30. The Biodemography of Human Health in Contemporary Non-industrial Populations: Insights from the Tsimane Health and Life History Project
© 2024 M. Gurven, H. Kaplan, B. Trumble & J. Stieglitz, CC BY 4.0 https://doi.org/10.11647/OBP.0251.30
The life history of human hunter-gatherers is characterized by an extended post-reproductive lifespan, prolonged juvenile growth, high fertility with multiple dependents and biparental care, and extensive intra- and inter-generational resource and information transfer. Long-term, in-depth study of contemporary non-industrial populations offers important glimpses into how these traits operate, and insights into how they may have evolved. The Tsimane Health and Life History Project is a large-scale bio-behavioural study of the human life course designed to help understand the bidirectional connections between life history, health and social behaviour in a high fertility, kin-based population lacking amenities of modern urban life. It seeks to document the epidemiology of health and mortality across the lifespan, and to understand how growth and investment, social structure, sharing networks and behaviour impact health and aging. It focuses on how pathogen burden influences health and well-being during development and adulthood, and addresses how modernization affects health and sociality. We reflect on the implications of current findings and highlight the need for more joint ethnographic and biomedical studies of subsistence populations to address unresolved questions not only in evolutionary anthropology or biodemography, but in public health, epidemiology, gerontology and medicine.
Introduction
Average human life expectancy has increased by almost three months per year over the past 160 years, surpassing 70 years well into the twenty-first century (UN, 2015). Improvements in sanitation, nutrition, and public health account for much of this change (Riley, 2001, Oeppen and Vaupel, 2003). Reductions in infant and child mortality have greatly increased life expectancy, and chronic degenerative diseases have become the main sources of morbidity and mortality in industrialized populations. In fact, it is commonly reported that chronic diseases of aging were rare or absent throughout much of human evolutionary history. These afflictions of industrialized society are viewed as examples of evolutionary “mismatch” due to rapid environmental and lifestyle changes (i.e. “modernization”) outpacing our evolved genetic heritage. According to this view, the widespread prevalence today of cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM), Alzheimer’s Disease, and other degenerative diseases (e.g. osteoporosis) result from our being “Stone Agers in the fast lane” (Eaton et al., 1988).
Here, we introduce a detailed case study to help improve understanding of the processes that shaped the evolution of the human life course, with a focus on health and aging. What does physical condition, health, biological aging and disease look like in non-market subsistence contexts more similar to how we lived prior to industrialization? How can the study of aging, health and social lives in remote rural populations provide insight into what ails (or does not ail) us in contemporary urban settings? While it would be useful to study multiple populations over evolutionary time, comparable fine-resolution data on health and aging do not exist for past populations, and thus data from extant modern groups offer an imperfect lens to view the past. The demography and life history of extant foraging populations was spearheaded by pioneering research among Dobe !Kung of Botswana and Namibia (Howell, 1979, Howell, 2010, Lee and DeVore, 1976), the Ache of Paraguay (Hill and Hurtado, 1996) and Hadza of Tanzania (Marlowe, 2010, Blurton Jones, 2016). These and other vital empirical studies conducted among non-industrial populations identified universal features of the evolved human life history: compared to other mammals, and even primates, an extended post-reproductive life span, high fertility with multiple dependents, delayed juvenile growth, extensive intra- and intergenerational resource transfers and cumulative culture — in concert with the co-evolution of a highly encephalised brain (Kaplan et al., 2000, Hill et al., 2009, Kaplan, 1997) (see Kramer, in this volume). The exact timing and context for the evolution of these traits remain difficult to ascertain, but the long-term study of contemporary non-industrial populations with limited access to modern amenities (e.g. sanitation, electricity) is one approach to better understand this human adaptive complex.
The Tsimane Health and Life History Project (THLHP) is one such endeavour designed to integrate traditional ethnography with advances in methods and concepts from other disciplines, including demography, biomedicine, gerontology, epidemiology, economics and psychology in a subsistence population of forager-horticulturalists in lowland Bolivia. By examining changes in physical growth, health, development and aging in relation to economic productivity, resource transfers and social networks, we seek to test competing models meant to explain the evolution of our long human lifespan and associated traits (Hawkes, 2003, Kaplan et al., 2000). This task, by nature, demands an inter-disciplinary, mixed method approach. A classic expectation from the branch of evolutionary biology known as life history theory is that low exogenous mortality acts as a prime driver shaping a slower life history — that is, prolonged maturation, greater energetic investments in somatic maintenance, and longer lifespan (Stearns, 1992). A more realistic approach treats mortality as endogenous and co-evolving with other life history traits, such as the role of learning in development (Kaplan and Robson, 2002). The learning-intensive human foraging niche shifted an already slow life history further in this direction, but numerous questions remain: How did increased investments in learning and mortality reduction co-evolve, and how are they related to mortality-reducing effects of human sociality, risk buffering and cumulative culture? How did ecological shifts impact human food production and sharing, risk preferences, and mating patterns? How do different sources of morbidity, such as exposure to a diverse array of pathogens and co-morbidities, impact growth rates, somatic maintenance costs, and aging? To what extent are human-specific traits a coordinated and coevolved bundle, versus a mix of adaptations and by-products? Addressing these questions requires careful study of directly observable patterns of behaviour, health and human-environment interactions, which is only possible by studying contemporary, small-scale subsistence populations.
Joint behavioural and biomedical inquiry among contemporary non-industrial societies like the Tsimane aids in reconstructing ancestral patterns of human aging, health, life history and sociality. It also provides insight into the respective roles that changing diets and other lifestyle characteristics (e.g. physical activity) play in affecting health. A broader range of societies is required to understand evolved human reaction norms across different environments over recent millennia. Subsistence horticulturalists like the Tsimane share many similarities with existing full-time hunter-gatherers: they also hunt, gather, and fish, exhibit natural fertility, have minimal access to modern sanitation or medicine, and show limited group size. The differences between foragers and horticulturalists like the Tsimane, however, can shed additional light on the impacts of plant and animal domestication on health and life history-relevant traits, including parasite burden, nutritional status, fertility, mobility, residence patterns and social structure. Bottlenecks and expansions of human populations during the advent of agriculture also had profound impacts on human population genetics (Hawks et al., 2007, Fumagalli et al., 2011, Karmin et al., 2015), further highlighting the importance of non-foragers when considering health and disease in contemporary populations.
“Modernization” (defined here as a trend toward greater participation in the market/cash economy) affects health and reshapes aspects of social ecology, but the mechanisms by which these changes occur are not well understood. Contemporary non-industrial populations like the Tsimane are experiencing rapid social, political, economic and cultural change. Socioeconomic transformation due to increasing access to cash economies, wage labour, schooling, sanitation, access to modern medicine and other amenities (e.g. savings accounts) adds layers of complexity to understand how changing conditions alter health, risk management and life histories. Careful study is necessary for evaluating whether chronic diseases of aging, such as CVD, T2DM, osteoporosis and Alzheimer’s disease, were common during human evolutionary history or are fairly novel, resulting from an evolutionary mismatch between our evolved heritage and rapid environmental and lifestyle change. Addressing complex questions of how modernization influences health and sociality requires long-term study of an appropriate reference group, in this case, small-scale subsistence populations with relatively limited access to modern markets.
In this chapter, we first describe the Tsimane population, and then introduce the goals and organization of the Tsimane Health and Life History Project (THLHP). The Tsimane are a useful case study to describe various aspects of physical and mental health in a subsistence population, in relation to other life history, economic and demographic patterns, and in marked contrast to industrialized, urban populations. The Tsimane are now beginning to experience an epidemiologic transition, where the overall burden of disease currently dominated by infectious sources of morbidity, may soon be replaced by chronic, degenerative diseases. We thus highlight the role of infection in shaping different aspects of health, including several chronic diseases.
The Tsimane of Bolivia
Bolivia is home to 36 indigenous groups which together constitute over 60% of the population (INE, 2012). Of the 30 groups inhabiting the tropical lowlands, the Tsimane are among the most isolated (along with the Yuqui, Araona and Siriono). The Tsimane are forager-horticulturalists of the Bolivian Amazon who subsist largely from slash-and-burn horticulture, including plantains, rice, sweet manioc and corn (Figure 1). They also fish in rivers, streams and lagoons, hunt a large array of neotropical mammals, and engage in seasonal gathering of fruits and other foods (e.g. honey, nuts). They inhabit over 90 villages numbering from 50 to 500 individuals along the Maniqui, Quiquibey and Mato Rivers and interfluvial terra firma (Figure 2). While early censuses in the late 1990s estimated a population size of 6,000 Tsimane, the most recent complete THLHP census in 2015 suggests closer to 16,000 (also see INE (2012)) and a population growth rate of over 3.5%. Unlike most extant foragers, the relatively large Tsimane population size provides the opportunity for study of all stages of the human life course, including late adulthood, which is essential to study competing models of human life history evolution and diseases of aging.

Fig. 1 The Tsimane of central Bolivia. A central feature enabling delayed childhood, high fertility and long life span is extensive sociality — manifesting in cooperative production, distribution and childcare. Photo credits: Michael Gurven.
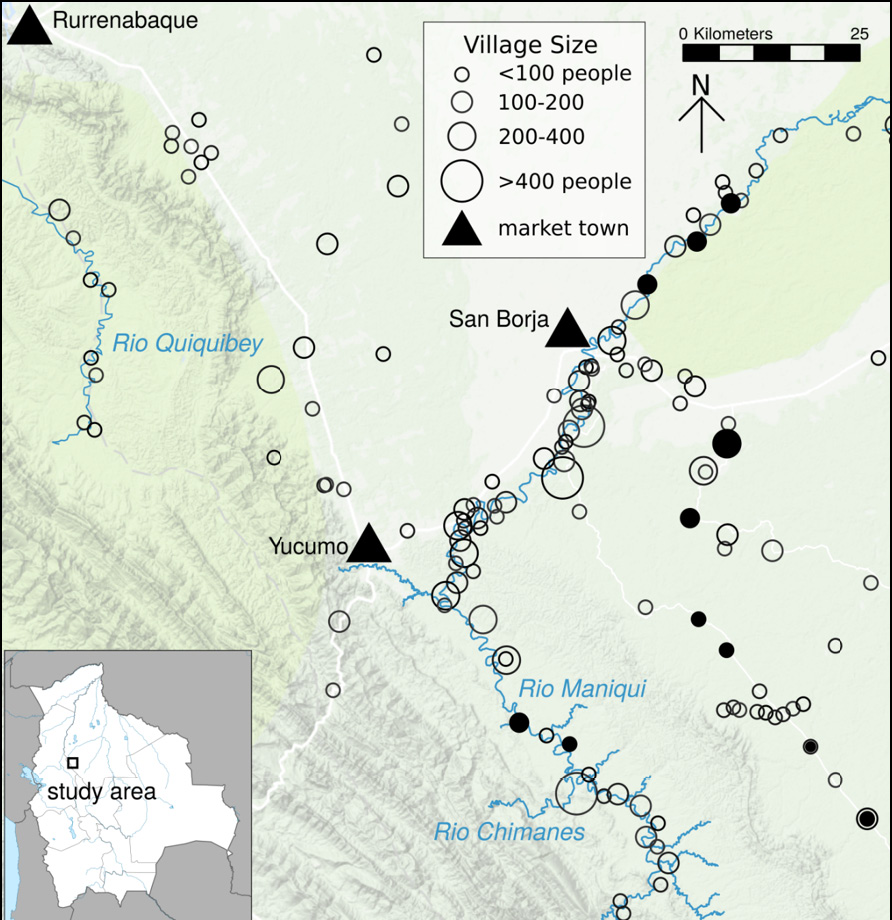
Fig. 2 Map of Tsimane territory and study villages. Solid circles signify “core” villages where relatively long-term study has occurred, empty circles are other villages visited by the biomedical team. Triangles reflect towns. Size of circles are proportional to village census size.
Throughout the first half of the twentieth century, Tsimane maintained a traditional lifestyle due to the relative absence of navigable roads in their territory. While road and river access has improved due to logging, development projects and new technologies (e.g. the recent boom of pequi outboard boat motors in the last five years), market access remains somewhat limited for many villages, especially during certain periods of the year when heavy rains wash out bridges and dirt roads, and make river travel dangerous. This variable access to the market and associated non-traditional cultural influences serve as a quasi-experiment — an opportunity for examining effects of socioeconomic change on health, fertility and social behavior (Gurven, 2012, Trumble et al., 2015, Gurven et al., 2012, Gurven et al., 2013).
Production and Reproduction
Total fertility rate is higher than most hunter-gatherers (nine births per woman), and the production and sharing network is multi-generational. Tsimane produce less food than they consume until late adolescence (Gurven et al., 2012, Hooper et al., 2015). Thus, the caloric burden on families can be substantial, especially for younger parents with multiple highly dependent offspring. For example, a married woman age 33 is at her peak dependency of 4.3 expected children younger than age 15. Food production efficiency peaks in the 30s-40s, especially for hunting and other difficult, skill-intensive activities (Gurven et al., 2009). Peak productivity extends long beyond peak strength (Gurven et al., 2006), suggesting the importance of skills-based practice and learning. Though delayed productivity is clear for hunting (Gurven et al., 2006, Walker et al., 2002), expertise in a wide range of production, manufacturing and other tasks (e.g. childcare, conflict mediation) is reported most frequently among middle-aged or older adults (Schniter et al., 2015). Nuclear families provide much of the daily calories, with older adults, including parents, grandparents and siblings providing substantial amounts of food to younger kin (Hooper et al., 2015). As strength and functional ability decline in later adulthood, Tsimane shift emphasis toward low strength and high skill subsistence and political activities, including hook-and-line fishing and horticulture, conflict mediation, village leadership roles and storytelling (Schniter et al., 2015). Older adults are also actively involved with various forms of pedagogy. Though caloric production declines at later ages, cognitive abilities remain relatively intact through the seventh decade of life (Gurven et al., 2017), helping to facilitate knowledge transfer.
Food sharing is widespread within extended families, but is more limited in scope than typically described among foragers. Kinship and relative need, as determined by recipient age, productivity and family size, and health status, largely determine the magnitude and direction of resource flows (Hooper et al., 2015, Gurven et al., 2012). Informal exchange networks help Tsimane manage multiple risks like sickness and injury, in addition to those from daily food shortfalls (Gurven et al., 2012, Jaeggi et al., 2016). The “prices” implicitly negotiated in these informal exchange networks partly reflect individual differences in supply and demand, which itself relates to household needs and abilities.
Whereas our nearest primate relatives, chimpanzees, show a rate of reproductive decline that is more closely linked to somatic decline and increasing risks of mortality, human reproductive aging precedes somatic senescence by roughly 20–25 years (Wood, 1994). After ceasing to reproduce, both men and women provide net economic transfers to children and grandchildren. By the time they reach their 70s, Tsimane rarely give food away, and so contribute less to sharing networks (Figure 3). The time delay between unproductivity due to physical deterioration and death appears to be short. During periods of productive decline, older adults may help in other ways. However, given this pattern of productivity and transfers, further delays in the age at menopause would produce net economic deficits within families because older adults would not be able to produce enough food for their own offspring (Kaplan et al., 2010).
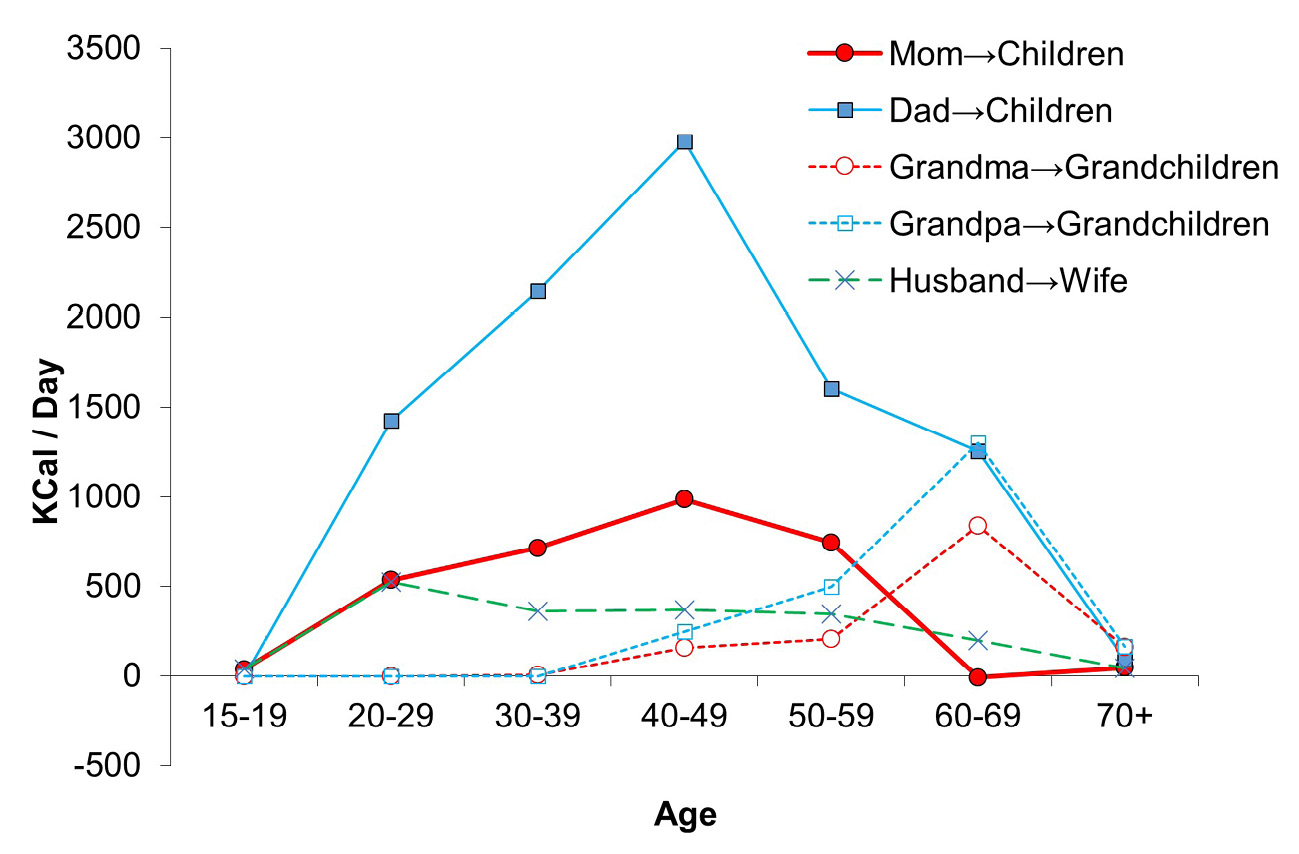
Fig. 3 Kin-based resource flows. Net contributions of food (measured in kilocalories transferred per day) from parents to children, from grandparents to grandchildren and husband to wife by age. Data come from over a thousand interviews on production and sharing behavior, and spot observations of consumption patterns.
Tsimane Health and Life History Project (THLHP)
From its inception in 2002, the research design of the THLHP has included a mobile biomedical team comprised of Bolivian physicians, biochemists, and Tsimane research assistants that visits each sampled village annually or once every other year to provide broad snapshots of physical condition and health, demography and socioeconomic life (see Gurven et al., 2017). A reduced “core team” composed of a few anthropologists and Tsimane research assistants complements this mobile team through more focused, longer-term sampling and more intensive socioeconomic data collection in core villages. These extended field sessions in core villages provide rich ethnographic study of economic and social behavior, and health. Our initial sample included 18 villages, expanded to 23 by 2005, 85 by 2009, and 90 by 2015 (Figure 2).
Our holistic bio-behavioral approach has the advantage that we can link information on multiple phenotypes for the same individual over time to better understand factors influencing aging, health and sociality. Our team has collected systematic baseline data at the individual level on many traits: demographic, behavioral, morbidity, biomarkers related to health and aging, infectious exposure, inflammation and other indicators of immune function, and measures of physical and functional status (see Gurven et al., 2017). The measures are derived from observation, surveys, medical exams, and biospecimens (blood, feces, urine, saliva). Several THLHP protocols were modified from prior life course epidemiological studies in high income countries (e.g. NHANES, Mexican Health and Aging Study, MacArthur Aging Study), permitting direct cross-cultural comparisons.
New THLHP findings replicate those from earlier studies of foragers and forager-horticulturalists: Tsimane exhibit a skill-intensive economic niche with all the hallmarks of our evolved life history, including a long learning period of juvenility and adolescence, biparental care, socially-mediated risk-buffering, multi-generational resource transfers by parents and grandparents to dependent young, high productivity of post-reproductive adults until the 8th decade of life, and a long adult lifespan. New research has enriched our understanding of the life history of production, consumption and cooperation, and shed new light on a variety of health-related themes: (a) lifespan, aging and psychological well-being, (b) the role of infectious disease in shaping different life history components including growth, senescence and fertility, (c) chronic disease and lifestyle change. In what follows, we discuss each theme in turn.
Lifespan, Aging and Well-being
From the period 1950–89, life expectancy at birth (e0) among Tsimane was 43 years; by 2002, e0 increased to about 53 years (Gurven et al., 2007) (Figure 4a). Despite recent improvement, Tsimane death rates at all ages are similar to those observed in Europe in the 1800s (Gurven et al., 2008). Infection is the largest cause of mortality, responsible for about half of all deaths, and a loss of almost 12 years of life expectancy at birth (Figure 5). Unlike many patterns observed historically, where initial increases in lifespan are largely due to reductions in infant and child mortality, the improvement in e0 from 1990–2002 was more a result of reduced death rates in adulthood than among infants or children (Figure 4b). We suspect that this is due to differences in access to medical interventions for adults and older children, since they have a greater ability than infants or young children to seek and survive treatment. Despite recent improvement in access to health care facilities, Tsimane cultural beliefs about sickness and death, coupled with some ethnic discrimination in town may still deter people from seeking treatment. Modal age of adult death is 70 years (SD=6.3), similar to that observed among hunter-gatherers and other horticulturalists, and 15 years earlier than that observed in high-income countries (Gurven and Kaplan, 2007).
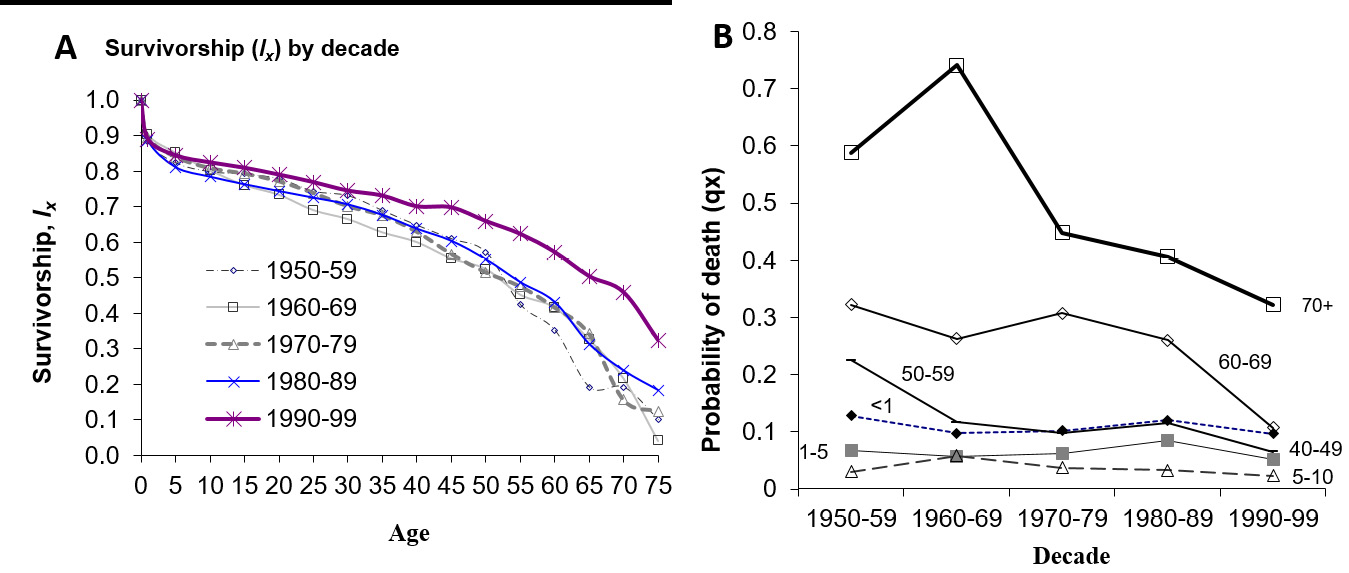
Fig. 4 Tsimane period mortality. (a) Tsimane survivorship (lx) by decade, spanning period from 1950–1999; (b) Mortality rates (qx) by decade for same time period for selected age groups. Late age mortality declined more substantially over the period 1950–99 than infant and child mortality. For details on mortality methodology, see Gurven et al. (2007).
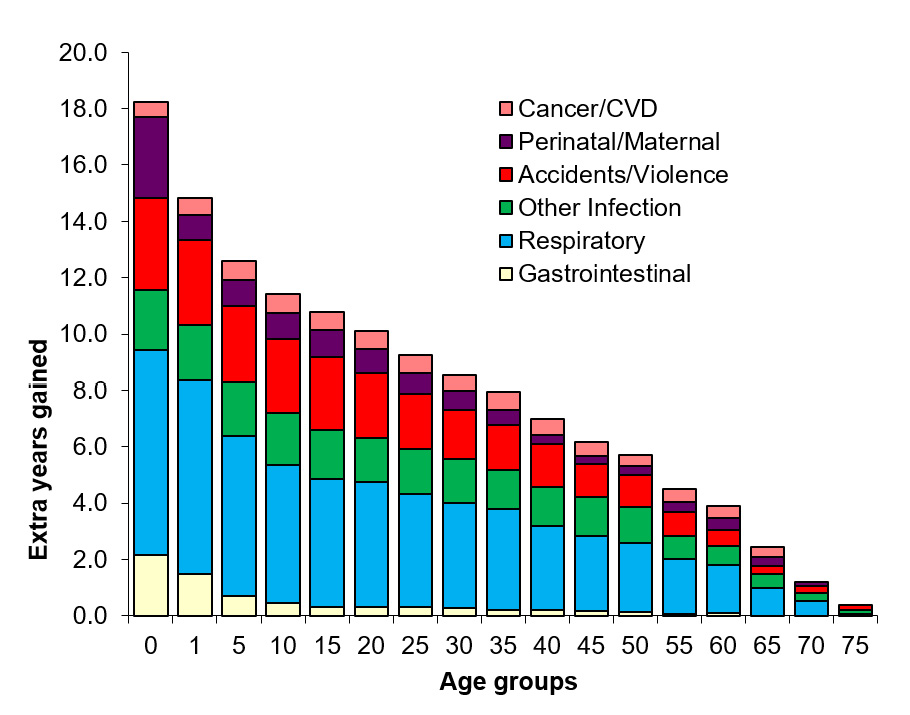
Fig. 5 Effect of mortality cause elimination on remaining life expectancy at age x (ex) during the period 1950–89, when e0 was 42 yrs. Using single decrement life tables offers a glimpse of the absolute increase in ex expected by eliminating six macro-causes of death. Causes of death were assessed by verbal autopsy.
By age 60, Tsimane show evidence of significant physical disability. Physical strength declines continuously by the fourth decade of life (Gurven et al., 2006). Over 60% of Tsimane over age 60 complain about hearing loss, over 80% have trouble seeing close distances, and over 70% can no longer chop large trees in their fields. About 50% of men and 70% of women over age 70 can no longer walk long distances, and complain frequently about painful arthritis in their legs, back, and hips. Over 70% of men no longer hunt by age 70; these men complain about weakness, lethargy, and having poor eyesight and hearing. Functional disability is a strong predictor of Tsimane depression: adults aged 50+ in the top decile of a composite disability measure score 14% higher on a depression scale than those in the bottom decile after controlling for multiple potential confounders (Stieglitz et al., 2014). We find that depression increases with age as disability increases and limits production and sharing ability (Stieglitz et al., 2014). This observation runs counter to the common claim that human depression is a modern mismatch disease, or that it universally peaks in mid adulthood (i.e. “mid-life crisis”) (Weiss et al., 2012).
Fluid cognitive abilities related to reasoning and processing speed also appear to decline in late life from their peak in early adulthood, whereas crystallized abilities based on cumulative experience and knowledge increase throughout the lifespan (Gurven et al., 2016, Trumble et al., 2015). While this pattern has been widely documented in Western contexts, it had never before been assessed systematically in a non-literate or non-industrial population. While the decline in fluid abilities seems to mirror changes in physical abilities with age, non-declining crystallized abilities are consistent with the functional role of middle-aged and older adults as mentors, instructors, and caregivers in Tsimane society. We also find that older Tsimane with the apolipoprotein E4 are protected against decline in cognitive performance, but only among those with heavy parasite burden (Trumble et al., 2017); E4 carriers without parasites and non-E4 carriers with parasites showed lower cognitive performance (Figure 6). These findings suggest that the E4 allele, the strongest risk factor for Alzheimer’s Disease and cognitive decline in industrialized populations, might have fitness-relevant advantages in a more infectious environment.
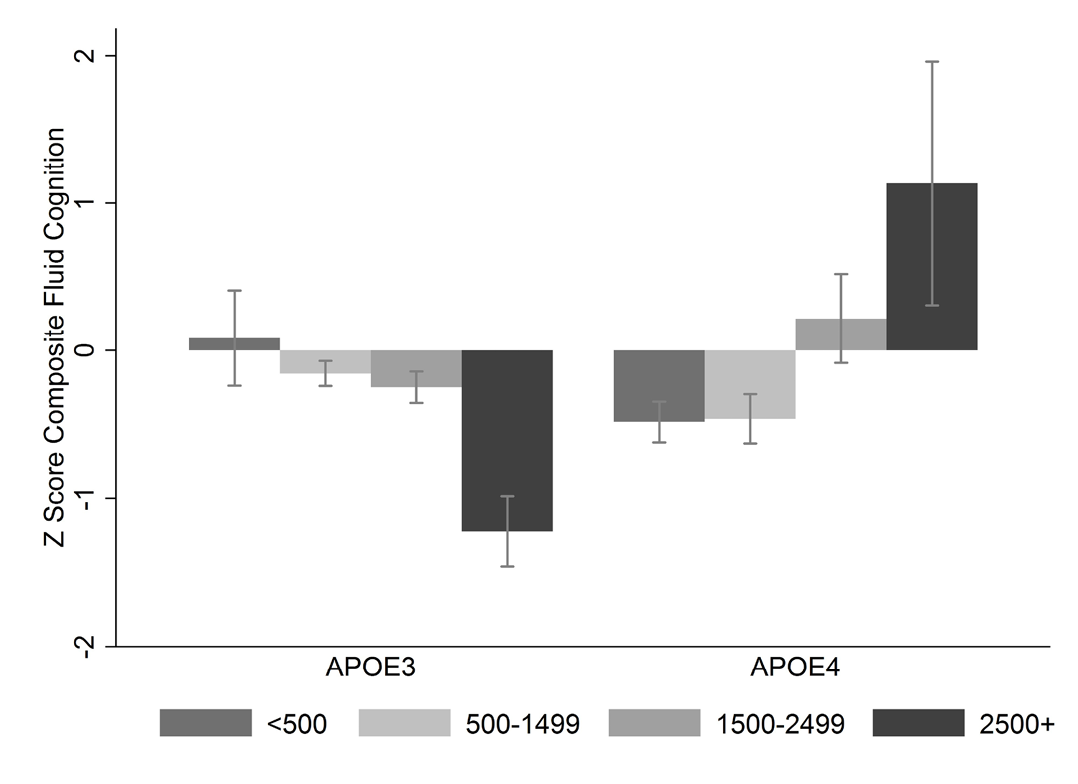
Fig. 6 Cognitive performance, apolipoprotein-E (APOE) allelic variation and parasitic infection. Predicted z-scores for composite fluid cognitive performance (n=242 adults aged 30+). APOE alleles include E3 and E4; parasite infection proxied by four levels of eosinophil count (“normal” <500/uL, “mild” eosinophilia 500–1499/uL, “marked” eosinophilia 1500–2499/uL, “very high” eosinophilia ≥2500 /uL). Model controls for age, sex, years of schooling, Spanish fluency, and community ID as a random effect. See Trumble et al. (2017) for more details.
Despite the belief that depression is largely a modern ailment unique to industrialized populations characterized by high inequality, intense social competition and eroded family ties (Nesse, 2000), Tsimane adults frequently report symptoms of persistent sadness that interferes with routine daily functioning. We find that depression in older adults is associated with reduced energetic status, greater physical limitations, reduced subsistence involvement and greater social conflict (Stieglitz et al., 2014), consistent with a human life history perspective emphasizing the importance of adult economic production surplus and downward net transfers. We also find that emotional, cognitive and somatic symptoms of depression are each associated with greater immune activation (i.e. pro-inflammatory cytokines), both at baseline and in response to ex vivo stimulation (Stieglitz et al., 2015) (Figure 7). This result is consistent with depression serving as a type of “sickness behavior” geared towards conserving energy to aid in immune defenses against infection. In Western populations, the association between immune activation and depression has instead typically been interpreted to reflect reduced cellular immunity, and immune dysregulation due, in part, to reduced pathogen exposure during child development.
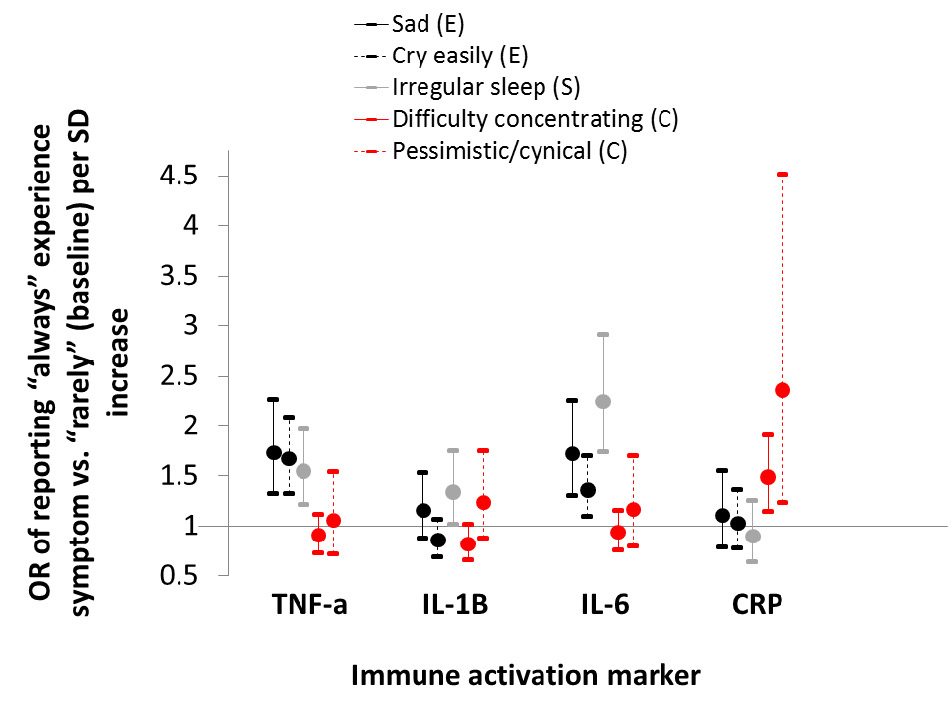
Fig. 7 Depression symptoms and immune activation. Odds ratio (OR with 95% CI) of reporting always vs. rarely experiencing a symptom per standard deviation unit increase in cytokine or lnCRP concentration. Symptoms are categorized by whether they include emotional (E, black), somatic (S, gray), or cognitive (C, red) components. Estimates derived from multinomial logistic regression controlling for age, age2, sex, body mass index and geographic region of residence. See Stieglitz et al. (2016) for more details.
In summary, mortality rate increases in late adulthood are linked to changes in physical condition due to aging and associated declines in muscularity and strength, aerobic fitness, sensory acuity, cognitive performance and immune function. Those changes, in turn, are linked to changes in economic productivity and psychological well-being. The productivity of Tsimane adults supports net economic transfers to descendants until the eighth decade, coinciding with the modal age of adult death observed among Tsimane and other subsistence populations (Gurven and Kaplan, 2007). These findings demonstrating declining fitness-related utility at late ages are compatible with the “disposable soma” theory of aging, which views aging as the result of compromised energy allocation favoring investments providing fitness benefits earlier in life (e.g. direct reproduction) in light of somatic maintenance costs that increase with age (Kirkwood and Westendorp, 2001). However, despite speculation, it has still not been clearly determined why humans live as long as they do, but not longer. One possibility is that the fitness benefits of costly investments in grandchildren outweigh the fitness costs of slowing down the aging process, but that these benefits diminish once grandchildren are past the high mortality period early in life. Given dispersal, migration and the dilution of genetic relatedness with each successive generation, the sum of fitness effects over all descendants may be too small to favor further delays in aging.
Pathogens and Life History
Throughout history, human populations were exposed to an array of pathogens, many of which were common to other wild primate species (Nunn et al., 2004). Ancestral humans may also have been exposed to additional pathogens due to the consumption of meat and fish (Finch and Stanford, 2004). Phylogenetic evidence for several pathogens, including smallpox, Plasmodium falciparum, and Mycobacteria tuberculosis suggests a pre-agricultural history of exposure (see review in Pearce-Duvet, 2006, Bos et al., 2014). Sexually transmitted diseases also likely have a long evolutionary history among humans (Donovan, 2000). Antibodies to viral infections, such as herpes simplex, Epstein-Barr and varicella-zoster virus (VZV) have been documented in isolated Amazonian groups, along with cytomegalovirus (CMV), intestinal helminths, herpes simplex viruses, hepatitis B and arboviruses (Black et al., 1970, Salzano and Callegari-Jacques, 1988).
Helminths, or intestinal worms, have coexisted with humans for millennia and represent a major feature of early human disease ecology (see Hurtado et al., 2008 for review). Non-human primates are widely infected with helminths, and infection with multiple species of soil-transmitted intestinal parasites has been documented in remote Amerindian populations (Lawrence et al., 1980, Confalonieri et al., 1991, Hurtado et al., 2008). Macro-parasites such as Enterobius vermicularis (pinworm) and hookworms (Necator americanus and Ancylostoma duodenale) have been discovered in coprolites from 7–10kya (Fry and Moore, 1969, Gonçalves et al., 2003). Throughout human history, helminth burdens have fluctuated, but it is likely that the absence of helminths is a very recent occurrence specific to industrialized, urban environs. Helminths have complex life cycles within human hosts, passing through numerous host tissues, and with intricate survival strategies that involve not only thwarting host immunity, but also competing with other helminths for host resources and creating a favorable niche by host manipulation (Maizels et al., 2004, Blackwell et al., 2013). This long history suggests that human immune systems have co-evolved with helminths and may occasionally produce maladaptive outcomes under the novel, mismatched conditions introduced in recent human history.
Tsimane exhibit high rates of diverse infections. Over 66% of Tsimane have at least one intestinal parasite, the most common being hookworm (Ancylostoma duodenale or Necator americanus, prevalence 56%), roundworm (Ascaris lumbricoides, 15%) and whipworm (Trichuris sp., 4%) (Blackwell et al., 2011). Protozoan infections are also common, including Giardia lambia (30%), and E. histolytica (5%). About half of men and women have anemia, with children and adolescents showing the highest risk (56% of girls, 63% boys). Polyparasitic co-infections are common. Several helminth species co-occur, whereas helminths such as hookworm and roundworm appear to have protective effects against giardia infection (Blackwell et al., 2013). Helminths also affect Tsimane fertility: hookworm is associated with reduced fertility, while roundworm is associated with shorter interbirth intervals, perhaps by increasing maternal immunological tolerance for a fetus (Blackwell et al., 2015). Given the high transmission rate of multiple pathogens in the Tsimane environment, the prevalence of several parasites and energetic investments in immune defenses against them (e.g. immunoglobulin-E, IgE production) peak earlier in childhood than in populations with lower transmission rates (Blackwell et al., 2011). This finding is consistent with the “peak shift” hypothesis, which suggests that in more infectious environments, immune defenses develop earlier and thus peak incidence rates occur at younger ages.
Perhaps as a consequence of earlier and consistent immune responses to diverse pathogens over the life course, several components of adaptive immune function show evidence of rapid senescence. Adaptive immunity refers to humoral and cell-mediated immune components that help build immunological memory to specific pathogens in order to mount an effective response upon re-exposure. Naïve CD4+ helper T-cells, essential for mobilizing immune defenses against unfamiliar pathogens, are considerably depleted by age 50, while natural killer cell counts are substantially elevated (Blackwell et al., 2016). Consistent with these patterns, a measure of epigenetic age acceleration — based in part on the estimation of immune cell counts — is higher among Tsimane relative to other populations (Horvath et al., 2016).
Indeed, infections are the main source of Tsimane morbidity and mortality over the life course (Gurven et al., 2007). Gastrointestinal illness and respiratory infections are frequent diagnoses: 30–40% of infants and young children suffer from each; 30–40% of adults suffer from gastrointestinal illness and 20–30% from respiratory infections. Living in a pathogenic environment likely favors pro-inflammatory (e.g. C-Reactive Protein [CRP], Interleukin-6 [IL-6]) alleles (Vasunilashorn et al., 2011), and higher levels of inflammation than in more hygienic environments. Levels of one indicator of inflammation, CRP, are higher than among Americans, especially in childhood (Blackwell et al., 2016). Cross-sectional estimates of life lived with high CRP indicate that by age 34, Tsimane have spent an average of 15 years (42% of life) with high CRP, compared to 6.8 years (19%) in the US. Tsimane CRP levels in early life are higher than those sampled among diverse populations, including Italians, Mexicans, Filipinos, and Native Americans in the USA (Gurven et al., 2008). CRP levels vary between and within individuals, with half of the total variance being between individuals; elevations thus likely do not represent only acute infections, and instead are moderately stable within individuals over time (Blackwell et al., 2016). In contrast to the geographical distribution of some sexually transmitted infections like trichomoniasis (where Tsimane living near town have higher prevalence than those living farther from town) (Stieglitz et al., 2012), Tsimane living farther from town show higher CRP levels than those living near town, suggesting higher exposure to other infectious ailments in remote villages. Other biomarkers also suggest high levels of immune activity throughout life: Tsimane have higher levels of white blood cells, erythrocyte sedimentation rate, B cells, and natural killer cells than Americans at all ages (Blackwell et al., 2016). On average, 20% of Tsimane white blood cells are eosinophils, consistent with high levels of parasitic infection, compared with the US reference range of <5%. Antibodies related to infection are also much higher among Tsimane: immunoglobulin-G (IgG) is about twice as high, and IgE, most relevant for helminthic infection, is about 100 times higher than typical US levels (Blackwell et al., 2011).
Perhaps as a consequence of high levels of infection and immune activation, Tsimane show elevated resting metabolic rates, with 10–15% of metabolism associated with immune activation (Gurven et al., 2016). The high prevalence of infection, and requisite energy shunted towards immune defenses, may help explain the slow somatic growth and stunting common among Tsimane and similar energy-limited populations experiencing high pathogen burden (Blackwell et al., 2017). Population differences in growth trajectories during childhood may reflect patterns of pathogen exposure and immune investment, since Tsimane show slower growth during periods of their early peak IgE production (Blackwell et al., 2011). The higher energetic cost of tolerating and/or defending oneself from parasites may be further offset by other shifts in energy use. Possibilities in the Tsimane context include lower physical activity, sickness behavior (Stieglitz et al., 2015), cachexia and osteopenia (Stieglitz et al., 2015), dyslipidemia and anemia (Gurven et al., 2016, Straub et al., 2010).
In summary, infectious disease has multiple phenotypic consequences. Not surprisingly, it appears to upregulate immune activity, resulting in greater energy expenditures throughout life and more rapid senescence of some immune cell populations. Variation in pathogen burden across human environments, and over time within individuals, seems to be associated with adaptive and plastic immune responses. The Tsimane, living in a warm, humid and tropical environment in relatively settled communities, may experience greater pathogen burden than other contemporary and ancestral human populations in drier, colder, or more mobile environments (Page et al., 2016). Yet their modal age at death is similar to that of subsistence-level populations living in drier environments (Gurven and Kaplan, 2007). Perhaps natural selection on human aging has resulted in a species-typical lifespan, despite sources of morbidity and death differing across populations.
Chronic Disease, Mismatch and Lifestyle Change
The THLHP provides an opportunity to test ideas about the role of environmental and socioeconomic change on health concerns believed to be either universal aspects of human aging, or consequences of an evolutionary mismatch between long-standing genetic adaptations and novel environments. We have found that several conditions common in urban areas of both high and low-income countries are rare or absent among Tsimane. As observed in other rural settings (Yazdanbakhsh et al., 2002, Rook, 2012), allergies, atopy and other auto-immune diseases are rare among Tsimane. This is to be expected according to the “hygiene” and “old friends” hypotheses, which propose that early pathogenic exposures, especially helminths, help promote improved immune regulation in ways that temper pro-inflammatory responses (Yazdanbakhsh et al., 2002, Rook, 2012). Benign prostatic hyperplasia is also rare, presumably due in large part to lower testosterone levels in early adulthood than those reported in Western populations (Trumble et al., 2015). Lower cumulative exposure to testosterone may be protective against benign prostatic hyperplasia. Reproductive cancers, such as endometrial, ovarian, breast and prostate cancers, are also often associated with high levels of cumulative exposure to reproductive hormones, and appear to be rare among Tsimane as suggested by our clinical data; other cancers of more infectious etiology, however, such as cervical cancer, are expected to be more common among Tsimane than in other populations due to higher infectious burden (Stieglitz et al., 2012).
Atherosclerosis, the main cause of CVD, also appears to be largely absent among Tsimane for several reasons (Figure 8). First, cardio-metabolic risk factors associated with greater heart disease and stroke risk in industrialized populations, such as obesity, high cholesterol, and hypertension, are rare among the Tsimane (Gurven et al., 2012). Even after adjusting for lower Tsimane body mass, rates of blood pressure increase in adulthood are lower among Tsimane than in 52 other populations from the INTERSALT study (Gurven et al., 2012). Second, despite living in semi-permanent villages with limited residential mobility, Tsimane are not sedentary; they engage in high levels of moderate physical activity, even at advanced ages (Gurven et al., 2013), and show strong cardiorespiratory fitness, as measured by VO2max (Pisor et al., 2013) and high prevalence of bradycardia (resting pulse<60). Third, Tsimane diet is lean but calorie rich, and abundant in fiber and omega-3 fatty acids (Martin et al., 2012, Kraft et al., 2018). Regular cigarette smoking is rare (Gurven et al., 2009).

Fig. 8 75th Percentile of Coronary Arterial Calcification (CAC) in women (A) and men (B) across populations. Tsimane data are compared to U.S. (Multi-Ethnic Study of Atherosclerosis [MESA], Mid America Heart Institute [MAHI], University of Illinois at Chicago [UIC]), Germany (Heinz Nixdorf RECALL Study [HNR]), Japan and Korea. A bootstrapped 95% CI is displayed for Tsimane data. See Kaplan et al. (2017) for more details and references.
On the other hand, CRP and IL-6 — two biomarkers of inflammation that independently predict CVD morbidity and mortality in industrialized populations — are elevated throughout life among Tsimane, likely as a result of consistent pathogenic exposure (Gurven et al., 2008, Blackwell et al., 2016). In industrialized contexts, inflammation has been linked to every stage of heart disease, from atheroma and plaque formation to myocardial infarction (Buckley et al., 2009). Yet despite elevated levels of CRP, IL-6 and erythrocyte sedimentation rate, Tsimane present with exceptionally low levels of atherosclerosis (Kaplan et al., 2017). Tsimane high density lipoproteins (HDL, “good cholesterol”) levels are also low (Vasunilashorn et al., 2010). However, peripheral arterial disease (PAD), a pre-cursor to fully developed atherosclerosis, is also not observed among adults, as assessed by ankle brachial blood pressure index; PAD increases with age in every other population studied to date (Gurven et al., 2009). Coronary artery calcification (CAC), a sensitive predictor of cardiovascular morbidity and mortality measured from thoracic computed tomography (CT) scans, is also extremely low among Tsimane (Kaplan et al., 2017) (Figure 8); only 8% of Tsimane by age 80 show evidence of moderate atherosclerosis, compared to 51% of “healthy” US adults. Several hundred “verbal autopsy” reports of recent and past deaths also reveal few cases of obvious cardiac or cerebrovascular events, and so mortality selection does not appear to be culling younger individuals with CVD. Ongoing cranial CTs will assess changes in cerebral morphology to help understand cognitive aging, dementia, and the link between CVD and dementia. Due to the relative absence of overt atherosclerosis and vascular disease, we expect to find lower rates of cognitive impairment and several types of cerebral atrophy in late adulthood among Tsimane than reported elsewhere. Alternatively, greater infection, inflammation and limited schooling may accelerate cerebral atrophy, cognitive decline and dementia.
Despite the systemic pro-inflammatory environment fostered by some bacterial and viral infections, other infections might offer protection against prominent chronic diseases of aging. A number of animal models provide evidence of protective effects of one type of parasitic infection on T2DM and CVD — helminths. These include mostly intestinal geohelminths such as hookworm and roundworm, but may also include water-borne helminths such as schistosomes and insect-borne filarial helminths such as Wuscheria bancrofti. The notion that helminths in particular might offer protection against atherosclerosis was first proposed in 2005 by the Israeli physician, Eli Magen (Magen et al., 2005). Exploring the role that pathogenic exposure, particularly helminths, plays in risk of atherosclerosis and T2DM is a relatively novel research direction that merits further attention.
As part of their own strategies to insure their own survival and reproduction, helminths have multiple effects on their host. They consume blood lipids and glucose, alter lipid metabolism, and modulate immune function towards greater TH2 (T helper cell type 2) anti-inflammatory activity. In combination, these conditions can lower blood cholesterol, reduce obesity, increase insulin sensitivity, decrease atheroma progression, and reduce likelihood of atherosclerotic plaque rupture (Wiria et al., 2014, Gurven et al., 2016). Consistent with these expectations, we find that biomarkers of helminthic infection (e.g. IgE, eosinophils) are inversely associated with total cholesterol, LDL, HDL, and obesity (Vasunilashorn et al., 2010) (Figure 9). Total cholesterol is almost 10 points lower among those with elevated CRP and IL-6, and 19 points lower among those with elevated IgE controlling for potential confounders (Vasunilashorn et al., 2010). Other human studies are consistent with potential cardio-protective effects of helminths. There was minimal clinical atherosclerosis in patients with schistosomal hepatic fibrosis (Assaad-Khalil et al., 1991), lower levels of T2DM with lymphatic filariasis (Aravindhan et al., 2010), and lower blood glucose, glycated hemoglobin (HbA1c), insulin resistance, triglycerides and LDL with prior Schistosomiasis japonicum infection (Chen et al., 2012). The Indonesian ImmunoSPIN project has found that helminth infections are associated with greater insulin sensitivity (Wiria et al., 2015). Lastly, an autopsy study of cadavers in the Khanty-Mansiisk region of Russia measured both Opisthochis felineus worm burden and area of atherosclerotic lesions in the thoracic and aortic arteries (Magen et al., 2013). Fatty streaks, fibrotic plaques and complicated lesions were inversely related to the number of worms per infected liver and were most common in uninfected individuals.
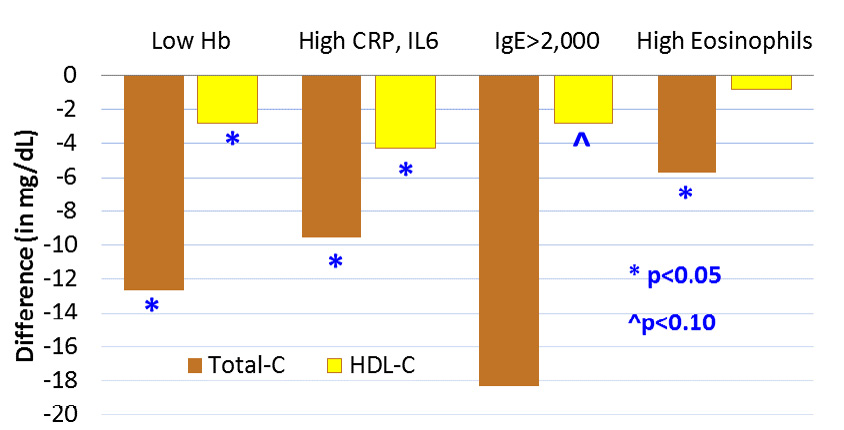
Fig. 9 Relationships between indicators of infection and immune activation on blood lipids. Low hemoglobin, high CRP and IL-6, high IgE and eosinophil count are all associated with lower total blood cholesterol, and to some extent with lower HDL cholesterol. Sample restricted to adults age 20+ from 17 Tsimane villages in 2004. Results based on multiple regression analyses of total-C (n=345) and HDL (n=318) that also control for age, sex, BMI. Low Hb refers to first quartile, high eosinophils refers to fourth quartile. Based on Vasunilashorn et al. (2010).
In summary, Tsimane have a very low frequency of chronic diseases typically found in Western populations. The relative roles of energetic expenditure, diet and pathogen burden in explaining differences in chronic disease risk among populations are still poorly understood due to the scarcity of detailed, longitudinal studies of appropriate populations in epidemiological transition, but each appears to play a contributory role. THLHP research shows that a more nuanced understanding of the role of infection-induced inflammation in the etiology of chronic diseases is needed. Perhaps high levels of inflammation are only atherogenic and diabetogenic in the context of high adiposity and minimal exercise. Alternatively, different sources of inflammation may have different effects on chronic disease, with infection in some cases actually lowering chronic disease risk.
Conclusion
Longevity is a feature of Homo sapiens living subsistence lifestyles, in direct contradiction to the Hobbesian view that human lifespan was “nasty, brutish and short” — a view also traditionally supported by paleodemographic lifetables of prehistoric populations. The THLHP provides an interesting case study with broad relevance to demography, and has contributed to critical debates in the social and life sciences. THLHP helps bridge theory with empirical data that establishes vital links between kin-directed cooperation, reciprocal exchange networks, age profiles of productivity and health, and human life history traits. Our research program thus attempts to advance a “whole organism” understanding of the aging process and health in environmental conditions more similar to the ones in which humans evolved, while also studying reaction norms and potential health-related mismatches as those environmental conditions change.
An ethnographic emphasis combined with an evolutionary and ecological focus has the ability to influence multiple disciplines by highlighting aspects of human diversity typically ignored by more conventional research traditions. For example, Tsimane findings support new ways of thinking about the role of inflammation on chronic disease etiology, and the potential benefits of helminth infection on immune regulation (as well as on cognitive performance, fertility and insulin resistance). Indeed, the long co-evolutionary history of helminths and other pathogens with humans highlights neglected potential mutualisms with beneficial effects on human health. The relative absence of many chronic diseases that afflict high income countries, including coronary heart disease, T2DM, osteoporosis, autoimmune diseases, prostate and breast cancer, provides new opportunities for study. A causal understanding of these and other mismatch diseases may be advanced by further study of groups like the Tsimane who are undergoing rapid changes in multiple ways that can be studied jointly.
Although the Tsimane represent a single case study, an important goal is to stimulate more cross-cultural comparative research using standardized methods. For example, a comparative approach will be needed to make broader inferences about the relative importance of infection, lifestyle factors and genetics in chronic disease morbidity. In this case, comparison of populations varying in parasite burden, diet and activity profile can provide insight into the role of immune dysregulation on chronic disease progression.
Acknowledgements
We are grateful and honored by the privilege of living and working with the Tsimane. Nothing would be possible without talented, responsible and committed THLHP logistic and biomedical personnel, especially Roberta Mendez, Carmen Mavis Ardaya, Daniel Eid Rodriguez, Edhitt Cortez Linares, and Raul Quispe Gutierrez. We thank Tsimane research assistants for their enormous efforts over the years, especially Juana Bani Cuata, Maguin Gutierrez Cayuba, Bacilio Vie Tayo, and Arnulfo Cari Ista. We also thank all student researchers and key collaborators, without whom the THLHP could not function. THLHP has been generously supported by the National Institutes of Health/National Institute on Aging (NIH/NIA) (R01AG024119, R56AG024119, P01AG022500, RF1AG054442) and the National Science Foundation (BCS0136274, BCS0422690, RAPID BCS1440212).
References1
Aravindhan, V., Mohan, V., Surendar, J., Rao, M.M., Pavankumar, N., Deepa, M., Rajagopalan, R., Kumaraswami, V., Nutman, T.B., and Babu, S., ‘Decreased Prevalence of Lymphatic Filariasis among Diabetic Subjects Associated with a Diminished Pro-Inflammatory Cytokine Response (Cures 83)’, PLoS Negl Trop Dis, 4 (2010), e707.
Assaad-Khalil, S., Lachine, N., Sidrak, M., Amara, F., Jacotot, B., and Fahmy, M., ‘Immuno-Metabolic Factors in Schistosomal Hepatic Fibrosis Modulating Atherogenesis’, Annales de biologie clinique, 50 (1991), 697–701.
Black, F.L., Woodall, J.P., Evans, A.S., Liebhaber, H., and Henle, G., ‘Prevalence of Antibody against Viruses in the Tiriyo, an Isolated Amazonian Tribe’, American Journal of Epidemiology, 91 (1970), 430–38.
Blackwell, A.D., Gurven, M.D., Sugiyama, L.S., Madimenos, F.C., Liebert, M.A., Martin, M.A., Kaplan, H.S., and Snodgrass, J.J., ‘Evidence for a Peak Shift in a Humoral Response to Helminths: Age Profiles of Ige in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. Nhanes’, PLoS Negl Trop Dis, 5 (2011), e1218.
Blackwell, A.D., Martin, M., Kaplan, H., and Gurven, M., ‘Antagonism between Two Intestinal Parasites in Humans: The Importance of Co-Infection for Infection Risk and Recovery Dynamics’, Proceedings of the Royal Society B: Biological Sciences, 280 (2013).
—, ‘Antagonism between Two Intestinal Parasites in Humans: The Importance of Co-Infection for Infection Risk and Recovery Dynamics’, Proceedings of the Royal Society of London B: Biological Sciences, 280 (2013), 20131671.
Blackwell, A.D., Tamayo, M.A., Beheim, B., Trumble, B.C., Stieglitz, J., Hooper, P.L., Martin, M., Kaplan, H., and Gurven, M., ‘Helminth Infection, Fecundity, and Age of First Pregnancy in Women’, Science, 350 (2015), 970–72.
Blackwell, A.D., Trumble, B.C., Maldonado Suarez, I., Stieglitz, J., Beheim, B.A., Snodgrass, J.J., Kaplan, H., and Gurven, M., ‘Immune Function in Amazonian Horticulturalists’, Annals of Human Biology, 43 (2016), 382–96.
Blackwell, A.D., Urlacher, S.S., Beheim, B.A., von Rueden, C., Jaeggi, A.V., Stieglitz, J., Trumble, B., Gurven, M., and Kaplan, H., ‘Growth References for Tsimane Forager-Horticulturalists of the Bolivian Amazon’, American Journal of Physical Anthropology, 162 (2017).
Blurton Jones, N., Demography and Evolutionary Ecology of Hadza Hunter-Gatherers. Vol. 71 (Cambridge University Press, 2016).
Bos, K.I., Harkins, K.M., Herbig, A., Coscolla, M., Weber, N., Comas, I., Forrest, S.A., Bryant, J.M., Harris, S.R., and Schuenemann, V.J., ‘Pre-Columbian Mycobacterial Genomes Reveal Seals as a Source of New World Human Tuberculosis’, Nature, 514 (2014), 494–97.
Buckley, D.I., Fu, R., Freeman, M., Rogers, K., and Helfand, M., ‘C-Reactive Protein as a Risk Factor for Coronary Heart Disease: A Systematic Review and Meta-Analyses for the Us Preventive Services Task Force’, Annals of internal medicine, 151 (2009), 483–95.
Chen, Y., Lu, J., Huang, Y., Wang, T., Xu, Y., Xu, M., Li, M., Wang, W., Li, D., and Bi, Y., ‘Association of Previous Schistosome Infection with Diabetes and Metabolic Syndrome: A Cross-Sectional Study in Rural China’, The Journal of Clinical Endocrinology & Metabolism, 98 (2012), E283-E87.
Confalonieri, U., Ferreira, L.F., and Araujo, A., ‘Intestinal Helminths in Lowland South American Indians: Some Evolutionary Interpretations’, Human Biology, 63 (1991), 863–73.
Donovan, B., ‘The Repertoire of Human Efforts to Avoid Sexually Transmissible Diseases: Past and Present. Part 1. Strategies Used before or Instead of Sex’, Sex Transm Infect, 76 (2000), 88–93.
Eaton, S.B., Konner, M.J., and Shostak, M., ‘Stone Agers in the Fast Lane: Chronic Degenerative Diseases in Evolutionary Perspective’, American Journal of Medicine, 84 (1988), 739–49.
Finch, C.E., and Stanford, C.B., ‘Meat-Adaptive Genes and the Evolution of Slower Aging in Humans’, Quarterly Review of Biology, 79 (2004), 3–50.
Fry, G.F., and Moore, J.G., ‘Enterobius Vermicularis: 10,000-Year-Old Human Infection’, Science, 166 (1969), 1620–20.
Fumagalli, M., Sironi, M., Pozzoli, U., Ferrer-Admettla, A., Pattini, L., and Nielsen, R., ‘Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution’, PLoS Genet, 7 (2011), e1002355.
Gonçalves, M.L.C., Araújo, A., and Ferreira, L.F., ‘Human Intestinal Parasites in the Past: New Findings and a Review’, Memorias do Instituto Oswaldo Cruz, 98 (2003), 103–18.
Gurven, M., ‘Infant and Fetal Mortality among a High Fertility and Mortality Population in the Bolivian Amazon’, Social Science & Medicine, 75 (2012), 2493–502.
Gurven, M., Blackwell, A.D., Rodríguez, D.E., Stieglitz, J., and Kaplan, H., ‘Does Blood Pressure Inevitably Rise with Age? Longitudinal Evidence among Forager-Horticulturalists’, Hypertension, 60 (2012), 25–33.
Gurven, M., Fuerstenberg, E., Trumble, B.C., Stieglitz, J., Beheim, B., Davis, H., and Kaplan, H., ‘Cognitive Performance across the Life Course of Bolivian Forager-Farmers with Limited Schooling’, Developmental Psychology, 53 (2017), 160–76.
—, ‘Cognitive Performance across the Life Course of Bolivian Forager-Farmers with Limited Schooling’, Developmental Psychology (2016), http://dx.doi.org/10.1037/dev0000175
Gurven, M., Jaeggi, A.V., Kaplan, H., and Cummings, D., ‘Physical Activity and Modernization among Bolivian Amerindians’, PLoS ONE, 8 (2013), e55679.
Gurven, M., and Kaplan, H., ‘Longevity among Hunter-Gatherers: A Cross-Cultural Comparison’, Population and Development Review, 33 (2007), 321–65.
Gurven, M., Kaplan, H., Crimmins, E., Finch, C., and Winking, J., ‘Lifetime Inflammation in Two Epidemiological Worlds: The Tsimane of Bolivia and the United States’, Journal of Gerontology Biological Sciences, 63A (2008), 196–99.
Gurven, M., Kaplan, H., and Gutierrez, M., ‘How Long Does It Take to Become a Proficient Hunter? Implications for the Evolution of Delayed Growth’, Journal of Human Evolution, 51 (2006), 454–70.
Gurven, M., Kaplan, H., Winking, J., Eid, D., Vasunilashorn, S., Kim, J., Finch, C., and Crimmins, E., ‘Inflammation and Infection Do Not Promote Arterial Aging and Cardiovascular Disease among Lean Tsimane Forager-Horticulturalists’, PLoS ONE, 4 (2009), e6590.
Gurven, M., Kaplan, H., and Zelada Supa, A., ‘Mortality Experience of Tsimane Amerindians: Regional Variation and Temporal Trends’, American Journal of Human Biology, 19 (2007), 376–98.
Gurven, M., Stieglitz, J., Hooper, P.L., Gomes, C., and Kaplan, H., ‘From the Womb to the Tomb: The Role of Transfers in Shaping the Evolved Human Life History’, Experimental Gerontology, 47 (2012), 807–13.
Gurven, M., Stieglitz, J., Trumble, B., Blackwell, A.D., Beheim, B., Davis, H., Hooper, P., and Kaplan, H., ‘The Tsimane Health and Life History Project: Integrating Anthropology and Biomedicine’, Evolutionary Anthropology: Issues, News, and Reviews, 26 (2017), 54–73.
Gurven, M., Winking, J., Kaplan, H., von Rueden, C., and McAllister, L., ‘A Bioeconomic Approach to Marriage and the Sexual Division of Labor’, Human Nature, 20 (2009), 151–83.
Gurven, M.D., Stieglitz, J., Trumble, B., Blackwell, A.D., Beheim, B., Davis, H., Hooper, P.L., and Kaplan, H., ‘The Tsimane Health and Life History Project: Integrating Anthropology and Biomedicine’, Evolutionary Anthropology, 26 (2017).
Gurven, M.D., Trumble, B.C., Stieglitz, J., Blackwell, A.D., Michalik, D.E., Finch, C.E., and Kaplan, H.S., ‘Cardiovascular Disease and Type 2 Diabetes in Evolutionary Perspective: A Critical Role for Helminths?’, Evolution, medicine, and public health, 2016 (2016), 338–57.
Gurven, M.D., Trumble, B.C., Stieglitz, J., Yetish, G., Cummings, D., Blackwell, A.D., Beheim, B., Kaplan, H.S., and Pontzer, H., ‘High Resting Metabolic Rate among Amazonian Forager‐Horticulturalists Experiencing High Pathogen Burden’, American Journal of Physical Anthropology (2016).
Hawkes, K., ‘Grandmothers and the Evolution of Human Longevity’, American journal of human biology, 15 (2003), 380–400.
Hawks, J., Wang, E.T., Cochran, G.M., Harpending, H.C., and Moyzis, R.K., ‘Recent Acceleration of Human Adaptive Evolution’, Proceedings of the National Academy of Sciences, USA, 104 (2007), 20753–58.
Hill, K., Barton, M., and Hurtado, A.M., ‘The Emergence of Human Uniqueness: Characters Underlying Behavioral Modernity’, Evolutionary Anthropology: Issues, News, and Reviews, 18 (2009), 187–200.
Hill, K., and Hurtado, A.M., Ache Life History: The Ecology and Demography of a Foraging People (New York: Aldine de Gruyter, 1996).
Hooper, P.L., Gurven, M., Winking, J., and Kaplan, H.S., ‘Inclusive Fitness and Differential Productivity across the Life Course Determine Intergenerational Transfers in a Small-Scale Human Society’, Proceedings of the Royal Society of London B: Biological Sciences, 282 (2015), 20142808.
Horvath, S., Gurven, M., Levine, M.E., Trumble, B.C., Kaplan, H., Allayee, H., Ritz, B.R., Chen, B., Lu, A., Sun, D., Berenson, G.S., Li, S., Chen, W., Tsao, P., Absher, D., and Assimes, T., ‘An Epigenetic Age Analysis of Race/Ethnicity, Gender and Coronary Heart Disease Addresses Several Paradoxes Surrounding Mortality’, Genome Biology, 17 (2016), 171.
Howell, N., Demography of the Dobe !Kung (New York: Academic Press, 1979).
—, Life Histories of the Dobe! Kung: Food, Fatness, and Well-Being over the Life Span. Vol. 4 (Univ of California Press, 2010).
Hurtado, A.M., Anderson Frey, M., Hurtado, I., Hill, K., and Baker, J., ‘The Role of Helminthes in Human Evolution: Implications for Global Health in the 21st Century’, in Medicine and Evolution: Current Applications, Future Prospects, ed. by Sarah Elton and Paul O’Higgins (New York: Taylor and Francis, 2008), pp. 151–78.
INE, ‘Bolivia Características De Población Y Vivienda: Censo Nacional De Población Y Vivienda 2012’, (La Paz: Instituto Nacional de Estadística, 2012).
Jaeggi, A.V., Hooper, P.L., Beheim, B., Kaplan, H., and Gurven, M., ‘Reciprocal Exchange Patterned by Market Forces Helps Explain Cooperation in a Small-Scale Society’, Current Biology (2016).
Kaplan, H., Gurven, M., Winking, J., Hooper, P.L., and Stieglitz, J., ‘Learning, Menopause, and the Human Adaptive Complex’, Annals New York Academy of Sciences (2010), 30–42.
Kaplan, H., Hill, K., Lancaster, J.B., and Hurtado, A.M., ‘A Theory of Human Life History Evolution: Diet, Intelligence, and Longevity’, Evolutionary Anthropology, 9 (2000), 156–85.
Kaplan, H., Thompson, R., Trumble, B.C., Wann, L.S., Allam, A.H., Beheim, B., Frolich, B., Sutherland, L., Sutherland, J., Stieglitz, J., Eid Rodriguez, D., Michalik, D.E., Rowan, C.J., Lombardi, G., Bedi, R., Garcia, A.R., Min, J.K., Narula, J., Finch, C.E., Gurven, M., and Thomas, G.S., ‘Indigenous South American Tsimane Demonstrate the Lowest Levels of Coronary Atherosclerosis’, Lancet, S0140 (2017), 30752–3.
Kaplan, H.S., ‘The Evolution of the Human Life Course’, in Between Zeus and Salmon: The Biodemography of Aging, ed. by K. Wachter and C. Finch (Washington, D.C.: National Academy of Sciences, 1997), pp. 175–211.
Kaplan, H.S., and Robson, A.J., ‘The Emergence of Humans: The Coevolution of Intelligence and Longevity with Intergenerational Transfers’, Proceedings of the National Academy of Sciences, 99 (2002), 10221–26.
Karmin, M., Saag, L., Vicente, M., Sayres, M.A.W., Järve, M., Talas, U.G., Rootsi, S., Ilumäe, A.-M., Mägi, R., and Mitt, M., ‘A Recent Bottleneck of Y Chromosome Diversity Coincides with a Global Change in Culture’, Genome research, 25 (2015), 459–66.
Kirkwood, T.B.L., and Westendorp, R.G.J., ‘Human Longevity at the Cost of Reproductive Success: Trade-Offs in the Life History’, in Sex and Longevity: Sexuality, Gender, Reproduction, Parenthood, ed. by J.-M. Robine, T.B.L. Kirkwood and M. Allard (Berlin: Springer, 2001), pp. Pp. 1–6
Kraft, T.S., Stieglitz, J., Trumble, B.C., Martin, M., Kaplan, H., and Gurven, M., ‘Nutrition Transition in 2 Lowland Bolivian Subsistence Populations’, The American journal of clinical nutrition, 108 (2018), 1183–95.
Lawrence, D.N., Neel, J.V., Abadie, S.H., Moore, L.L., Adams, L.J., Healy, G.R., and Kagan, I.G., ‘Epidemiologic Studies among Amerindian Populations of Amazonia. Iii. Intestinal Parasitoses in Newly Contacted and Acculturating Villages’, The American journal of tropical medicine and hygiene, 29 (1980), 530–37.
Lee, R.B., and DeVore, I., Kalahari Hunter-Gatherers: Studies of The! Kung San and Their Neighbors (Harvard Univ Pr, 1976).
Magen, E., Borkow, G., Bentwich, Z., Mishal, J., and Scharf, S., ‘Can Worms Defend Our Hearts? Chronic Helminthic Infections May Attenuate the Development of Cardiovascular Diseases’, Medical hypotheses, 64 (2005), 904–09.
Magen, E., Bychkov, V., Ginovker, A., and Kashuba, E., ‘Chronic Opisthorchis Felineus Infection Attenuates Atherosclerosis–an Autopsy Study’, International journal for parasitology, 43 (2013), 819–24.
Maizels, R.M., Balic, A., Gomez‐Escobar, N., Nair, M., Taylor, M.D., and Allen, J.E., ‘Helminth Parasites–Masters of Regulation’, Immunological reviews, 201 (2004), 89–116.
Marlowe, F., The Hadza: Hunter-Gatherers of Tanzania. Vol. 3 (Univ of California Press, 2010).
Martin, M.A., Lassek, W.D., Gaulin, S.J., Evans, R.W., Woo, J.G., Geraghty, S.R., Davidson, B.S., Morrow, A.L., Kaplan, H.S., and Gurven, M.D., ‘Fatty Acid Composition in the Mature Milk of Bolivian Forager‐Horticulturalists: Controlled Comparisons with a Us Sample’, Maternal & child nutrition, 8 (2012), 404–18.
Nesse, R.M., ‘Is Depression an Adaptation?’, Archives of General Psychiatry, 57 (2000), 14–20.
Nunn, C.L., Altizer, S., Sechrest, W., Jones, K.E., Barton, R.A., and Gittleman, J.L., ‘Parasites and the Evolutionary Diversity of Primate Clades’, American Naturalist, 164 (2004), S90-S103.
Oeppen, J., and Vaupel, J.W., ‘Broken Limits to Life Expectancy’, Science, 296 (2003), 1029–31.
Page, A.E., Viguier, S., Dyble, M., Smith, D., Chaudhary, N., Salali, G.D., Thompson, J., Vinicius, L., Mace, R., and Migliano, A.B., ‘Reproductive Trade-Offs in Extant Hunter-Gatherers Suggest Adaptive Mechanism for the Neolithic Expansion’, Proceedings of the National Academy of Sciences, 113 (2016), 4694–99.
Pearce-Duvet, J.M.C., ‘The Origin of Human Pathogens: Evaluating the Role of Agriculture and Domestic Animals in the Evolution of Human Disease’, Biological Reviews, 81 (2006), 369–82.
Pisor, A.C., Gurven, M., Blackwell, A.D., Kaplan, H., and Yetish, G., ‘Patterns of Senescence in Human Cardiovascular Fitness: Vo2max in Subsistence and Industrialized Populations’, American Journal of Human Biology, 25 (2013), 756–69.
Riley, J., Rising Life Expectancy: A Global History (Cambridge: Cambridge University Press, 2001).
Rook, G.A., ‘Hygiene Hypothesis and Autoimmune Diseases’, Clinical reviews in allergy & immunology, 42 (2012), 5–15.
Salzano, F.M., and Callegari-Jacques, S., ‘South American Indians: A Case Study in Evolution’, in Research Monographs on Human Population Biology (Oxford University Press, 1988).
Schniter, E., Gurven, M., Kaplan, H.S., Wilcox, N.T., and Hooper, P.L., ‘Skill Ontogeny among Tsimane Forager-Horticulturalists’, American Journal of Physical Anthropology (2015).
Stearns, S.C., The Evolution of Life Histories (Oxford: Oxford University Press, 1992).
Stieglitz, J., Beheim, B.A., Trumble, B.C., Madimenos, F.C., Kaplan, H., and Gurven, M., ‘Low Mineral Density of a Weight-Bearing Bone among Adult Women in a High Fertility Population’, American Journal of Physical Anthropology, 156 (2015), 637–48.
Stieglitz, J., Blackwell, A.D., Gutierrez, R.Q., Linares, E.C., Gurven, M., and Kaplan, H., ‘Modernization, Sexual Risk-Taking, and Gynecological Morbidity among Bolivian Forager-Horticulturalists’, PLoS ONE, 7 (2012), e50384.
Stieglitz, J., Schniter, E., Von Rueden, C., Kaplan, H., and Gurven, M., ‘Functional Disability and Social Conflict Increase Risk of Depression in Older Adulthood among Bolivian Forager-Farmers’, The Journals of Gerontology Series B: Psychological Sciences and Social Sciences (2014), gbu080.
Stieglitz, J., Trumble, B.C., Thompson, M.E., Blackwell, A.D., Kaplan, H., and Gurven, M., ‘Depression as Sickness Behavior? A Test of the Host Defense Hypothesis in a High Pathogen Population’, Brain, Behavior, and Immunity, 49 (2015), 130–39.
Straub, R.H., Cutolo, M., Buttgereit, F., and Pongratz, G., ‘Energy Regulation and Neuroendocrine–Immune Control in Chronic Inflammatory Diseases’, Journal of Internal Medicine, 267 (2010), 543–60.
Trumble, B.C., Stieglitz, J., Blackwell, A.D., Allayee, H., Beheim, B., Finch, C.E., Gurven, M., and Kaplan, H., ‘Apolipoprotein E4 Is Associated with Improved Cognitive Function in Amazonian Forager-Horticulturalists with a High Parasite Burden’, The FASEB Journal, 31 (2017), 1508–15.
Trumble, B.C., Stieglitz, J., Rodriguez, D.E., Linares, E.C., Kaplan, H.S., and Gurven, M.D., ‘Challenging the Inevitability of Prostate Enlargement: Low Levels of Benign Prostatic Hyperplasia among Tsimane Forager-Horticulturalists’, The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (2015), glv051.
Trumble, B.C., Stieglitz, J., Thompson, M.E., Fuerstenberg, E., Kaplan, H., and Gurven, M., ‘Testosterone and Male Cognitive Performance in Tsimane Forager-Horticulturalists’, American Journal of Human Biology, 27 (2015), 582–86.
UN, ‘World Population Prospects: 2015 Revision’, in United Nations Department of Economic and Social Affairs (2015).
Vasunilashorn, S., Crimmins, E.M., Kim, J.K., Winking, J., Gurven, M., Kaplan, H., and Finch, C.E., ‘Blood Lipids, Infection, and Inflammatory Markers in the Tsimane of Bolivia’, American Journal of Human Biology, 22 (2010), 731–40.
Vasunilashorn, S., Finch, C.E., Crimmins, E.M., Vikman, S.A., Stieglitz, J., Gurven, M., Kaplan, H., and Allayee, H., ‘Inflammatory Gene Variants in the Tsimane, an Indigenous Bolivian Population with a High Infectious Load’, Biodemography and Social Biology, 57 (2011), 33–52.
Walker, R., Hill, K., Kaplan, H., and McMillan, G., ‘Age-Dependency in Skill, Strength and Hunting Ability among the Ache of Eastern Paraguay’, Journal of Human Evolution, 42 (2002), 639–57.
Weiss, A., King, J.E., Inoue-Murayama, M., Matsuzawa, T., and Oswald, A.J., ‘Evidence for a Midlife Crisis in Great Apes Consistent with the U-Shape in Human Well-Being’, Proceedings of the National Academy of Sciences, 109 (2012), 19949–52.
Wiria, A.E., Hamid, F., Wammes, L.J., Prasetyani, M.A., Dekkers, O.M., May, L., Kaisar, M.M., Verweij, J.J., Guigas, B., and Partono, F., ‘Infection with Soil-Transmitted Helminths Is Associated with Increased Insulin Sensitivity’, PLoS ONE, 10 (2015), e0127746.
Wiria, A.E., Sartono, E., Supali, T., and Yazdanbakhsh, M., ‘Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome’, PLoS Pathogens, 10 (2014).
Wood, J.W., Dynamics of Human Reproduction: Biology, Biometry and Demography (New York: Aldine de Gruyter, 1994).
Yazdanbakhsh, M., Kremsner, P.G., and van Ree, R., ‘Allergy, Parasites, and the Hygiene Hypothesis’, Science, 296 (2002), 490–94.
1 Note this chapter has been posted on the Open Science Framework website since 28/06/2019, after it was accepted for publication, so the references will reflect when the chapter was written and not the OBP publication date.
