth evolutionary demographic knowledge (Mcgraw and Caswell, 1996; Jones and Bird, 2014) with ongoing advances in molecular and quantitative genetics research (Barban and others, 2016; Tropf and others, 2015b; Guo and others, 2018).
Towards Gene x Environment Interaction
We acknowledged that reproductive behaviour is not only genetic but largely shaped by individual-level factors, but also family (meso-) and societal-level (macro-) forces, which we reviewed. We noted that until now, the majority of demographic and sociological research on this topic has focussed almost primarily on these aspects. We likewise acknowledged that they explain the majority of these complex outcomes and will continue to do so. Promising new approaches, however, should focus on how reproductive PGSs interact with these socio-environmental characteristics. It may be, for instance, that fertility issues arising from genetic predispositions for reproductive health problems, such as endometriosis or sperm defects, are more relevant in populations with higher average age at childbirth than in populations with younger ages at childbirth. Or, someone might be more genetically “hard-wired” to have more children, but if they come of age in an economic recession or work in precarious jobs, this might be more important than these predispositions. Likewise, it remains important to understand the physiological and psychological mechanisms, how genes influence fertility behaviour and outcomes. We anticipate that it is not the socio-environmental or the genetic predictors that will uncover fundamentally new findings, but rather a combined sociogenomic approach.
References1
Balbo, N., and N. Barban. 2014. ‘Does Fertility Behavior Spread among Friends?’, American Sociological Review, 79.3: pp. 412–31, https://doi.org/10.1177/0003122414531596
Balbo, Nicoletta, Francesco C. Billari, and Melinda Mills. 2013. ‘Fertility in Advanced Societies: A Review of Research’, European Journal of Population / Revue Européenne de Démographie, 29.1: pp. 1–38, https://doi.org/10.1007/s10680-012-9277-y
Balbo, Nicoletta, and Melinda Mills. 2011. ‘The Effects of Social Capital and Social Pressure on the Intention to Have a Second or Third Child in France, Germany, and Bulgaria, 2004–05’, Population Studies, 65.3: pp. 335–51, https://doi.org/10.1080/00324728.2011.579148
Barban, N., R. Jansen, R. De Vlaming, A. Vaez, J. J. Mandemakers, and others. 2016. ‘Genome-Wide Analysis Identifies 12 Loci Influencing Human Reproductive Behavior’, Nature Genetics, 48.12, https://doi.org/10.1038/ng.3698
Barban, Nicola, Rick Jansen, Ronald De Vlaming, Ahmad Vaez, Jornt J. Mandemakers, and others. 2016. ‘Genome-Wide Analysis Identifies 12 Loci Influencing Human Reproductive Behavior’, Nature Genetics, 48.12: pp. 1–7, https://doi.org/10.1038/ng.3698
Beauchamp, Jonathan P. 2016. ‘Genetic Evidence for Natural Selection in Humans in the Contemporary United States’, Proc Natl Acad Sci US A, 113.28: pp. 7774–79, https://doi.org/10.1073/pnas.1600398113
Becker, Gary Stanley. 2009. A Treatise on the Family (Cambridge, Massachusetts: Harvard university press).
Billari, F., and H-P. Kohler. 2002. ‘The Impact of Union Formation Dynamics on First Births in West Germany and Italy: Are There Signs of Convergence?’, Dynamics of Fertility and Partnership in Europe: Insights and Lessons from Comparative Research, 2: pp. 43–58.
Blundell, Renald. 2007 ‘Causes of Infertility’, International Journal of Molecular Medicine in Advance Sciences, 3.1: pp. 63–65. https://www.um.edu.mt/library/oar/bitstream/123456789/22304/1/Causes%20of%20Infertility.pdf
Bongaarts, J. 1978. ‘A Framework for Analyzing the Proximate Determinants of Fertility’, Population and Development Review: pp. 105–32, https://doi.org/10.2307/1972149
Bongaarts, J., and G. Feeney. 2000. ‘On the Quantum and Tempo of Fertility: Reply’, Population and Development Review, 26.3: pp. 560–64, https://doi.org/10.1111/j.1728-4457.2000.00560.x
Briley, Daniel A., F. C. Tropf, and Melinda C. Mills. 2017. ‘What Explains the Heritability of Completed Fertility? Evidence from Two Large Twin Studies’, Behavior Genetics, 47.1: pp. 36–51, https://doi.org/10.1007/s10519-016-9805-3
Byars, Sean G., Douglas Ewbank, Diddahally R. Govindaraju, and Stephen C. Stearns. 2010. ‘Natural Selection in a Contemporary Human Population’, Proceedings of the National Academy of Sciences, 107.suppl 1: pp. 1787–92, https://doi.org/10.1073/pnas.0906199106
Courtiol, A., F. C. Tropf, and M. C. Mills. 2016. ‘When Genes and Environment Disagree: Making Sense of Trends in Recent Human Evolution’, Proc Natl Acad Sci US A, 113.28: pp. 7693–95, https://doi.org/10.1073/pnas.1608532113
Daw, J., and G. Guo. 2011. ‘The Influence of Three Genes on Whether Adolescents Use Contraception, USA 1994–2002’, Population Studies, 65.3: pp. 253–71, https://doi.org/10.1080/00324728.2011.598942
Easterlin, R. A. 1976. ‘The Conflict between Aspirations and Resources’, Population and Development Review, 2.3/4: pp. 417–25, https://doi.org/10.2307/1971619
Elks, C. E., J. R. B. Perry, P. Sulem, D. Chasman, N. Franscechini, and others. 2010. ‘Thirty New Loci for Age at Menarche Identified by a Meta-Analysis of Genome-Wide Association Studies’, Nature Genetics, 42.12: pp. 1077–85, https://doi.org/10.1038/ng.714
Fisher, R. A. 1930. The Genetical Theory of Natural Selection (Oxford: Clarendon Press).
Freese, J. 2008. ‘Genetics and the Social Science Explanation of Individual Outcomes’, American Journal of Sociology, 114.S1: pp. S1–S35, https://doi.org/10.1086/592208
Guo, G., Y. Tong, and T. Cai. 2008. ‘Gene by Social Context Interactions for Number of Sexual Partners Among White Male Youths: Genetics-Informed Sociology’, AJS; American Journal of Sociology, 114.S1: p. S36, https://doi.org/10.1086/592207
Guo, Jing, Jian Yang, and Peter M. Visscher. 2018. ‘Leveraging GWAS for Complex Traits to Detect Signatures of Natural Selection in Humans’, Current Opinion in Genetics and Development, 53: pp. 9–14, https://doi.org/10.1016/j.gde.2018.05.012
Halpern, C.T. K. Joyner, J. R. Udry, and C. Suchindran. 2000. ‘Smart Teens Don’t Have Sex (or Kiss Much Either)’, Journal of Adolescent Health, 26.3: pp. 213–25, https://doi.org/10.1016/S1054-139X(99)00061-0
He, Chunyan, Peter Kraft, Constance Chen, Julie E Buring, Guillaume Paré, and others. 2009. ‘Genome-Wide Association Studies Identify Loci Associated with Age at Menarche and Age at Natural Menopause’, Nature Genetics, 41.6: pp. 724–28, https://doi.org/10.1038/ng.385
Ioannidis, J. P. A. 2005. ‘Why Most Published Research Findings Are False’, Chance, 18.4: pp. 40–47, https://doi.org/10.1080/09332480.2005.10722754
Joffe, M. 2010. ‘What Has Happened to Human Fertility?’, Human Reproduction, 25.2: pp. 295–307, https://doi.org/10.1093/humrep/dep390
Jones, James Holland, and Rebecca Bliege Bird. 2014. ‘The Marginal Valuation of Fertility’, Evolution and Human Behavior, 35.1: pp. 65–71, https://doi.org/10.1016/j.evolhumbehav.2013.10.002
Kahn, J. R., and K. E. Anderson. 1992. ‘Intergenerational Patterns of Teenage Fertility’, Demography, 29.1: pp. 39–57, https://doi.org/10.2307/2061362
Kim, Yuri, and James J. Lee. 2019. ‘The Genetics of Human Fertility’, Current Opinion in Psychology, 27: pp. 41–45, https://doi.org/10.1016/j.copsyc.2018.07.011
Kirk, K. M., S. P. Blomberg, D. L. Duffy, A. C. Heath, I. P. F. Owens, and others. 2001. ‘Natural Selection and Quantitative Genetics of Life‐history Traits in Western Women: A Twin Study’, Evolution, 55.2: pp. 423–35, https://doi.org/10.1554/0014-3820(2001)055[0423:NSAQGO]2.0.CO;2
Kohler, H-P., and J. L. Rodgers. 2003. ‘Education, Fertility and Heritability: Explaining a Paradox’, Offspring: Human Fertility Behavior in Biodemographic Perspective: pp. 46–90. https://doi.org/10.17226/10654
Kohler, H-P., J. L. Rodgers, and K. Christensen. 1999. ‘Is Fertility Behavior in Our Genes? Findings from a Danish Twin Study’, Population and Development Review, 25.2: pp. 253–88, https://doi.org/10.1111/j.1728-4457.1999.00253.x
Kong, A. et al. 2017. ‘Selection against Variants in the Genome Associated with Educational Attainment’, Proceedings of the National Academy of Science, 114.5: pp. E727–32, https://doi.org/10.1073/pnas.1612113114
Lesthaeghe, Ron. 1995. ‘The Second Demographic Transition in Western Countries: An Interpretation’, Gender and Family Change in Industrialized Countries: pp. 17–62.
Levine, P., and A. Bashford. 2010. ‘Introduction: Eugenics and the Modern World’, in The Oxford Handbook of the History of Eugenics, ed. Alison Bashford, and Philippa Levine (Oxford: Oxford University Press), https://doi.org/10.1093/oxfordhb/9780195373141.001.0001
Mathieson, Iain, Felix R. Day, and others. Forthcoming. ‘Genome-wide analysis identifies genetic effects on reproductive success and ongoing natural selection at the FADS locus’, Nature Human Behaviour.
Mcgraw, James B., and Hal Caswell. 1996. ‘Estimation of Individual Fitness from Life-History Data’, The American Naturalist, 147.1, https://doi.org/10.1086/285839
Mills, M. C., N. Barban, and F. C. Tropf. 2018. ‘The Sociogenomics of Polygenic Scores of Reproductive Behavior and Their Relationship to Other Fertility Traits’, RSF: The Russell Sage Foundation Journal of the Social Sciences, 4.4, https://doi.org/DOI: 10.7758/RSF.2018.4.4.07
Mills, M.C., F.C. Tropf, and others. 2021. ‘Large-Scale Genome-Wide Association Study Identifies 370 Loci for Onset of Sexual and Reproductive Behaviour Implicated with Psychiatric Disorders, Infertility and Longevity’, Nature Human Behaviour, 5.12: pp. 1717–30.
Mills, M. C., and H. P. Blossfeld. 2005. ‘Globalisation, Uncertainty and the Early Life Course: A Theoretical Framework’, in Globalisation, Uncertainty and Youth in Society, ed. by H. P. Blossfeld, E. Klijzing, M. Mills, & K. Kurz (London, New York: Routledge), pp. 1–24.
Mills, M. C., H. P. Blossfeld, and E. Klijzing. 2005. ‘Becoming an Adult in Uncertain Times: A 14th Century Comparison of the Losers of Globalization’, in Globalization, Uncertainty and Youth in Society (London, New York: Routledge).
Mills, M. C., & Mathieson, I. (2022). The challenge of detecting recent natural selection in human populations. Proceedings of the National Academy of Sciences, 119.15: e2203237119. https://doi.org/10.1073/pnas.2203237119
Mills, M. C., Ronald R. Rindfuss, Peter McDonald, and Egbert te Velde. 2011. ‘Why Do People Postpone Parenthood? Reasons and Social Policy Incentives’, Human Reproduction Update, 17.6: pp. 848–60, https://doi.org/10.1093/humupd/dmr026
Mills, M. C., and F. C. Tropf. 2016. ‘The Biodemography of Fertility: A Review and Future Research Frontiers’, Kölner Zeitschrift Für Soziologie Und Sozialpsychologie, 67 (Special Issues: Demography – Forschung an der Schnittstelle von Soziologie und Demografie): pp. 397–424, https://doi.org/10.1007/s11577-015-0319-4
Mills, Melinda, Letizia Mencarini, Maria Letizia Tanturri, and Katia Begall. 2008. ‘Gender Equity and Fertility Intentions in Italy and the Netherlands’, Demographic Research, 18.1: pp. 1–26, https://doi.org/10.4054/demres.2008.18.1
Milot, E., F. M. Mayer, D. H. Nussey, M Boisvert, F Pelletier, and others. 2011. ‘Evidence for Evolution in Response to Natural Selection in a Contemporary Human Population’, Proceedings of the National Academy of Sciences, 108.41: pp. 17040–45, https://doi.org/10.1073/pnas.1104210108
Montgomery, G. W., K. T. Zondervan, and others. 2014. ‘The Future for Genetic Studies in Reproduction’, Molecular Human Reproduction, 20.1: pp. 1–14, https://doi.org/10.1093/molehr/gat058
Mostafavi, Hakhamanesh, Tomaz Berisa, Felix R. Day, John R. B. Perry, Molly Przeworski, and others. 2017. ‘Identifying Genetic Variants That Affect Viability in Large Cohorts’, PLOS Biology, 15.9, ed. by Nick Barton: p. e2002458, https://doi.org/10.1371/journal.pbio.2002458
Murphy, M., and D. Wang. 2001. ‘Family-Level Continuities in Childbearing in Low-Fertility Societies’, European Journal of Population, 17.1: pp. 75–96, https://doi.org/10.1023/A:1010744314362
Neiss, Michelle, David C. Rowe, and J. L. Rodgers. 2002. ‘Does Education Mediate the Relationship between IQ and Age of First Birth? A Behavioural Genetic Analysis’, Journal of Biosocial Science, 34.2: pp. 259–75, https://doi.org/10.1017/S0021932002002596
Nisén, Jessica, Pekka Martikainen, Jaakko Kaprio, and Karri Silventoinen. 2013. ‘Educational Differences in Completed Fertility: A Behavioral Genetic Study of Finnish Male and Female Twins’, Demography, 50.4: pp. 1399–420, https://doi.org/10.1007/s13524-012-0186-9
Nolte, Ilja Maria, Felix Christian Tropf, and Harold Snieder. 2019. ‘Missing Heritability of Complex Traits and Diseases’, ELS, pp. 1–9, https://doi.org/10.1002/9780470015902.a0028223
Okbay, Aysu, Jonathan P. Beauchamp, Mark Alan Fontana, James J. Lee, Tune H. Pers, and others. 2016. ‘Genome-Wide Association Study Identifies 74 Loci Associated with Educational Attainment’, Nature (AAAS), 10: pp. 1467–71, https://doi.org/10.1038/nature17671
Painter, Jodie N., Carl A. Anderson, Dale R. Nyholt, Stuart Macgregor, Jianghai Lin, and others. 2011. ‘Genome-Wide Association Study Identifies a Locus at 7p15.2 Associated with Endometriosis’, Nature Genetics, 43.1: pp. 51–54, https://doi.org/10.1038/ng.731
Perry, J. R. B., T. Corre, and T. Esko, and others. 2013. ‘A Genome-Wide Association Study of Early Menopause and the Combined Impact of Identified Variants’, Human Molecular Genetics, 22.7: pp. 1465–72, https://doi.org/10.1093/hmg/dds551
Polderman, Tinca J. C., Beben Benyamin, Christiaan A de Leeuw, Patrick F Sullivan, Arjen van Bochoven, and others. 2015. ‘Meta-Analysis of the Heritability of Human Traits Based on Fifty Years of Twin Studies’, Nature Genetics, 47.7: pp. 702–9, https://doi.org/10.1038/ng.3285
Rijken, Arieke J., and Aart C. Liefbroer. 2009. ‘Influences of the Family of Origin on the Timing and Quantum of Fertility in the Netherlands’, Population Studies, 63.1: pp. 71–85, https://doi.org/10.1080/00324720802621575
Rindfuss, Ronald R., Vandenheuvel, A. 1990. ‘Cohabitation: A Precursor to Marriage or an Alternative to Being Single?’, Population and Development Review, 16.4: pp. 703–26, https://doi.org/10.2307/1972963
Robinson, Gene E., Russell D. Fernald, and David F. Clayton. 2008. ‘Genes and Social Behavior’, Science, 322.5903: pp. 896–900, https://doi.org/10.1126/science.1159277
Rodgers, J. L., and H-P. Kohler. 2012. The Biodemography of Human Reproduction and Fertility.
Rodgers, J. L., H. P. Kohler, K O. Kyvik, and K. Christensen. 2001. ‘Behavior Genetic Modeling of Human Fertility: Findings from a Contemporary Danish Twin Study’, Demography, 38.1: pp. 29–42, https://doi.org/10.1353/dem.2001.0009
Rodgers, J. L., H. P. Kohler, M. McGue, J. R. Behrman, I. Petersen, and others. 2008. ‘Education and Cognitive Ability as Direct, Mediating, or Spurious Influences on Female Age at First Birth: Behavior Genetic Models Fit to Danish Twin Data’, American Journal of Sociology, 114.S1: p.S202, https://doi.org/10.1086/592205
Rodgers, J. L., D. C. Rowe, and M. Buster. 1999. ‘Nature, Nurture and First Sexual Intercourse in the USA: Fitting Behavioural Genetic Models to NLSY Kinship Data’, Journal of Biosocial Science, 31.1: pp. 29–41, https://doi.org/10.1017/S0021932099000292
Sanjak, Jaleal S., Julia Sidorenko, Matthew R. Robinson, Kevin R. Thornton, and Peter M. Visscher. 2017. ‘Evidence of Directional and Stabilizing Selection in Contemporary Humans’, Proceedings of the National Academy of Sciences of the United States of America, 115.1: pp. 151–56, https://doi.org/10.1073/pnas.1707227114
Snieder, H., A. J. MacGregor, and T. D. Spector. 1998. ‘Genes Control the Cessation of a Woman’s Reproductive Life: A Twin Study of Hysterectomy and Age at Menopause’, Journal of Clinical Endocrinology & Metabolism, 83.6: pp. 1875–80, https://doi.org/10.1210/jcem.83.6.4890
Snieder, H., Xiaoling Wang, and A. J. MacGregor. 2010. ‘Twin Methodology’, in Encyclopedia of Life Sciences, ed. by Ltd JohnWiley & Sons (Chichester: John Wiley & Sons), https://doi.org/10.1002/9780470015902.a0005421.pub2
Sobotka, Tomáš, Skirbekk, V., Philipov, D. 2011. ‘Economic Recession and Fertility in the Developed World’, Population and Development Review, 37.2: pp. 267–306, https://doi.org/10.1111/j.1728-4457.2011.00411.x
Sobotka, Tomáš. 2004. ‘Is Lowest‐Low Fertility in Europe Explained by the Postponement of Childbearing?’, Population and Development Review, 30.2: pp. 195–220, https://doi.org/10.1111/j.1728-4457.2004.010_1.x
Stearns, Stephen C., Sean G. Byars, Diddahally R. Govindaraju, and Douglas Ewbank. 2010. ‘Measuring Selection in Contemporary Human Populations’, Nature Reviews Genetics, 11.9: pp. 611–22, https://doi.org/10.1038/nrg2831
Stulp, G., L. Barrett, F. C. Tropf, and M. C. Mills. 2015. ‘Does Natural Selection Favour Taller Stature among the Tallest People on Earth?’, Proceedings of the Royal Society of London B: Biological Sciences, 282.1806: p. 20150211, https://doi.org/10.1098/rspb.2015.0211
Sulem, Patrick, Daniel F. Gudbjartsson, Thorunn Rafnar, Hilma Holm, Elinborg J. Olafsdottir, and others. 2009. ‘Genome-Wide Association Study Identifies Sequence Variants on 6q21 Associated with Age at Menarche’, Nature Genetics, 41.6: pp. 734–38, https://doi.org/10.1038/ng.383
Tropf, F. C., and J. J. Mandemakers. 2017. ‘Is the Association Between Education and Fertility Postponement Causal? The Role of Family Background Factors’, Demography, 54.1: pp. 71–91, https://doi.org/10.1007/s13524-016-0531-5
Tropf, F. C., G Stulp, N Barban, PM Visscher, J Yang, and others. 2015. ‘Human Fertility, Molecular Genetics, and Natural Selection in Modern Societies’, PloS One, 10.6: p. e0126821, https://doi.org/10.1371/journal.pone.0126821
Tropf, F. C., Renske M. Verweij, Peter J van der Most, Gert Stulp, Andrew Bakshi, and others. 2017. ‘Hidden Heritability Due to Heterogeneity across Seven Populations’, Nature Human Behaviour, 1: pp. 757–65, https://doi.org/10.1038/s41562-017-0195-1
Tropf, F. C., N. Barban, M. C. Mills, Harold Snieder, and Jornt J. Mandemakers. 2015a. ‘Genetic Influence on Age at First Birth of Female Twins Born in the UK, 1919–68’, Population Studies, 69.2: pp. 129–45, https://doi.org/10.1080/00324728.2015.1056823
Tropf, F. C., G. Stulp, N. Barban, P. M. Visscher, J. Yang, and others. 2015b. ‘Human Fertility, Molecular Genetics, and Natural Selection in Modern Societies’, PloS One, 10.6: p. e0126821, https://doi.org/10.1371/journal.pone.0126821
Udry, J. R. 1996. ‘Biosocial Models of Low-Fertility Societies’, Population and Development Review, 22: pp. 325–36, https://doi.org/10.2307/2808017
Verweij, Renske M., Melinda C. Mills, Felix C. Tropf, René Veenstra, Anastasia Nyman, and others. 2017. ‘Sexual Dimorphism in the Genetic Influence on Human Childlessness’, European Journal of Human Genetics, 25.9: pp. 1067–74, https://doi.org/10.1038/ejhg.2017.105
Visscher, P. M., W. G. Hill, and N. R. Wray. 2008. ‘Heritability in the Genomics Era — Concepts and Misconceptions’, Nature Reviews Genetics, 9.4: pp. 255–66, https://doi.org/10.1038/nrg2322
Visscher, P. M., Naomi R. Wray, Qian Zhang, Pamela Sklar, Mark I. McCarthy, and others. 2017. ‘10 Years of GWAS Discovery: Biology, Function, and Translation’, The American Journal of Human Genetics, 101.1: pp. 5–22, https://doi.org/10.1016/j.ajhg.2017.06.005
Wachter, K. W. 2008. ‘Biodemography Comes of Age’, Demographic Research, 19: pp. 1501–12, https://doi.org/10.4054/demres.2008.19.40
Wachter, K. W. 2003. ‘Biodemography of Fertility and Family Formation’, in Offspring: Human Fertility Behavior in Biodemographic Perspective, ed. by K. W. Wachter, and R.A. Bulata (Washington: National Academies Press).
Wray, Naomi R, Jian Yang, Ben J. Hayes, Alkes L. Price, Michael E. Goddard, and others. 2013. ‘Pitfalls of Predicting Complex Traits from SNPs’, Nature Reviews. Genetics, 14.7: pp. 507–15, https://doi.org/10.1038/nrg3457
Yang, Jian, S. Hong Lee, Michael E. Goddard, and Peter M. Visscher. 2011. ‘GCTA: A Tool for Genome-Wide Complex Trait Analysis’, The American Journal of Human Genetics, 88.1: pp. 76–82, https://doi.org/10.1016/j.ajhg.2010.11.011
1 Note this chapter has been posted on the Open Science Framework website since 17/02/2021, after it was accepted for publication, so the references will reflect when the chapter was written and not the OBP publication date.
