27. The Impact of Social Dynamics on
Life History Trajectory and Demographic Traits: Insights from the “Producer-Scrounger” Game
© 2024 Jonathan Wells, CC BY 4.0 https://doi.org/10.11647/OBP.0251.27
Evolutionary demography applies models and theories from evolutionary biology to understand variability in fertility and mortality patterns. Many important ecological influences derive from the natural environment, such as the burden of infectious disease, or the availability of energy and other nutrients. However, human society is itself a source of diverse stimuli and stresses that may generate profound impacts on demographic traits. On this issue, much attention to date has focused on the benefits of social interaction, in particular “cooperative breeding” through which the costs of reproduction are shared among kin or others. In contrast, this chapter will use a simple model of social inequality, based on the ecological “producer-scrounger” game, to shed light on how social hierarchy, through the key medium of nutrition, can shape diversity in life history trajectories. Life history trade-offs shape both physiological and behavioural characteristics of individuals, which in turn affect both fertility and mortality profiles. In every society, it is ultimately through relationships embedded in the context of nutrition that different groups within social hierarchies interact. The key insight from the producer-scrounger game is that in social hierarchies, the life history strategies of producers and scroungers are structurally inter-related. This results in contrasting phenotypes and demographic outcomes between the two groups. Those lower in social hierarchies have higher risks, and fewer opportunities to acquire resources, and may adapt through trade-offs that favour immediate survival and reproduction over growth and long-term health maintenance. In contrast, those with priority access to resources may demonstrate trade-offs that favour growth and long-term health maintenance, leading to greater longevity, a lengthier reproductive career and higher quality offspring. These contrasting life history strategies may emerge through the direct control of subordinates by high-ranked individuals, or through indirect control over the resources that subordinates struggle to access. This simple conceptual approach can help understand both contemporary variability within and between populations in demographic traits, and also their historical divergence or convergence over time.
Introduction
Demography is the study of the size, structure, and distribution of populations, and the variability that they exhibit in association with patterns of births, migration, ageing, and deaths. Until recently, the discipline drew little insight from evolutionary theory, and instead focused on describing demographic patterns, and unravelling the proximate mechanisms that underlie variability in fertility, ageing and mortality. Such mechanisms include behaviours, cultural values and the hormonal regulation of reproductive function.
The sub-discipline of evolutionary demography emerged specifically to improve understanding of why individuals exhibit variability in demographic outcomes. Patterns of reproduction represent the consequences of a series of decisions (Sear, Lawson et al. 2016), some made consciously, others effectively “made by the body” through hormone-regulated mechanisms of physiological plasticity, through which traits relevant to reproduction or survival respond to ecological conditions (Wade, Schneider et al. 1996, Schneider 2004). Patterns of ageing, and hence mortality risk, can be considered through the same lens. Ultimately, these mechanisms are assumed to have been shaped by the action of natural selection on ancestral populations, so that both the pattern of producing offspring, and their characteristics (including reproductive potential and likely lifespan), have evolved to enhance the odds of parental genes passing to future generations.
It is well recognised that human reproduction is inherently social, perhaps best expressed in the phrase that “it takes a village” to raise children (Hrdy 2009). In recent decades, many researchers have considered how cooperative behaviours can help spread the various costs of reproduction over a larger pool of individuals, often kin, thereby reducing the pressures facing individual mothers. For example, several studies have evaluated potential support from grandmothers, as discussed in more detail below.
However, less attention has been paid to the impact of human relationships that are far from cooperative, and might therefore pose challenges for survival and reproduction. Some work has focused on the absence of individuals such as fathers who, if present, would likely have been beneficial for their offspring (Webster, Graber et al. 2014, Sear, Sheppard et al. 2019). This approach still assumes that fathers generally contribute beneficially to the development of their offspring. Others have considered whether parents-in-law, who do not share genes with the mother, may prioritise paternal interests at the expense of maternal outcomes (Leonetti, Nath et al. 2008).
Overall, aspects of the social environment that may prove unsupportive of women’s reproduction remain relatively unexplored, and there is no over-arching framework through which different types of antagonistic relationships may be investigated. In this chapter, I aim to provide such a framework, by showing how hierarchical relations can affect biological and behavioural traits with relevance to demographic outcomes. I will consider two types of hierarchical relations: those within a social group, and those between social groups (whether defined at the level of class or caste, ethnicity or nationality). In order to integrate my approach with the work of others, however, I begin by reviewing several theoretical approaches used in evolutionary demography.
Theoretical Perspectives in Evolutionary Demography
Evolutionary demography draws heavily on life history theory (Stearns 1992, Hill 1993), which assumes that energy is a limited resource for every organism, and must be allocated across four competing functions (maintenance, growth, reproduction, and defence against pathogens or predators). Allocation “decisions” or trade-offs between these functions are the means through which individuals can respond to stimuli or stresses to maximise fitness. A key principle of evolutionary medicine is that natural selection shapes organisms to maximise survival and fitness, at potential costs to health or longevity (Nesse and Williams 1994). Accordingly, allocation trade-offs between maintenance and reproduction shape both longevity and fertility, and these trade-offs intensify in harsh conditions.
Using this framework, several important issues have been addressed. For example, fertility and mortality patterns have a complex connection. Reproduction is metabolically costly for the mother and may accelerate the physiological rate of aging, potentially shortening her lifespan (Westendorp and Kirkwood 1998, Penn and Smith 2007). However, greater investment in reproduction may either increase or decrease the risk of specific maternal diseases, depending on the underlying physiological mechanisms (Jasienska 2017). Counterbalancing such risks is the possibility that adult offspring might eventually care for their parents in old age, prolonging the lifespan of those who have reproduced. Overall, these inter-generational trade-offs vary in association with ecological and social conditions.
Another key issue is that kin may cooperate to share the costs of reproduction (Hrdy 2009). “Pooled energy budgets” help distribute costs that would otherwise fall entirely on the mother (Kramer and Elison 2010), thereby reducing the magnitude of inter-generational trade-offs. Grandmothers may be particularly important in this context, both reducing child mortality rates (Sear and Mace 2008) and promoting child growth (Gibson and Mace 2005, Meehan, Helfrecht et al. 2014), though in general this applies more to maternal than to paternal grandmothers (Sear and Mace 2008). The notion that human reproduction is typically a “cooperative enterprise” is now well established, and this strategy may have been an important factor favouring both encephalization and greater longevity in the genus Homo, though different explanatory theories have been presented (Isler and van Shaik 2012, Wells 2012).
Another approach rapidly gaining momentum is the “developmental origins of adult health and disease” (DOHaD) hypothesis (Barker 2004). This focuses on how ecological exposures early in the life-course shape the quality of adult phenotype. Pioneering work showed that early growth patterns predict the risk of conditions such as diabetes and cardiovascular disease in adulthood, thus contributing to variability in longevity (Barker 1992). This approach can be broadened to encompass other demographic outcomes, including maturation rate, fertility, migration and the rate of aging.
For example, a high infectious disease burden during infancy is associated with delayed menarche, indicating reduced availability of energy for early growth (Ellison 1981). In chronically under-nourished Indian populations, growth continues past the age of 20 years (Satyanarayana, Nadamuni et al. 1981), yet Indians still remain one of the shortest populations globally (N. C. D. Risk Factor Collaboration 2016). In this scenario, growth proceeds slowly and ceases late in terms of “time on the clock” but early in terms of the “final size attained”. In settings with high vaccination rates and low infection risk, however, rapid infant weight gain is associated with earlier menarche (Dunger, Ahmed et al. 2006, Ong, Emmett et al. 2009), while high mortality risk can also favour earlier maturation provided that there is adequate energy for growth (Walker, Gurven et al. 2006). In this scenario, growth ceases early in terms of the “time on the clock”. Both of these scenarios are nonetheless consistent with life history theory, by showing that life history trajectory is sensitive to cues of both energy supply and mortality risk (Figure 1).
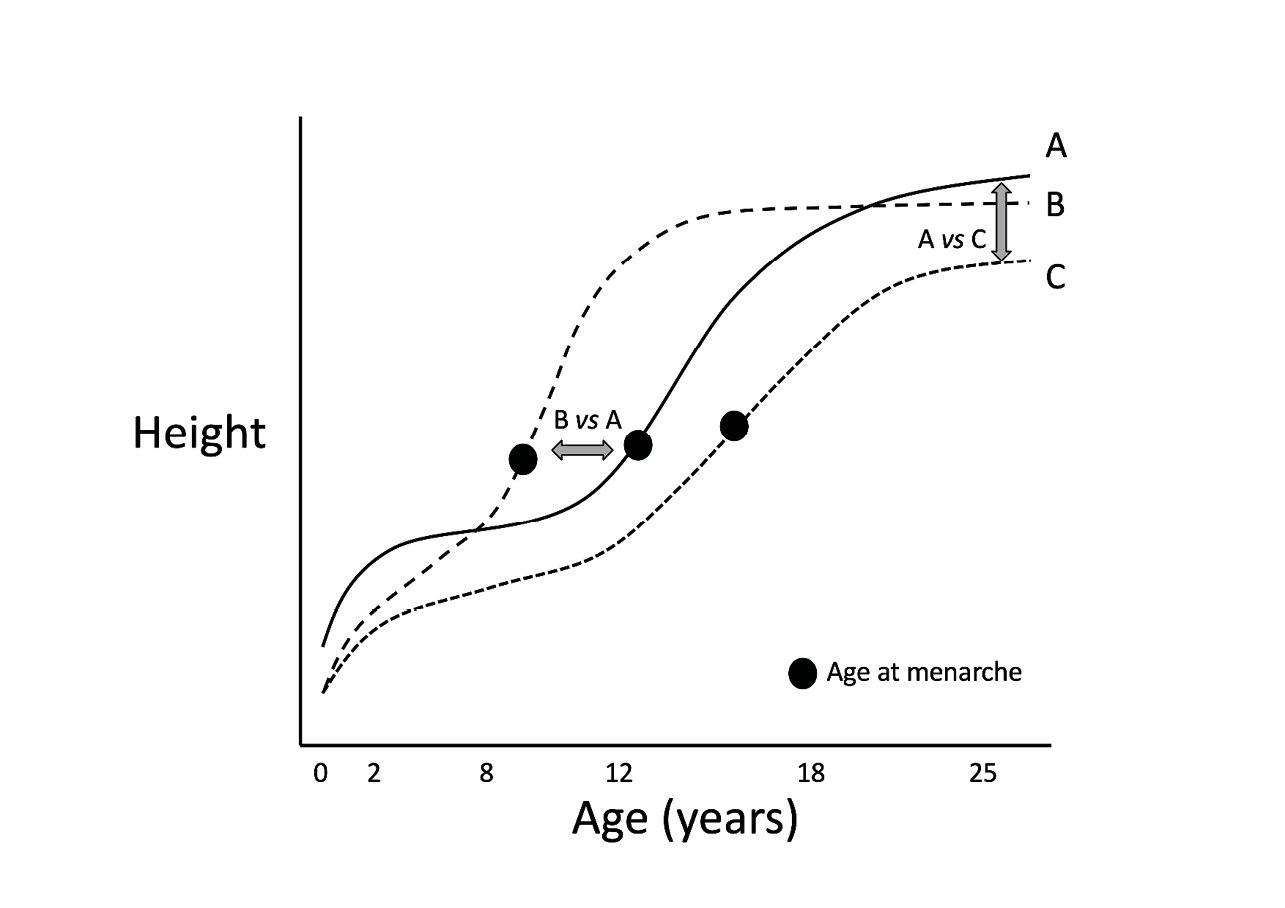
Fig. 1 Schematic diagram, illustrating three contrasting patterns whereby developmental trajectory response to ecological cues. Relative to a healthy growth trajectory in a benign environment (A), high mortality risk, in association with adequate energy supply, favours earlier maturation (B), whereas inadequate energy supply, in the absence of high mortality risk, can favour delayed maturation (C). The arrows highlight contrasts between individuals on these trajectories on one axis, while showing similar values on the other axis.
The “DOHaD” approach can be extended to an inter-generational time-frame, as variability in developmental trajectory is associated with parental nutritional status (Barker 1992, Monaghan 2008). Below, I discuss how these insights can be developed further, through the lens of “maternal capital”, allowing me to develop a new perspective on the association between demographic traits and social hierarchy.
The Maternal Capital Model
Mothers maximise their fitness by allocating some of their resources to their offspring, a scenario known as “parental investment” (Trivers 1972). Consequently, life history trade-offs in each generation are initially shaped by many components of maternal behaviour and biology, that determine the level and timing of investment (Wells 2019) Each offspring passes through a succession of “critical periods” during development, and a given level of maternal investment during foetal life may have different effects on the offspring, compared to the same level of investment at a later stage of development (Wells 2018).
The concept of “embodied capital” provides a broad framework with which to investigate how individuals accumulate diverse fitness-enhancing characteristics through the life-course (Kaplan, Lancaster et al. 2003). Building on this approach, I defined maternal capital as “any aspect of maternal phenotype, whether somatic or behavioural, which enables differential investment in offspring” (Wells 2010). This approach was developed to help integrate evolutionary approaches with the DOHaD hypothesis, by emphasising maternal phenotype as the key niche experienced by the offspring during its most plastic period of development, the “first thousand days of life”.
Much attention has focused on physical components of maternal capital, such as energy stores, body size, micronutrient status, and the burden of infections. Across countries, for example, maternal height is inversely associated with the risk of stunting, underweight and early mortality in the offspring (Ozaltin, Hill et al. 2010). Many other components of maternal nutritional or physiological status show similar associations (Wells 2010).
However, other types of resource are also relevant, though they may all ultimately impact the offspring via nutritional pathways. For example, education in its broadest sense (social learning) can enhance parenting success, and can help improve the success of reproductive behaviours such as lactation, which in humans is not instinctive (Wells 2006). Social capital may be equally important, ameliorating the costs of producing and nurturing offspring as discussed above. Beyond grandmothers, other beneficial groups include siblings who may act as “helpers at the nest”, whereas the benefits of fathers for child survival are more variable (Sear and Mace 2008). In Ethiopia, support from maternal grandmothers was associated with better growth of grandchildren through reducing the mother’s workload (Gibson and Mace 2005). Beyond the contribution emanating directly from these helpers, we should also recognise the agency of mothers in nurturing such supportive networks.
Similarly, material capital may contribute to physical shelter, the capacity to produce food directly, or in market economies the ability to purchase food, accommodation and medical care. These resources are typically influenced by other members of a woman’s household, such as brothers, parents, uncles, husbands or parents in law. In patrilocal and patrilineal societies, women have little or no agency in selecting the characteristics of the household in which they will produce their offspring, or how material resources are allocated, as these choices tend to be made by other family members. Nonetheless, the resulting resources still have major implications for maternal fitness, and hence the phenotypic quality of offspring.
The availability of maternal capital also depends on reproductive scheduling, and can vary in association with maternal age, parity, inter-birth interval and the sex of the offspring, as well as time-varying factors such as household composition, financial assets and so on. A broad conceptual model of maternal capital is presented in Figure 2.
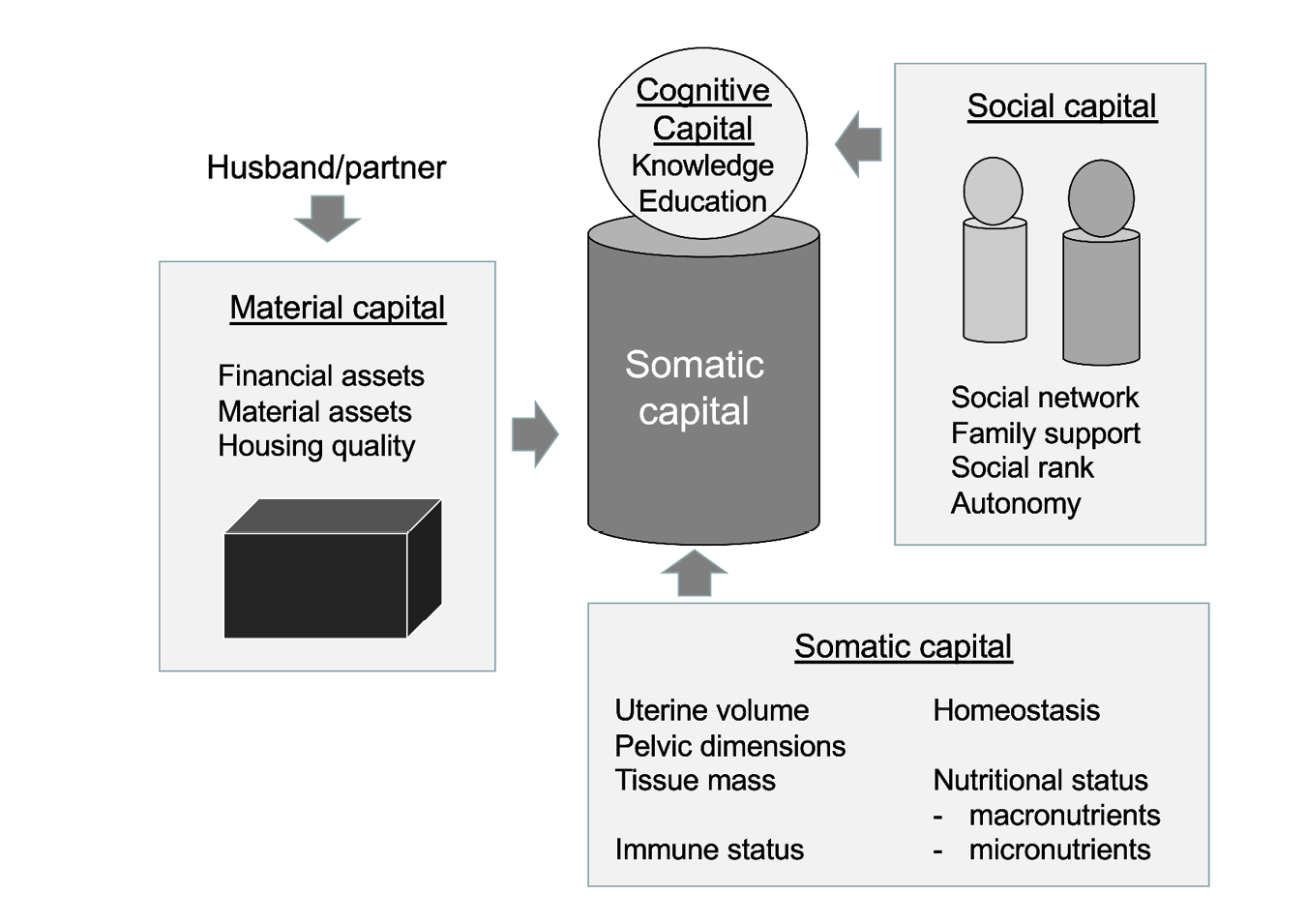
Fig. 2 Components of maternal capital, a composite trait that maternal promotes investment in the offspring. The central cylinder represents the maternal body, through which diverse tissues, organs and skeletal structures contribute to a wide range of types of somatic capital. The upper circle represents the brain, and cognitive capital, through which mothers can also access social capital, in the form of supportive individuals and networks, and material capital, such as housing or financial support.
It is no coincidence, I have argued, that the primary window of plasticity in each life-course — the first thousand days — coincides broadly with physical exposure to maternal capital through placental nutrition and lactation (Wells 2003, Wells 2014). The offspring benefits from substantial buffering against ecological stresses during its most sensitive periods of development, though this protection is imperfect. However, this benefit is obtained at a cost: offspring phenotype is strongly influenced by maternal phenotype, and such effects may benefit maternal fitness, potentially at the expense of offspring fitness. In this way, maternal capital is the critical determinant of the life history strategy initially adopted by the offspring (Wells 2016). While some maternal-offspring correspondence in life history strategy is likely to reflect shared genes, there is ample evidence that plastic maternal traits also exert strong effects on the phenotype of the offspring.
The way in which maternal phenotype imprints the composite life history strategy of the next generation was recently shown by a study of South Asian women living in the UK (Wells, Yao et al. 2016). Birth weight, a simple proxy for maternal nutritional investment in early life, was associated with a series of life history traits, indicative of life history “decisions” or trade-offs, in the daughters: lower birth weight predicted earlier menarche, shorter adult stature, higher levels of body fat, and higher blood pressure (Figure 3). Collectively, therefore, lower maternal investment in early life obliges the daughter to shunt energy towards rapid maturation and reproduction, at a cost to somatic growth and the long-term capacity for homoeostasis.
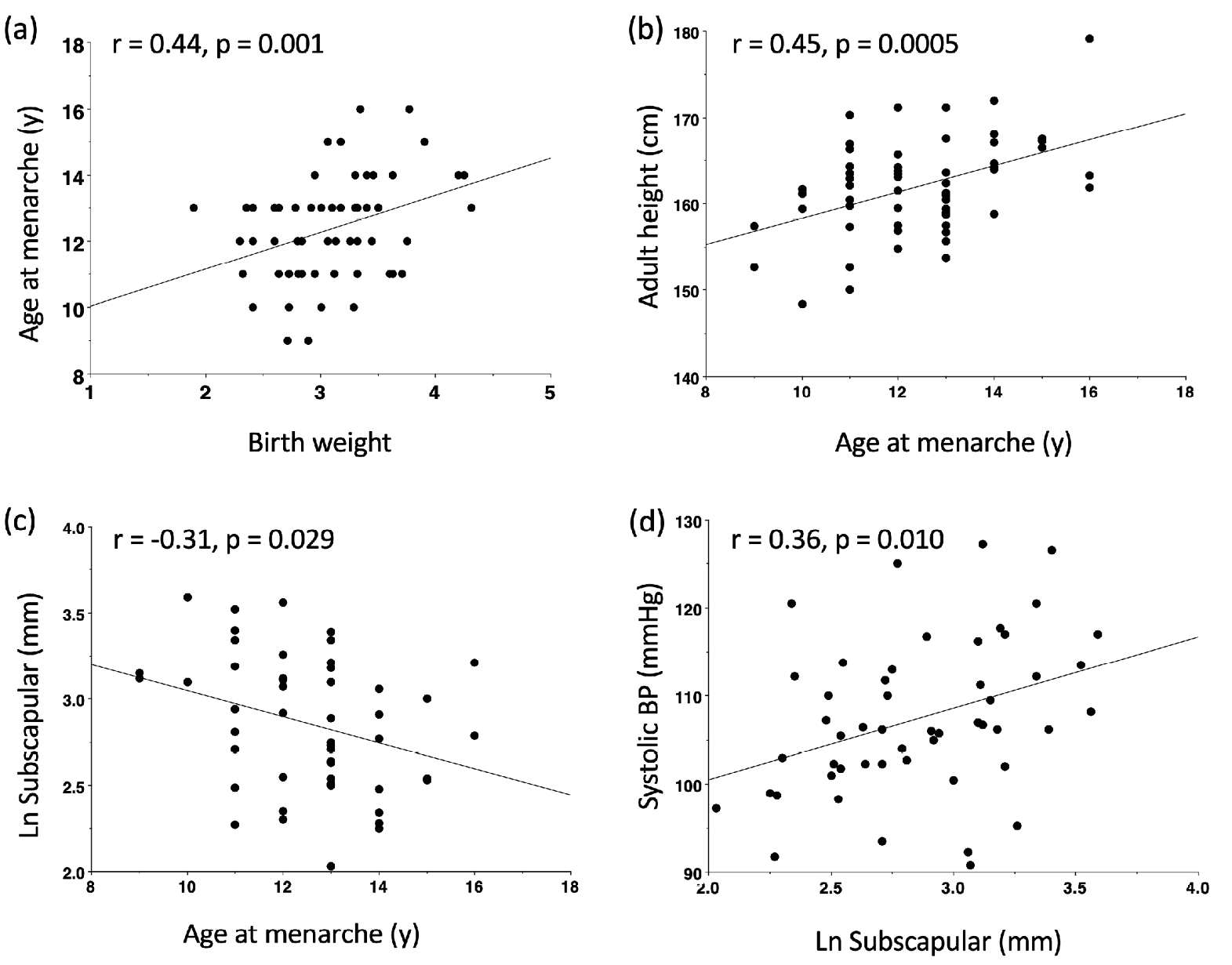
Fig. 3 A chain of life history decisions in South Asian women living in the UK. (a) Birth weight (indicating maternal investment) is inversely associated with age at menarche. (b) Earlier menarche is associated with lower adult stature. (c) Earlier menarche is associated with higher adult body fat. (d) Body fat is positively associated with systolic blood pressure. Reproduced with permission from (Wells, Yao et al. 2016).
Similar findings emerged from a larger study of mothers and their adult daughters in southern Brazil. Maternal capital was assessed using a composite index, integrating data on height, nutritional status, education and household income. Mothers with low capital had daughters who grew poorly in height from foetal life onwards, and who instead developed a more central (unhealthy) distribution of body fat by adulthood. In this setting, daughters receiving low maternal investment did not undergo early puberty, but were still more likely to have reproduced by 18 years, in comparison with those receiving high maternal investment. Once again, therefore, low maternal investment in early life induced trade-offs in the daughter that favoured short-term survival and reproduction at a cost to somatic growth and homeostasis, highlighting that the overall trade-off between health and fitness has its basis in developmental plasticity (Wells, Cole et al. 2019). As yet, it remains unclear whether sons would show similar or contrasting trade-offs.
We can therefore see two key periods of developmental plasticity in the offspring. The first component enables the adjustment of early growth trajectory to the magnitude of maternal investment, while external stresses are buffered. The second component allows the reorganisation of life history strategy in response to the external resources and stresses encountered in postnatal life (Wells 2019). Trade-offs enacted during the second period are strongly shaped by those occurring in the first (Wells 2010, Wells, Yao et al. 2016).
Once we understand the crucial role of maternal capital on life history trajectory of the offspring, we gain new insight into the association between social hierarchy and demographic outcomes. Most human populations show diverse forms of social inequality, so that the magnitude of maternal capital is inherently associated with the mother’s place in the social order (Wells 2010). The more severe the hierarchy, the greater the imprint of society on maternal capital, and the more strongly development of the next generation is imprinted by maternal social rank. If high-ranked mothers can direct more resources to each offspring, then those of low-ranked mothers are exposed to depleted maternal capital.
Precisely because maternal rank is relatively stable, offspring are consistently exposed to its metabolic correlates throughout early development. Thus, the very buffering systems that reduce exposure of the offspring to ecological stresses such as food insecurity and infections increase the exposure to maternal rank (Wells 2010, Wells 2016). To understand these associations, it is helpful to draw on evolutionary models that explicitly acknowledge the dynamic relationships that characterise hierarchy and the mediating role of nutrition.
The Producer-Scrounger Game
The physiological mechanisms through which hierarchical relations impact life history strategies can be examined through the lens of the “producer-scrounger” game (Wells 2016). This dynamic game was developed by ecologists, to understand how social interactions result in unequal distributions of food among organisms. The approach may be particularly valuable in humans, because nutritional dynamics lie at the heart of all forms of human hierarchy and inequality (Wells 2016). The fundamental insight of game theory is that the best strategy for one individual depends on what others in the population are doing.
One of the first games used to study interactions over resources was the Hawk-Dove game (Maynard Smith 1982). In this approach, hawks use aggressive tactics to obtain resources, whereas Doves avoid aggressive interactions. When Hawks compete with Doves, Hawks almost inevitably obtain the resource, but when Hawks compete with each other (regardless of winning, losing or sharing the pay-off) they have a high risk of physical injury. In a population of hawks and doves, the crude pay-offs of Hawk-Hawk and Dove-Dove interactions are equal, but paired Doves ultimately do better than paired Hawks because they do not experience physical injury.
The Hawk-Dove game can be applied to many social interactions in humans. One way or another, power imbalances always involve unequal access to resources, which ultimately resolve to energy. In social species, therefore, power relations are directly relevant to life history theory as they mediate the conversion of energy into fitness (Wells 2016). Nevertheless, foraging would be a very costly activity if a large proportion of individuals regularly indulged in overt aggression to obtain their energy supply. A more suitable game for modelling access to resources is therefore the “producer-scrounger” game (Barnard and Sibly 1981), which addresses more subtle forms of competition, and unlike the Hawk-Dove game takes into account how resources are obtained in the first place.
Producing and scrounging are considered discrete and incompatible tactics: an individual cannot engage in both simultaneously (Morand-Ferron, Giraldeau et al. 2007). Directly producing a resource may lead to an immediate payoff, known as the “finder’s share”. However, unless the resource is immediately consumed, it may be stolen by another individual, a scrounger. At the simplest level, the potential payoff for either strategy depends on the proportion of the population engaged in each activity. The fewer the producers, the fewer the opportunities for scrounging, and the lower the average payoff. As producers increase in number, so do the returns the scrounging, but if there are too many scroungers then the equilibrium tips back in favour of producing. This frequency-dependent interaction thus shapes the distribution of the two strategies in the population (Figure 4).
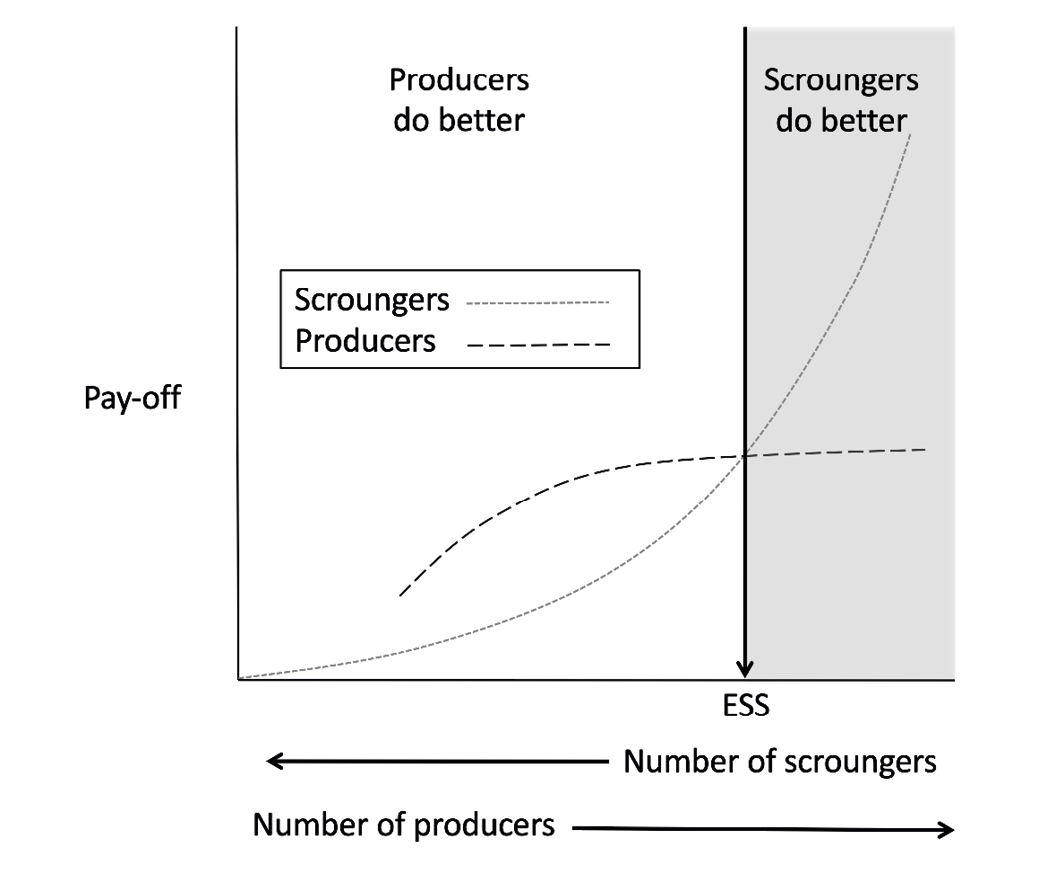
Fig. 4 Schematic diagram illustrating a simple dynamic model of two types of foraging strategy, producing and scrounging. The pay-off from scrounging increases as the relative number of producers rise. Redrawn with permission, Anim. Behav. Vol 29, Barnard and Sibly, ‘Producers and scroungers: A general model and its application to captive flocks of house sparrows’ pp. 543–50, Copyright (1981) with permission from Elsevier.
Producing and scrounging could be addressed entirely at the level of behaviour, representing alternative tactics to obtain a food package. Whether producers or scroungers obtain a higher pay-off depends on the nature of the resource. For ubiquitous resources, such as a common food plant, producing may far more efficient. Where substantial effort is needed to locate and access resources, however, scroungers can potentially outsource the costs of foraging by exploiting producers, hence increasing their own pay-off.
Producing and scrounging can represent alternative tactics for every individual, and each could select the behaviour offering the best returns on a moment-by-moment basis. Scrounging is predicted to be favoured when producers are unable to prevent it, when food packages are visible and high quality, and when a given resource has greater value to the scrounger (for example, if they are hungrier than the producer, and prepared to take more risks to obtain the resource) (Brockmann and Barnard 1979).
However, if individuals were to commit systematically to producing or scrounging, persistent variability in the supply of energy among individuals could emerge. In a study of bats, for example, individuals consistently produced or scrounged from other animals over several months, and individual scroungers repeatedly targeted specific producers (Harten, Matalon et al. 2018). On this basis, the two life history strategies of the two types of forager would be predicted to diverge. Producing and scrounging would no longer be ad hoc behavioural tactics, but more fundamental strategies that become “locked” into components of physical phenotype such as growth rate, adult size, body composition and sexual signalling (Wells 2016). The next sections consider evidence for this scenario in primates and humans.
Hierarchy and Life History Trajectory in Non-human Primates
Evidence from primate research supports three relevant hypotheses — that primate societies typically demonstrate significant social hierarchies; that a mother’s social rank affects her maternal capital, and therefore shapes the life history trade-offs demonstrated by her offspring; and that individual primates within a population may act either temporarily or more consistently as producers or scroungers.
In mammals in general, the effects of high social rank on fitness vary by sex. Theoretically, the fitness of male mammals is limited most strongly by mating opportunities, whereas that of females is constrained by the costs of pregnancy and lactation. On this basis, females are expected to prioritize access to nutritional resources, whereas males are expected to prioritize gaining access to fertile females. Within any species, we would expect higher-ranked members of social groups to align more closely with these ideal strategies than those of lower rank. In other words, high-ranked males might use their status to achieve greater body size and thereby increase access to fertile females, while high-ranked females might use their status to obtain the best quality foods or reliable support networks. In each case, high status would promote fitness, and this prediction is increasingly supported. Across mammals in general, for example, dominant females are younger at first conception, have shorter birth intervals, produce more offspring, have better offspring survival rates, and may even suppress the reproduction of lower-ranked rivals, thereby diverting resources towards themselves (Ellis 1995).
In primates, female rank appears as expected to be relatively independent of body size, and arises through coalitions and alliances, which may be inherited through the matriline. This means first that social skills are essential for attaining high rank, (Chadwick-Jones 1998), and second that social networks are a key conduit through which the pay-offs of high rank emerge. Among Chacma baboons, for example, higher-ranked females with greater social capital live longer and produce more surviving offspring (Silk, Beehner et al. 2009, Silk, Beehner et al. 2010).
In general, high-ranking mothers across diverse primate species transmit more nutritional resources to their offspring, often supported by priority access to the best foraging areas (Wells 2010). The greater nutritional investment results in faster offspring growth, and accelerated maturation of female offspring (Pusey, Williams et al. 1997). Overall, therefore, there is substantial evidence from non-human primates that maternal position in the hierarchy generates major inter-generational effects, through the medium of greater maternal capital.
Specific application of the producer-scrounger game in primates remains rare, however in studies of baboons, females were more likely to scrounge from co-feeding neighbours of subordinate status (King, Isaac et al. 2009), and low-ranked animals tended to experience lower food intakes when resources were scarce (Marshall, Carter et al. 2015). These studies therefore provide early evidence that variability in maternal capital may relate to competitive foraging dynamics, through which some animals obtain more energy at the expense of others.
Hierarchy and Life History Trajectory in Humans
In humans, there is likewise substantial evidence that social hierarchy is associated with variability in life history traits and demographic outcomes. Many studies have shown that children from more advantaged backgrounds are larger in size at birth, and remain so in adulthood (Eveleth and Tanner 1976). For example, family income was strongly associated with birth weight and infant weight gain in a population from southern Brazil (Victora, Barros et al. 1987). These differences attenuate only slowly when secular changes in living conditions occur (Kuh, Power et al. 1991), indicating powerful inter-generational effects (Wells and Stock 2011). Constraints on social mobility mean that maternal capital runs in families, propagating height inequality over time. Already by birth, therefore, the mother’s position in the hierarchy has profoundly impacted the life history strategy of her offspring.
As economic development occurs, nutritional constraint during foetal life and infancy may be followed by compensatory catch-up growth. Sometimes this results in larger size in adulthood (Siervo, Stephan et al. 2011), but in other cases catch-up represents an acceleration only in tempo, resulting in adulthood being achieved sooner but at smaller body size. An extreme version of this scenario is shown by Indian girls adopted in early life into Swedish families. Substantially smaller than the Swedish girls at birth, the Indian girls underwent precocious puberty without resolving the growth deficit, leading to short adult height (Proos 2009). A similar pattern on a broader scale is evident in many middle/higher income populations, where age at menarche is decreasing over time while adult height remains relatively static (N. C. D. Risk Factor Collaboration 2016, Wells 2016).
Collectively, these studies show that life history traits vary in association with social rank in a range of settings, but that the nature of the association depends on the risks and resource availability of the setting.
The Fundamental Role of Nutrition in Hierarchy
The nature of social hierarchy has varied substantially across time and geography, but as I argue below, the producer scrounger game can be applied to many different types of human society, and to many different types of behavioural interaction. If this single ecological game has such widespread application, it is because nutrition and power are fundamentally connected (Wells 2016).
In contemporary societies, position in the hierarchy is often assessed in terms of the capacity to participate in markets, reflecting financial wealth. However, nutrition is the ultimate context in which all hierarchies are generated and maintained (Wells 2016). Reflecting the emphasis of life history theory on energy dynamics, I use the term nutrition to refer not only to food intake but also to physical activity patterns and the condition of the body in terms of its growth, composition and ability to resist infectious diseases. Likewise, while the producer-scrounger game has been primarily applied to feeding interactions, its potential for understanding the consequences of social inequality is much broader. According to life history theory, the resources that are subjected to scrounging should not be limited in concept to discrete food parcels, but rather to energy dynamics in general. In non-human apes, for example, we could apply this lens to activities such as parental care and allo-mothering, while for humans the remit could be extended to a huge range of activities, such as work, taking risks, material resources and so on (Wells 2016).
It is generally accepted that the least hierarchical form of human society is foraging. Ethnographic studies indicate that foragers actively suppress hierarchy, in part by sharing food and other resources on a routine basis (Kelly 1995, Wiessner and Schiefenhovel 1997). Foragers never know who will fail to find adequate food on any given day, hence the available food tends to be redistributed relatively equally, and over time everyone is a net contributor, i.e. a producer, whilst those experiencing shortfalls are merely temporarily scroungers. Foragers tend to use several “levelling strategies” to reduce the emergence of social differences, and promote mutually supportive relations with neighbouring groups. Overall, therefore, systematic scrounging is suppressed, though subtle forms may persist including differences in status and gender inequality. Simple horticultural societies also show modest hierarchies, for the role of human labour in producing food limits the production of resources available for scrounging (Gurven, Borgerhoof Mulder et al. 2010).
Pastoralist societies contrast with foragers in maintaining tangible assets, in the form of animal herds that are potentially more susceptible to scrounging. In one sense, animals now represent the primary producers, and humans are generically the scroungers. Beyond that, certain social groups often attempt to raid the herds of other groups. Such competition may lead to long-term inequality between groups, since power and status are closely associated with the size of the animal herd (Borgerhoff Mulder, Fazzio et al. 2010). Again, nutrition is central to this relationship, since animals are the primary food source for pastoralists.
With the emergence of intensive agriculture, crops and stock animals became resources more susceptible to scrounging. Agriculture also encompasses a high element of risk, due to the possibility of harvest failure. This results in farmers regularly borrowing from each other, in order to recover from ecological shocks. Should they fail to repay their debt, they are often obliged to forego access to the land, and become tenant farmers or other forms of servile worker (Graeber 2011). Landowners and tenant farmers thus emerge as a new form of hierarchical society, in which food production remains central to the unequal status. This transition is associated with the emergence of major differentiation in body size and other life history traits (Shenk, Borgerhoff Mulder et al. 2010, Wells 2016).
In early industrial society, many of those low in the hierarchy shifted from agricultural production to manufacturing, in part through their displacement from common lands that terminated their capacity for direct food production. Those higher in the hierarchy could exploit this scenario by offering low wages, so that landless workers must work long hours in the new factories in order to purchase adequate food through markets (Hobsbawm 1968). Differential control of nutrition was thus a key element in the transformation to early industrial society, which saw the producer-scrounger relationship “reinvented” in the context of markets without negating its underlying logic. Ever since, the market has remained the primary medium of access to food in high-income countries, introducing a new interface between life history strategy and wealth inequality.
Thus, human hierarchy always involves groups that emerge in the context of each other, operationalized primarily through the medium of nutrition. The brief summary presented in this section does not discuss the heterogeneity that manifests within each broad mode of subsistence, or the variable associations that social inequality may show with life history traits in different settings. My broader argument is that it is through systematically obtaining food and other resources from producers that scroungers acquire and maintain their dominant status, with implications for life history traits and demographic outcomes (Wells 2016). Whilst this allows us to explore social hierarchy within populations, the same approach can be applied to relations between populations.
Social Hierarchy Within Groups and Demographic Outcomes
According to the notion of “evolutionary stable strategies”, the frequency of producers and scroungers stabilises when the average pay-off of each strategy is the same (Barnard and Sibly 1981). In human societies, however, scroungers may use various forms of power to coerce the producers. Consequently, the greater the degree of hierarchy, the greater the trade-off between health and fitness in the lower ranking group.
Figure 5 illustrates this scenario in a hypothetical population, in which a small number of dominant scroungers co-exist with a large number of subordinate producers, who provide the food requirements of the entire population. Over time, the size of each sub-population may remain constant, such that the average fitness of producers and scroungers is equal. However, this apparent equality may co-exist with substantial differences in demographic outcomes and health status. Producers may show high levels of mortality in early life, which translates into shorter average lifespan. Those that survive to adulthood may compensate for the higher juvenile mortality by having higher fertility rates. In combination, exposure to high-risk environments in early life and the elevated metabolic burden of reproduction (and labour) in adult life is expected to accelerate metabolic markers of ageing. This will result in poorer metabolic health and shorter life-span in those producers who reach adulthood.
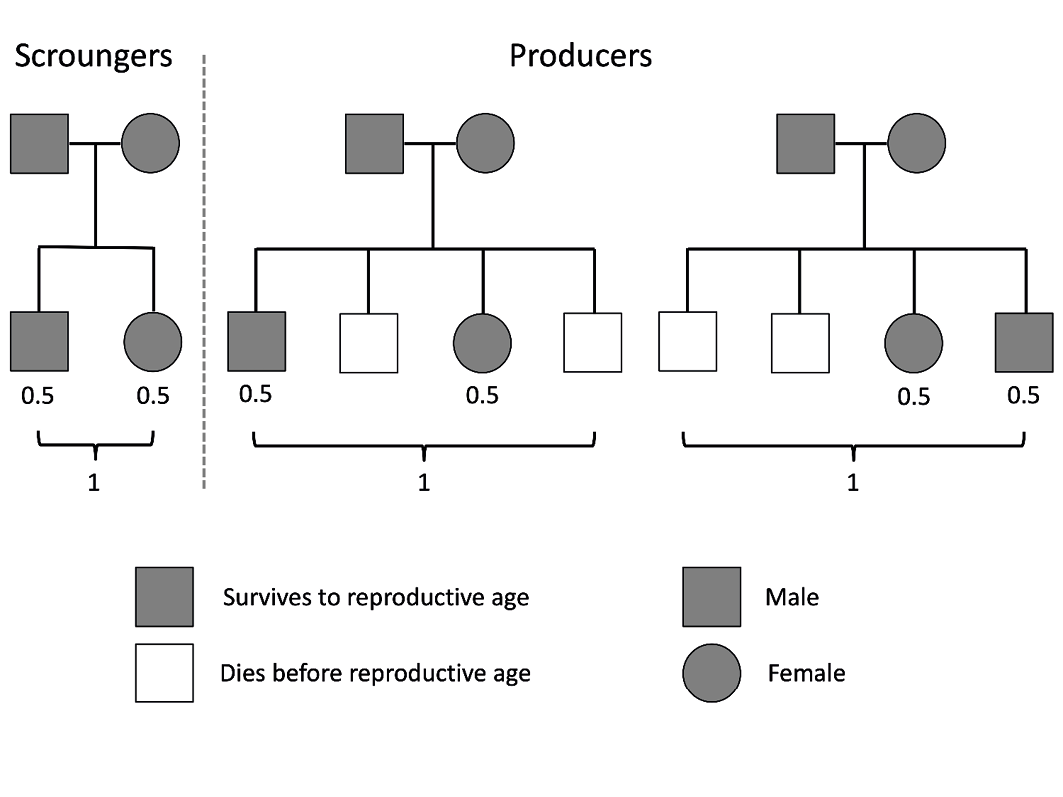
Fig. 5 Schematic diagram illustrating fitness and demographic outcomes in a hypothetical population of producers (common and subordinate) and scroungers (rare and dominant). Scroungers have negligible juvenile mortality and produce small numbers of high-quality offspring. Producers have high juvenile mortality, and compensate through higher fertility rates. The total fitness of producers and scroungers is similar, but producers have shorter average lifespan.
Given their superior health and longevity, one might ask why the scroungers do not also show high fertility. However, it is well established that greater height, wealth and education, all markers of higher social rank, do not typically drive higher fertility, but rather result in a higher quality of offspring.
Recent data from the UK provide support for this model. As argued above for human societies in general, the living standards of wealthier and poorer groups in the UK are structurally related. Comparing across levels of deprivation, poorer groups show higher levels of infant mortality (Kershenbaum, Fu et al. 2017), and shorter total life-span as well as shorter healthy life-span (the years spent free of chronic disease) (Office for National Statistics 2014), while the age of first reproduction also tends to decrease with the level of deprivation (Chipman and Morrison 2013). Collectively, these data support the over-arching hypothesis that demographic patterns emerge through variability in life history trade-offs, that in turn reflect the position of a given individual in the social hierarchy.
Social Hierarchy Between Groups
The producer-scrounger game can also be applied to relationships between populations, whether these are defined as castes, ethnic groups or countries. In each case, dominant groups acquire resources from, and in the process maintain power over, subordinate groups. Once again, the consequence is expressed in health inequalities that impact demographic outcomes such as fertility and life expectancy.
At the broadest level, the producer-scrounger game can be applied at an international level, thus helping understand demographic differences across countries. In the modern globalised market, unequal trading relations that emerged on the back of past relations of imperialism and colonisation maintain some countries as producers of cheap food, where large sections of the population continue to suffer poverty, high burdens of infectious disease, food insecurity and chronic under-nutrition. High-income countries have priority access overall to high quality foods and low burdens of infectious disease, though they may also maintain high levels of social inequality within the country. In turn, this helps explain why “producer countries” have higher average fertility than high-income countries, and lower average longevity.
Once again, the key point is that the demographic outcomes of different countries are structurally linked through their unequal access to resources, and the resulting life history trade-offs that emerge depending on their position in the international hierarchy.
Conclusion
In this chapter, I have explored the utility of a dynamic game theory model of social inequality for integrating across several fields of academic enquiry, in order to improve understanding of life history variability within and between populations. The resulting conceptual approach can in turn help understand contemporary variability within and between populations in demographic traits, and also their historical divergence or convergence over time. I propose that this framework offers new opportunities to explore the demographic consequences of relations within and between populations that are not cooperative, but rather exploitative. To date, interest in sociality and life history theory has focused almost exclusively on cooperative relationships, which has produced an unbalanced perspective on the relationship between human society and biological and demographic outcomes.
I give particular emphasis to nutrition because it is the key link between the various components of the over-arching framework. First, the central component of nutrition is energy supply, which fits well with the focus of life history theory on energy allocation strategies, which incorporate developmental adjustments in early life. However, my definition of nutrition is very broad, and relates to diverse forms of energy use and storage as well as energy income. Second, I have argued that all human hierarchies ultimately reduce to unequal access to energy as the most fundamental resource, that underpins all human activities. Third, I argue that it is because nutrition is the primary medium in which human hierarchies are generated and maintained, as recognised explicitly in the producer-scrounger game, that nutritional health is also where the primary benefits and costs of social inequality manifest at the level of individuals and populations (Wells 2016). This theoretical framework is illustrated in Figure 6.
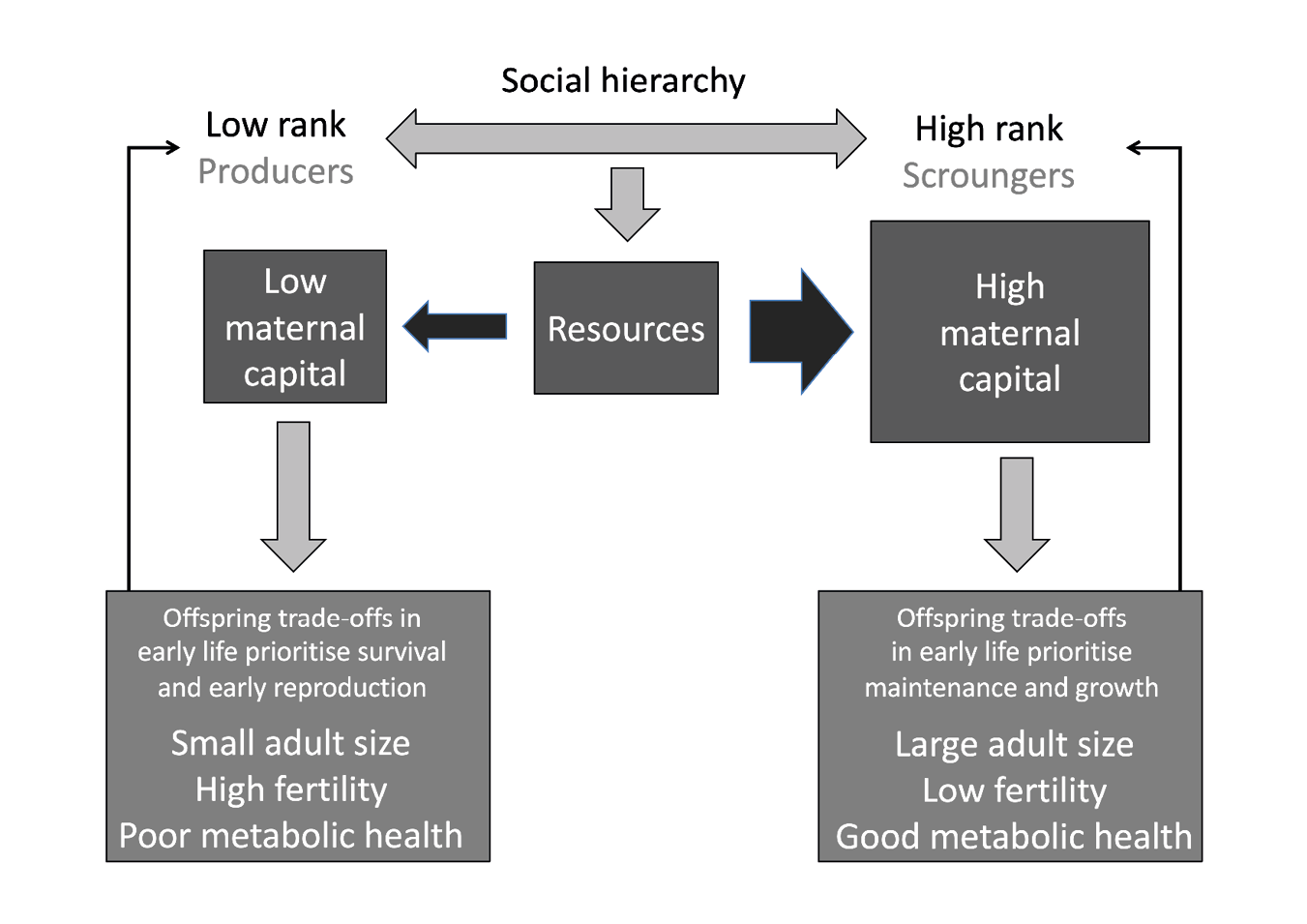
Fig. 6 The over-arching framework linking the interactions of the producer-scrounger game with variability in maternal capital and hence with life-history trade-offs in successive generations. Producers acquire lower levels of resources and hence lower levels of maternal capital. This leads to trade-offs favouring immediate survival and reproduction in the next generation, at a cost to growth and health. Smaller adult size then renders the next generation susceptible to becoming producers too. In contrast, scroungers gain more resources and acquire more maternal capital. This results in trade-offs favouring growth and health in the next generation, leading to large body size and a high chance of becoming scrounger.
Recent studies provide empirical evidence for links between position in the hierarchy, variability in maternal capital, life history trade-offs in the offspring and metabolic and demographic outcomes (Wells, Cole et al. 2019). However, the dynamic game may also be used directly in future, to test in silico how the life history trade-offs of producers and scroungers interact, through the medium of contests over energy availability. A strength of this approach is that it may be applied to any form of human society across time, geographical space, subsistence mode and culture.
Acknowledgements
I very much appreciate valuable constructive criticisms from Rebecca Sear, Daniel Nettle and Mhairi Gibson.
References1
Barker, D. J. 1992. ‘Fetal and infant origins of adult disease’. British Medical Journal. 301.6761. https://doi.org/10.1136/bmj.301.6761.1111
Barker, D. J. 2004. ‘The developmental origins of adult disease’. J Am Coll Nutr, 23.6 Suppl: pp. 588S-595S. https://doi.org/10.1080/07315724.2004.10719428
Barnard, C. J. and R. M. Sibly 1981. ‘Producers and scroungers: A general model and its application to captive flocks of house sparrows’. Anim. Behav. 29.2: pp. 543–50. https://doi.org/10.1016/s0003-3472(81)80117-0
Borgerhoff Mulder, M., I. Fazzio, W. Irons, R. L. McElreath, S. Bowles, A. C. Bell, T. Hertz and L. Hazzah. 2010. ‘Pastoralism and wealth inequality: revisiting an old question’. Curr. Anthropol. 51.1: pp. 35–48. https://doi.org/10.1086/648561
Brockmann, H. J. and C. J. Barnard. 1979. ‘Kleptoparasitism in birds’. Anim. Behav. 27: pp. 487–514. https://doi.org/10.1016/0003-3472(79)90185-4
Chadwick-Jones, J. 1998. Developing a social psychology of monkeys and apes. Hove, UK, Psychology Press, Taylor and Francis.
Chipman, A. and E. Morrison. 2013. ‘The impact of sex ratio and economic status on local birth rates’. Biol Lett. 9.2: p. 20130027. https://doi.org/10.1098/rsbl.2013.0027
Dunger, D. B., M. L. Ahmed and K. K. Ong. 2006. ‘Early and late weight gain and the timing of puberty’. Mol.Cell Endocrinol. 254–55: pp. 140–45. https://doi.org/10.1016/j.mce.2006.04.003
Ellis, L. 1995. ‘Dominance and reproductive success among non-human animals: a systematic literature review’. Ethol. Sociobiol. 16.4: pp. 257–333. https://doi.org/10.1016/0162-3095(95)00050-u
Ellison, P. T. 1981. ‘Morbidity, morality, and menarche’. Hum Biol, 53.4: pp. 635–43. https://pubmed.ncbi.nlm.nih.gov/7327544/
Eveleth, P. B. and J. M. Tanner. 1976. Worldwide variation in growth. Cambridge, Cambridge University Press.
Gibson, M. A. and R. Mace. 2005. ‘Helpful grandmothers in rural Ethiopia: A study of the effect of kin on child survival and growth’. Evol. Hum. Behav. 26.6: pp. 469–82. https://doi.org/10.1016/j.evolhumbehav.2005.03.004
Graeber, D. 2011. Debt: the first 5,000 years. London, Melville House.
Gurven, M., M. Borgerhoof Mulder, P. L. Hooper, H. Kaplan, R. Quinlan, R. Sear, E. Schniter, C. von Rueden, S. Bowles, T. Hertz and A. C. Bell. 2010. ‘Domestication alone does not lead to inequality: intergenerational wealth transmission among horticulturalists’. Curr. Anthropol. 51.1: pp. 49–64. https://doi.org/10.1086/648587
Harten, L., Y. Matalon, N. Galli, H. Navon, R. Dor and Y. Yovel. 2018. ‘Persistent producer-scrounger relationships in bats’. Sci Adv. 4.2: p. e1603293. https://doi.org/10.1126/sciadv.1603293
Hill, K. 1993. ‘Life history theory and evolutionary anthropology’. Evol. Anthropol. 2.3: pp. 78–89. https://doi.org/10.1002/evan.1360020303
Hobsbawm, E. 1968. Industry and empire. Harmondsworth, Penguin Books.
Hrdy, S. B. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA, Belknap Press.
Isler, K. and C. P. van Shaik. 2012. ‘How our ancestors broke through the grey ceiling: comparative evidence for cooperative breeding in early Homo’. Curr. Anthropol. 53.S6: pp. S453-S465. https://doi.org/10.1086/667623
Jasienska, G. 2017. ‘Lancet reproduction paper’. Lancet. 1111: pp. 11–111.
Kaplan, H., J. Lancaster and A. Robson. 2003. Embodied capital and the evolutionary economics of the human life span. Life Span: Evolutionary, Ecological, and Demographic Perspectives. J. R. Carey and S. Tuljapurkar. New York, Population Council.
Kelly, R. L. 1995. The foraging spectrum. Washington, Smithsonian Institution Press.
Kershenbaum, A., B. Fu and R. I. Gilbert. 2017. ‘Three decades of inequality in neonatal and early childhood mortality in singleton births in Scotland’. J Public Health. 39.4: pp. 712–19. https://doi.org/10.1093/pubmed/fdw114
King, A. J., N. J. Isaac and G. Cowlishaw. 2009. ‘Ecological, social and reproductive factors shape producer-scrounger dynamics in baboons’. Behav. Ecol. 20.5: pp. 1039–49. https://doi.org/10.1093/beheco/arp095
Kramer, K. L. and P. T. Elison. 2010. ‘Pooled energy budgets: Resituating human energy -allocation trade-offs’. Evol. Anthropol. 19.4: 136–47. https://doi.org/10.1002/evan.20265
Kuh, D. L., C. Power and B. Rodgers. 1991. ‘Secular trends in social class and sex differences in adult height’. Int J Epidemiol, 20.4: pp. 1001–09. https://doi.org/10.1093/ije/20.4.1001
Leonetti, D. L., D. C. Nath and H. S. Hemam. 2007. ‘In-law conflict: women’s reproductive lives and the roles of their mothers and husbands among the matrilineal Khasi’. Curr. Anthropol. 48.6: pp. 861–90. https://doi.org/10.1086/520976
Marshall, H. H., A. J. Carter, A. Ashford, J. M. Rowcliffe and G. Cowlishaw. 2015. ‘Social effects on foraging behavior and success depend on local environmental conditions’. Ecol Evol. 5.2: pp. 475–92. https://doi.org/10.1002/ece3.1377
Maynard Smith, J. 1982. Evolution and the theory of games. Cambridge, Cambridge University Press.
Meehan, C. L., C. Helfrecht and R. J. Quinlan. 2014. ‘Cooperative breeding and Aka children’s nutritional status: is flexibility key?’ Am J Phys Anthropol. 153.4: pp. 513–25. https://doi.org/10.1002/ajpa.22415
Monaghan, P. 2008. ‘Early growth conditions, phenotypic development and environmental change’. Philos Trans R Soc Lond B Biol Sci, 363.1497: pp. 1635–45. https://doi.org/10.1098/rstb.2007.0011
Morand-Ferron, J., L.-A. Giraldeau and L. Lefebvre. 2007. ‘Wild Carib grackles play a producer–scrounger game’. Behav. Ecol. 18.5: pp. 916–21. https://doi.org/10.1093/beheco/arm058
N. C. D. Risk Factor Collaboration. 2016. ‘A century of trends in adult human height’. eLife, 5. https://doi.org/10.7554/elife.13410
Nesse, R. M. and G. C. Williams. 1994. Why we get sick: the new science of Darwinian medicine. New York, Times Books.
Office for National Statistics. 2014. Inequality in healthy life expectancy at birth by national deciles of area deprivation: England, 2009–11, Office for National Statistics.
Ong, K. K., P. Emmett, K. Northstone, J. Golding, I. Rogers, A. R. Ness, J. C. Wells and D. B. Dunger. 2009. ‘Infancy weight gain predicts childhood body fat and age at menarche in girls’. J Clin. Endocrinol. Metab. 94.5: pp. 1527–32. https://doi.org/10.1210/jc.2008-2489
Ozaltin, E., K. Hill and S. V. Subramanian. 2010. ‘Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries’. JAMA, 303.15: pp. 1507–16. https://doi.org/10.1001/jama.2010.450
Penn, D. J. and K. R. Smith. 2007. ‘Differential fitness costs of reproduction between the sexes’. Proc Natl Acad Sci U. S. A., 104.2: pp. 553–58. https://doi.org/10.1073/pnas.0609301103
Proos, L. A. 2009. ‘Growth & development of Indian children adopted in Sweden’. Indian J Med Res. 130.5: pp. 646–50. https://pubmed.ncbi.nlm.nih.gov/20099404/
Pusey, A., J. Williams and J. Goodall. 1997. ‘The influence of dominance rank on the reproductive success of female chimpanzees’. Science, 277.5327: pp. 828–31. https://doi.org/10.1126/science.277.5327.828
Satyanarayana, K., N. A. Nadamuni, M. C. Swaminathan and B. S. Narasinga Rao. 1981. ‘Effect of nutritional deprivation in early childhood on later growth--a community study without intervention’. Am J Clin. Nutr. 34.8: pp. 1636–37. https://doi.org/10.1093/ajcn/34.8.1636
Schneider, J. E. 2004. ‘Energy balance and reproduction’. Physiol Behav. 81.2: pp. 289–317. https://doi.org/10.1016/j.physbeh.2004.02.007
Sear, R., D. W. Lawson, H. Kaplan and M. K. Shenk. 2016. ‘Understanding variation in human fertility: what can we learn from evolutionary demography?’ Philos Trans R Soc Lond B Biol Sci, 371.1692: p. 20150144. https://doi.org/10.1098/rstb.2015.0144
Sear, R. and R. Mace. 2008. ‘Who keeps children alive? A review of the effects of kin on child survival’. Evol. Hum. Behav. 29.1: pp. 1–18. https://doi.org/10.1016/j.evolhumbehav.2007.10.001
Sear, R., P. Sheppard and D. A. Coall. 2019. ‘Cross-cultural evidence does not support universal acceleration of puberty in father-absent households’. Philos Trans R Soc Lond B Biol Sci. 374.1770: p. 20180124. https://doi.org/10.1098/rstb.2018.0124
Shenk, M. K., M. Borgerhoff Mulder, J. Beise, G. Clark, W. Irons, D. Leonetti, B. S. Low, S. Bowles, T. Hertz, A. C. Bell and P. Piraino. 2010. ‘Intergenerational wealth transmission among agriculturalists: foundations of agrarian inequality’. Curr. Anthropol. 51.1: pp. 65–83. https://doi.org/10.1086/648658
Siervo, M., B. C. Stephan, A. Colantuoni and J. C. Wells. 2011. ‘First-borns have a higher metabolic rate and carry a higher metabolic risk in young women attending a weight loss clinic’. Eat Weight Disord, 16.3: pp. e171–76. https://doi.org/10.1007/bf03325128
Silk, J. B., J. C. Beehner, T. J. Bergman, C. Crockford, A. L. Engh, L. R. Moscovice, R. M. Wittig, R. M. Seyfarth and D. L. Cheney. 2009. ‘The benefits of social capital: close social bonds among female baboons enhance offspring survival’. Proc Biol Sci, 276.1670: pp. 3099–3104. https://doi.org/10.1098/rspb.2009.0681
Silk, J. B., J. C. Beehner, T. J. Bergman, C. Crockford, A. L. Engh, L. R. Moscovice, R. M. Wittig, R. M. Seyfarth and D. L. Cheney. 2010. ‘Strong and consistent social bonds enhance the longevity of female baboons’. Curr Biol, 20.15: pp. 1359–61. https://doi.org/10.1016/j.cub.2010.05.067
Stearns, S. C. 1992. The evolution of life histories. Oxford, Oxford University Press.
Trivers, R. L. 1972. Parental investment, and sexual selection. B. Campbell. Chicago, Aldine: pp. 139–79.
Victora, C. G., F. C. Barros, J. P. Vaughan, J. C. Martines and J. U. Beria. 1987. ‘Birthweight, socio-economic status and growth of Brazilian infants’. Ann Hum Biol. 14.1: pp. 49–57. https://doi.org/10.1080/03014468700008831
Wade, G. N., J. E. Schneider and H. Y. Li. 1996. ‘Control of fertility by metabolic cues’. Am J Physiol, 270.1: pp. E1-19. https://doi.org/10.1152/ajpendo.1996.270.1.e1
Walker, R., M. Gurven, K. Hill, A. Migliano, N. Chagnon, S. R. De, G. Djurovic, R. Hames, A. M. Hurtado, H. Kaplan, K. Kramer, W. J. Oliver, C. Valeggia and T. Yamauchi. 2006. ‘Growth rates and life histories in twenty-two small-scale societies’. Am J Hum Biol, 18.3: pp. 295–311. https://doi.org/10.1002/ajhb.20510
Webster, G. D., J. A. Graber, A. N. Gesselman, B. S. Crosier and T. O. Schember. 2014. ‘A life history theory of father absence and menarche: a meta-analysis’. Evol Psychol, 12.2: pp. 273–94. https://doi.org/10.1177/147470491401200202
Wells, J. C. 2003. ‘The thrifty phenotype hypothesis: thrifty offspring or thrifty mother?’ J. Theor. Biol. 221.1: pp. 143–61. https://doi.org/10.1006/jtbi.2003.3183
Wells, J. C. 2006. ‘The role of cultural factors in human breastfeeding: adaptive behaviour or biopower?’ J Hum Ecol, 2006.14: pp. 39–47. https://www.researchgate.net/publication/228627043_The_role_of_cultural_factors_in_human_breastfeeding_Adaptive_behaviour_or_biopower
Wells, J. C. 2010. ‘Maternal capital and the metabolic ghetto: An evolutionary perspective on the transgenerational basis of health inequalities’. Am J Hum Biol, 22.1: pp. 1–17. https://doi.org/10.1002/ajhb.20994
Wells, J. C. 2012. ‘Ecological volatility and human evolution: a novel perspective on life history and reproductive strategy’. Evol. Anthropol. 21.6: pp. 277–88. https://doi.org/10.1002/evan.21334
Wells, J. C. 2014. ‘Adaptive variability in the duration of critical windows of plasticity: Implications for the programming of obesity’. Evol Med Public Health, 2014.1: pp. 109–21. https://doi.org/10.1093/emph/eou019
Wells, J. C. 2016. The metabolic ghetto: an evolutionary perspective on nutrition, power relations and chronic disease. Cambridge, Cambridge University Press.
Wells, J. C. 2019. ‘Developmental plasticity as adaptation: adjusting to the external environment under the imprint of maternal capital’. Philos Trans R Soc Lond B Biol Sci. 374.1770: p. 20180122. https://doi.org/10.1098/rstb.2018.0122
Wells, J. C., T. J. Cole, M. Cortina-Borja, R. Sear, D. A. Leon, A. A. Marphatia, J. Murray, F. C. Wehrmeister, P. D. Oliveira, H. Gonçalves, I. O. Oliveira and A. M. Menezes. 2019. ‘Low Maternal Capital Predicts Life History Trade-Offs in Daughters: Why Adverse Outcomes Cluster in Individuals’. Front Public Health, 7: p. 206. https://doi.org/10.3389/fpubh.2019.00206
Wells, J. C. and J. T. Stock. 2011. ‘Re-examining heritability: genetics, life history and plasticity’. Trends Endocrinol Metab, 22.10: pp. 421–28. https://doi.org/10.1016/j.tem.2011.05.006
Wells, J. C., P. Yao, J. E. Williams and R. Gayner. 2016. ‘Maternal investment, life-history strategy of the offspring and adult chronic disease risk in South Asian women in the UK’. Evol Med Public Health, 2016.1: pp. 133–45. https://doi.org/10.1093/emph/eow011
Wells, J. C. K. 2018. ‘Life history trade-offs and the partitioning of maternal investment: Implications for health of mothers and offspring’. Evol Med Public Health, 2018.1: pp. 153–66. https://doi.org/10.1093/emph/eoy014
Westendorp, R. G. and T. B. Kirkwood. 1998. ‘Human longevity at the cost of reproductive success’. Nature, 396.6713: pp. 743–46. https://doi.org/10.1038/25519
Wiessner, P. and W. Schiefenhovel. 1997. Food and the status quest: an interdisciplinary perspective. Providence, RI, Berghahn Books.
1 Note this chapter has been posted on the Open Science Framework website since 02/11/2019, after it was accepted for publication, so the references will reflect when the chapter was written and not the OBP publication date.
