4. Anthropological and Evolutionary Demography
© 2024 Kim Hill, CC BY 4.0 https://doi.org/10.11647/OBP.0251.04
Demography was once a subfield of the social sciences dedicated to the statistical study of birth and death rates, and the mathematical description of these vital rates (function fitting). This also included an empirical examination of proximate factors that affect vital rates. Anthropological demography focused mainly on small-scale (non-Western) societies, and employed interpretations drawn from so-called “anthropological theory” (e.g., Howell, 1986; Campbell and Wood, 1998; Kertzer and Fricke, 1997; Bernardi, 2007). Cross-cultural comparisons were a mainstay of the field. In the past thirty years, however, anthropological demography changed significantly to become a theoretically informed study of mortality and fertility, and other age-related biological features. The theory is based on an evolutionary perspective that can unite human demographic studies with those of other primates, mammals and vertebrate species (e.g., Hill, 1993; Kaplan, 1996; Vaupel, 2010, Blurton-Jones, 2016). This transition expanded the field from the study of vital rates to one including research on growth, development, ageing patterns, etc. (physiological, cognitive, emotional mechanisms) that are strongly theoretically tied to mortality and fertility schedules (e.g., Ketterson and Nolan, 1992; Rickleffs and Wikelski, 2002; Kaplan and Gangestad, 2005; Kirkwood and Austad, 2000). These important changes in the field emerged primarily from the injection of life history theory from biology into the social sciences. A fundamental proposition of evolutionary biology is the recognition that fertility and mortality are the two components of individual fitness. Hence, all phenotypic adaptations that act on one or both of these components will evolve via natural selection. From this view, it is clear that the mechanisms of fertility and survival are key biological adaptations and can only be fully understood in the context of evolution.
Mammalian Life History Theory
The area of biology that focuses on mortality and fertility is called life history theory (LHT). LHT is a field that examines phenotypic traits whose expression at one age has implications for fertility and mortality rates at other ages. Temporal tradeoffs are therefore central to LHT. The goal of LHT is not just to describe demographic rates (fit them with mathematical equations), but to explain and predict the shape of mortality and fertility functions as adaptive outcomes of natural selection (Stearns, 1976, 1992; Charnov, 1991, 1993; Charlesworth, 1994). Natural selection produces organisms that effectively convert limited resources into gene copies at the highest possible rate in competitive environments. LHT, therefore, concerns the optimal timing of developmental events, investment in growth, somatic maintenance and reproduction, such that living organisms maximize their genetic contribution over time. In short, LHT predicts the fitness maximizing combinations of mortality and fertility investment trajectories that should emerge via natural selection, and the optimal timing of related phenotypic investments given the unavoidable mortality risks of an environment in combination with the ecological opportunities for nutrient capture.
Charnov has described the adaptive LH problem as “growth confronting landscapes of death” (Charnov, 2011). Both nutrient capture profiles and mortality risk are considered to have “extrinsic” components, or “constraints” that determine optimal life histories, just as constraints determine optimal phenotypes for all biological features (Parker and Maynard Smith, 1990). Specifically, some risks of mortality and opportunities for nutrient capture cannot normally be changed with reasonable investments (given species’ general phenotypic design and their econiche); hence populations of organisms can be expected to adapt to those facts as if they were “extrinsic” determinants of optimal investment patterns.
The general design of each mammalian species means that each has a living and feeding niche to which it is adapted. As they grow, mammals are able to harvest more total energy (advantages of body size and strength), but they become less efficient at biological “throughput” (absorbing, transporting and utilizing that energy for growth or reproduction) of that energy (universal metabolic and growth scaling laws). Proportional growth slows with body size (change in weight with time unit is proportional to body weight to the 3/4 power in mammals). This is possibly due to the branching nature of energy transport and distribution through the body (Case, 1978; Kleiber, 1932; West et al., 1997). Since energy for growth is diverted to reproduction during adulthood (see Charnov, 1991, 1993), growth laws imply that the proportional total energy expended on reproduction per unit time also declines with body size in mammals, both across individuals and species. Note, however, that while proportional energy throughput declines, absolute energy harvest and throughput increases monotonically with body size, such that larger females can produce more of the same size offspring per unit time than can smaller females. Finally, for a given feeding niche, there is often an optimal body size. As organisms approach that size they obtain fewer productive advantages from continued growth. The absolute cessation of growth at reproductive maturity in determinant growers such as mammals defines adulthood. At sexual maturity, growth ceases and reproductive function activates. The regular relationships between weight, growth, energy harvest and potential reproductive output are the reason that body mass, and not height or some other anthropometric measure, is the most important life history variable. Importantly, since growth itself is a function of the ecology of energy capture, ecological variation in environmental quality will change optimal life history trajectories.
Given the distributions and availability of food resources and the way that body size effects energy for reproduction, the mortality landscape of an animal’s living environment is the other major determinant of optimal life history. For simplicity, mortality can be divided into two types of hazards: those that can be reduced substantially with reasonable investment (disease, ageing, exposure, etc.), and those that cannot be easily avoided even with reasonable levels of investment (predation, accidents, etc.). The unavoidable hazards in an animal’s environment constitute what is termed “extrinsic mortality”. The two types of mortality hazard are mainly conceptual, because most causes of mortality can be partially avoided with some investment. By appropriate investment, some mortality reduction can be achieved, but a baseline hazard remains that is essentially “unavoidable” (e.g., conspecific violence is probably an unavoidable cause of some death in human societies, yet certain investments can make individuals less susceptible to becoming victims of violent aggression).
While growth in body size increases total energy throughput available for reproduction in adulthood, there are also other potential gains that come from a longer juvenile development period, body growth and delayed reproduction. These gains come from increased “embodied capital” (Kaplan et al., 2000). Some of the most common gains from delaying reproduction include greater safety from predators through increased body size, time to grow and program larger brains, building effective social alliances and the advantages that can be gained through learning and experience prior to adulthood. This then sets up the most basic life history trade-off: reproducing earlier or reproducing later.
How long should a mammal grow before diverting energy to reproduction (age “α” in life history terminology)? Since there are gains from extending the development and growth period, but there is also some probability of death with each interval that is pre-reproductive, natural selection should favor an “optimal juvenile period” that maximizes gene contribution. In general, when mortality is high, or growth to an optimal adult size is rapid, earlier reproduction is favored. If lifetime fitness (w) can be simplified as the product of survival to age α (lα), and the reproductive value at alpha (Vα), the optimal age at first reproduction is precisely when proportional gains in Vα from growing and developing one more time interval are precisely matched by the proportional decrease in probability of survival to the age of first reproduction by waiting one more time interval.
|
w = lα Vα |
(eqn. 1) |
|
w(max), when log dVα = −log dlα |
(eqn. 2) |
Since the right-hand term in eqn. 2 is simply the instantaneous mortality rate, this means that the end of the juvenile growth and development period should take place when the proportional increases in body size and other multiplicative components of reproductive value are exactly matched by the yearly mortality rate (proportional loss in probability of survival to age of first reproduction). Higher mortality around the age of sexual maturity will favor earlier maturity.
While the primary life history traits are yearly survival and reproductive rates, LHT can best be thought of in economic terms with LH investments allocated to “embodied capital” rather than material capital (see Kaplan et al., 2000). The LH that allocates energy in a way that results in greatest inclusive fitness is the one that becomes prevalent over time. Hence, LHT is a biological investment theory analogous to optimizing investment strategies in micro-economics. The fitness-maximizing problem for living organisms that can invest temporally in different life functions is analogous to the problem facing a hypothetical financial investor, endowed with an initial factory that extracts resources from the environment (e.g., mining, logging, fishing, etc.), and who must strategize to maximize total productive income over time, derived from that starting endowment (see Figure 1). Such a factory owner could invest all resources in immediate short-term profit (harvest and sell as much as possible now), or instead invest in growth of the facility and replication of other factories at the expense of maximum short-term productive gain. Most importantly, our hypothetical investor’s time and resources are always finite and divisible such that investment in one facility or function directly reduces the amount that can be invested in alternative operations. Hence, economic investment theory and LHT are both about the study of optimal trade-offs in investment patterns to maximize productive gain over time.
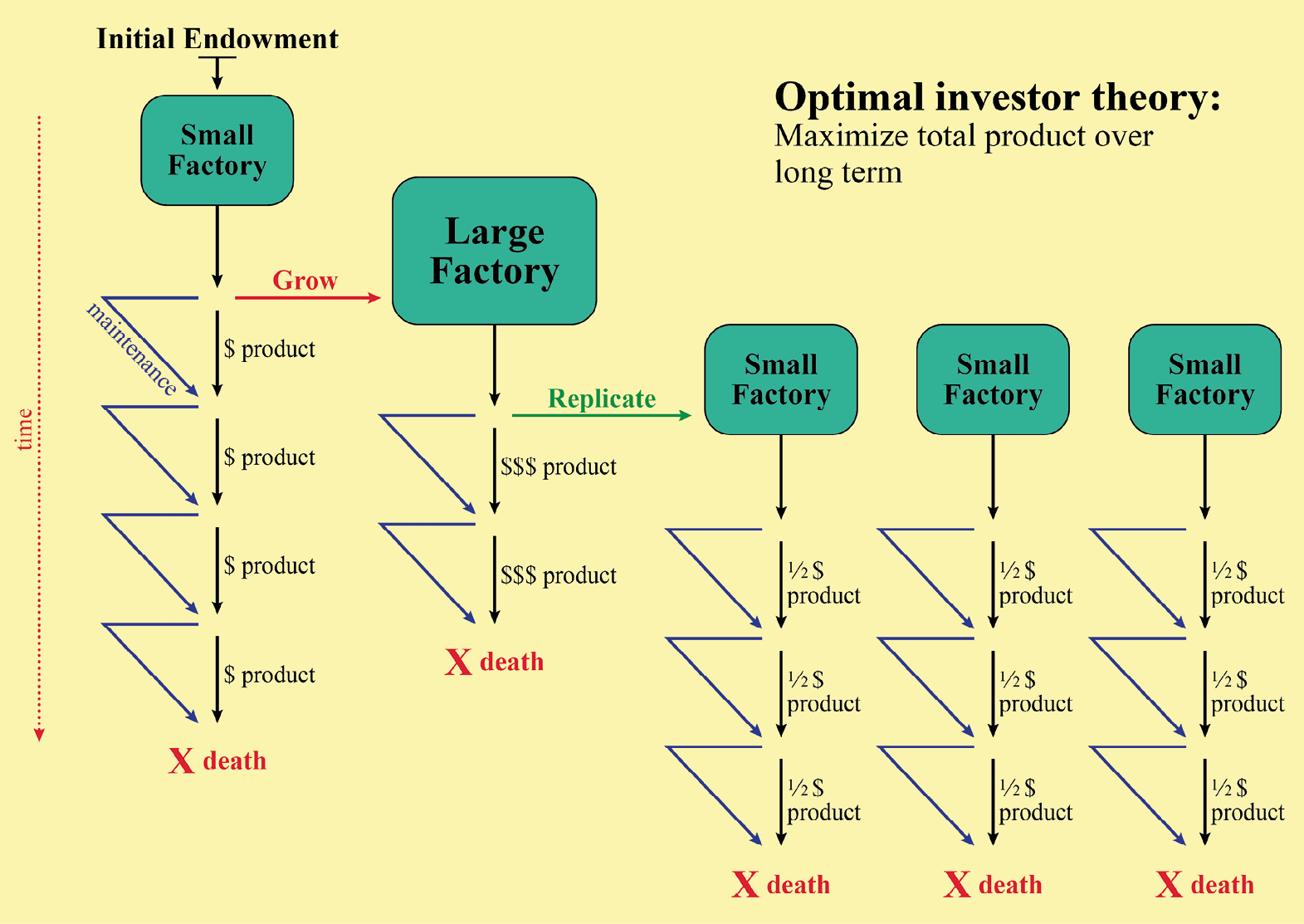
Figure 1: Optimal investor theory suggests that the initial founder of a small factory can either invest in growth, in factory maintenance, or take profits (product) after each production interval. Large factory owners can also convert some production into multiple small factories in order to ameliorate the risk of total loss if the large factory is destroyed by unavoidable circumstances.
Continuing the economic analogy with LHT, our hypothetical factory owner can either extract maximum profits in the short run, or re-invest some resources in expanding the size of the original facility for longer-term profits. Sometimes, he or she will do better to delay taking profits now and instead expand his or her operation, if a larger factory size will substantially increase mean productive harvest per unit time. In our hypothetical scenario, imagine that there is also some finite chance that any factory might be destroyed suddenly or shut down by an unavoidable or unpredictable natural disaster. That threat means that a wise investor should not keep reinvesting into a single expanding factory indefinitely. To do so might risk losing the whole large factory before anticipated long-term profits could be extracted. Instead, it may be wiser to establish several smaller, dispersed factories that will all continue to produce income, even if one of the factories were destroyed. Likewise, an old facility might ultimately deteriorate so much over time that upgrading all the defects would cost more than simply building a new factory from scratch (readers who have purchased a new car rather than continue to pay higher and higher mechanical service costs to repair a failing older vehicle will recognize this dilemma).
Continuing this analogy, our hypothetical investor might discover that the marginal increases in productive harvest with increasing factory size diminish progressively as the factory grows larger (perhaps due to logistical problems of supplying and transporting goods within large factories). This means that higher returns on investment (% gain per dollar of capital investment) are expected if the investor builds several new small factories, growing them to medium size, rather than continuing to expand the size of the original factory indefinitely. In our example, the establishment of new facilities is analogous to biological reproduction, if we stipulate that new factories are always built with a partner who splits the profits 50:50 (analogous to the genetic relationship between parents and offspring). Given the curve of factory productivity with size, deterioration with age and the chances of being destroyed by a natural disaster, the investor must calculate how large to grow his or her factory before investing profits in building a string of smaller factories (and then expanding them in turn). The ultimate goal is to maximize the net worth and total productive harvest capacity of all factories over time. The optimal investment trajectory should maximize total income over time indefinitely.
To finish our analogy between economics and LHT, our hypothetical investor must determine how much to reinvest on repairing and maintaining each factory that he or she builds. The optimal solution must take into account that maintenance costs reduce immediate factory output but allow existing factories to continue producing for longer periods of time. Of course, too much maintenance would be foolhardy if a disaster is likely to destroy any factory in a statistically known time span, but too little maintenance is wasteful of the investment to build the facility in the first place.
The investor analogy illustrates that LHT is an investment theory, just as other biological theories are similar to already developed microeconomic theories (for example Optimal Foraging Theory and models in microeconomics). The investment perspective of LHT allows us to organize trade-offs into major categories that are experienced over time. For humans and all mammals characterized by determinant growth (where captured energy devoted to growth during a juvenile phase is diverted to reproduction in the adult phase), life history trade-offs can be divided into three major categories: (1) growth vs. somatic maintenance; (2) growth vs. reproduction; and (3) reproduction vs. somatic maintenance. The tradeoff perspective also implies that adults face a trade-off within their reproductive budget: using available resources to produce more offspring per reproductive bout, or to produce fewer offspring of higher reproductive value (the quantity vs. quality trade-off) over time.
The entire suite of LH trade-offs can best be conceptualized as a single basic fitness trade-off between present and future reproduction (Bell and Koufopanou, 1986; Harshman and Zera, 2007). This trade-off implies that higher survival or higher fertility (for self or offspring) can only be achieved at the expense of the other (Gadgil and Bossert, 1970). Higher vital rates at one point in time come at the expense of lower rates at another point in time; or increased fitness of ego comes at the expense of decreased fitness of offspring and other close kin. Specific LH tradeoffs can often be detected empirically with careful research. Some nice examples are recent research with wild chimpanzees showing that maternal fertility and offspring growth trade-off against each other (Emery Thompson, 2016); field evidence from traditional human societies showing that fertility often trades off against offspring survival (Strassman & Gillespie, 2002); and studies showing that childhood growth trades off against activity (Urlacher and Kramer, 2018) or investment in disease resistance (immune function) (Urlacher et al., 2018). Finally, studies of ageing in a large number of living organisms strongly suggest that longer lifespan and fitness benefits earlier in life directly trade off against each other as well (Austad and Hoffman, 2018). The laws of conservation of matter and energy imply that investment trade-offs should be ubiquitous in living organisms.
Within each major life history trade-off category there are many sub-trade-offs as well (e.g. invest in immune function vs. invest in DNA repair; grow in mass vs. grow in height; invest more in helping current offspring A vs. current offspring B, etc.). Early life history tradeoffs in humans include: evolutionary decisions such as how long to grow in utero, when to shift from lactational dependence to other foods (decisions made by mothers about optimal gestation time and age of weaning), how much to invest in somatic maintenance and repair during childhood, how long to learn before using knowledge to produce resources or engage in social competition with adults and when to stop growing and start reproducing. Later trade-offs in human adulthood include: how frequently to produce an offspring, how long and how much to invest in each offspring based on age, sex and other qualities, how much energy to dedicate to avoiding illness and injury, when to stop reproducing and focus on helping close kin, how much to invest in somatic repair (anti-senescence) vs. assisting descendant kin. This short list can be expanded into an even larger set of specific trade-off decisions between virtually all energetic investments in survival vs. reproduction, for individuals and their close kin.
Fast and Slow Life Histories
Organisms sometimes experience high “extrinsic” mortality due to causes that cannot be fully avoided (e.g., accidents, predation, extreme variation in food availability or weather) even with reasonable investment. This favours speeding up the timing of events in the life history of the organism in order to complete more reproduction before the opportunity is lost forever through death. Species in such circumstances are said to have a “fast life history”, because they evolve to grow fast, reproduce early and expend greater reproductive effort in each adult time period. The initial difference between high and low extrinsic mortality risk is further compounded due to differential investment in survival. High extrinsic mortality favours less investment in somatic maintenance and repair (less DNA repair, anti-oxidant activity, cell repair, etc.), which results in earlier degenerative death and amplifies the extrinsic differences between species with high and low mortality risk. Because all organisms face natural risks that cannot be avoided by reasonable investment, the overall pace of a life history must be adapted to the chances that reproductive capacity will not be fully realized before the organism is destroyed.
Since optimal investment in somatic maintenance (lifespan), age at sexual maturity and rate of reproductive effort are all strongly affected by extrinsic mortality, we can talk about “fast life histories” as those characterized by short lifespans (early onset of senescence), early age at sexual maturity and high reproductive effort per unit time in adulthood (Promislow and Harvey, 1990). It is well established that a correlation between these LH traits is found across mammal species, hence the “fast-vs-slow” model outlined above is strongly supported empirically (see Purvis and Harvey, 1995; M. Oli, 2004). It is also generally true that smaller animals have faster life histories than larger animals, because predation risk is generally higher for smaller animals (also growing large takes time, so large body size is already an indicator of a longer juvenile period and hence slower LH). However, it is important to note that high or low reproductive effort per unit time does not always mean higher or lower observed fertility rate. The number of offspring produced per unit time (fertility rate) is determined by a combination of how much investment is put into each individual offspring (the “quality-quantity trade-off” in LH theory), and how much energy is invested in reproduction during each time period. The optimal level of investment per offspring, however, is determined by the marginal impact of parental investment (change in offspring reproductive value with additional investment) not by the chances of the adult reproductive dying each year. Since it is possible for parental investment to be efficient (large gains in reproductive value with increased parental investment) or inefficient, the observed fertility rate is determined by the ratio: [Total Reproductive Effort/Investment per Offspring]. It is quite possible for an organism to evolve a long lifespan and a very slow life history (due to low extrinsic mortality) but have a high annual fertility rate (because low investment per offspring is favoured). Examples are found among some large reptiles (e.g., alligators, Lance, 2003) and fish (e.g. ocean sunfish, Pope et al., 2010), which grow large and slowly, have long lifespans, but produce hundreds to thousands of small offspring each reproductive bout (this is also common in large trees). Only when there is low adult extrinsic mortality, and the effect of increased parental investment on offspring reproductive value is large, should we find slower life histories also being characterized by lower fertility rates.
Finally, fast-slow LH adaptations are expected to vary predictably with mortality landscape only when “all else is equal”. Sometimes other factors determine age at maturity, lifespan, or reproductive effort. For example, in mammals (and humans) males universally show higher extrinsic mortality than females at every age, yet they also grow longer and achieve sexual maturity and first reproduction at later ages than females. This is because the gains in reproductive value from waiting an extra year are much steeper for adolescent males than females (the effect of greater body size or social “experience” in intra-sexual competition). Hence, the difference between males and females in the onset of reproduction is the opposite of what a simple “fast-slow” view of life histories might predict.
Likewise, there are no simple fast-slow life history predictions related to changes in long-term or short-term resource abundance. When resource availability increases, mammals do not respond with slower life histories (cf. Baumard 2015, 2018). Instead, they grow faster, reach sexual maturity at younger ages but with larger body size, and show higher fertility rates (these are evolved reaction norms). Lifespan generally changes very little, and, if it does, it changes in the direction of an increase. Hence the reaction to resource abundance is a mix of faster (early maturity, higher fertility) and slower (longer lifespan) LH traits.
Finally, how evolved life histories react to “harsh” and “risky” conditions depends on what we mean by “harsh” and “risky”. It is not possible to generalize that harsh environments will result in fast life histories (cf. Brumbach et al., 2009). High mortality landscapes favour the kind of fast life history described above. However, when “harsh” is used to refer to resource shortage or variation, it is often the case that age at maturity will be delayed, because body size remains small and greater proportional gains can be achieved through further growth. This is most likely the reason why human populations that experience food shortage show delayed onset of menarche worldwide (Thomas et al., 2001). In that scenario, “harsh” conditions lead to components of a “slower” life history (delayed sexual maturity). With food shortage we also get lower fertility rates, because the total energy budget for reproduction is decreased. Once again, some would interpret this as indicative of a slower life history (lower fertility). Finally, if a “harsh” environment refers to a “variable” or “risky” environment, the adaptive LH response will be determined by exactly what kind of variability or risk is experienced (high risk of food shortage may lead to delayed maturity and lowered fertility, but high risk of injury or death may lead to a faster LH), and whether the risk can be ameliorated by more somatic investment in self or offspring. If an organism can survive temporal variation by investing more in energy storage, for example, then a variable environment might favor slow growth and reproduction, and a longer lifespan. On the other hand, if variation is frequently lethal, despite strategies to minimize the impact of fluctuation, then populations may evolve very fast life histories, even including semelparous reproduction (a single reproductive episode followed by death) if there is a poor chance of surviving until the next available breeding season. The key issue is whether variation is likely to be lethal, not the environmental fluctuation per se. “Risk” can favour either faster or slower life histories depending on the details of the risk.
By mammalian standards, human beings have a very slow life history due to exceptionally low mortality risk across much of the lifespan compared even with other large slow-growing mammals (Harvey and Zammuto, 1985). This is due to a series of cultural and behavioural traits that emerged during hominin evolution, such as the use of fire, projectile weapons against predators and food transfers during illness and injury that lead to exceptionally high survival (Kaplan et al., 2000; Hill and Hurtado, 2009). As a result, human children grow slowly, adolescents reach sexual maturity at a late age compared with other mammals, investment in anti-ageing mechanisms is outstanding (e.g., Hart and Setlow, 1974; MacRae et al., 2015) and onset of senescence takes place at a later age than other great apes (Emery Thompson and Sabbi, this volume). On the other hand, alloparental provisioning allows for exceptionally high female reproductive output in early adulthood despite the slow human life history (somewhat like queen ants, who produce large numbers of offspring but have very long lifespans). Note again that slow life history does not always imply low fertility rate.
The Derived Human Life History
People living in traditional societies (and probably ancestral Homo sapiens) exhibit a series of life history traits, reflected in demographic parameters, that are unique and different from those of our closest phylogenetic relatives (chimpanzees and bonobos). These differences are especially notable because mammalian life history traits tend to be strongly correlated with body size (Western, 1979; Promislow & Harvey, 1990; Charnov, 1993), and many chimpanzee populations are very similar in body size to many tropical hunter-gatherers (Walker et al., 2006; Emery Thompson and Sabbi, this volume). A quick comparison of human and ape life histories suggests four human characteristics: (1) a longer juvenile period; (2) a longer adult lifespan; (3) high early fertility that ends long before the lifespan; and (4) assisted reproduction by post-reproductive adults (Kaplan, 1997). Here I present a detailed comparison of human and ape life histories that suggests many other interesting differences as well. In my view, the derived human life history includes the following:
- Human beings are born at greater birthweight and after longer gestation despite a smaller maternal pelvic opening (Emery Thompson and Ellison, 2018). This is favorable to human infant survival, but dangerous to mothers, and is possible only because of assistance for human mothers during childbirth. A side effect of this trait is reasonably common death in childbirth among human beings but not apes (no deaths in childbirth have ever been reported among chimpanzees, Emery Thompson, personal communication).
- Earlier age at weaning for humans due to allomaternal provisioning, extensive food processing and low-fibre, high-protein lipid nutrient harvest by adults.
- Infant motor skills are more slowly developed in human beings because intense allomaternal caretaking allows for extremely altricial offspring to thrive. Early energy allocation mainly goes to brain growth and learning rather than physical activity and motor skills (Kuzawa et al., 2014).
- A long post-weaning period of juvenile food dependence among human beings (typically >15 years before food independence- Kaplan et al 2000).
- Lower proportional body growth by human beings after weaning and until adolescence. Primates have low growth constants (this means little yearly weight gain each year relative to initial body size) among mammals. But human beings have exceptionally low growth constants even for primates (Walker et al., 2006). Childhood energy is used for other functions: not growth (e.g., neural development, cognitive function, immunological competence, etc.). Since juveniles are provisioned by adults, slow growth is not due to their own food production capabilities. There is good evidence that slow childhood growth is partially due to the metabolic costs of brain growth and function (Kuzawa et al., 2014). However, the adolescent growth spurt (and sex differences in its timing), despite no corresponding age-specific increase in energy harvest (Walker et al., 2006), demonstrates that children probably could grow faster, and provisioners could subsidize faster growth if it were fitness maximizing. Human children might have evolved slow body growth to save energy during juvenile years while the brain is being programmed (some birds show programmed weight loss between reproductive seasons to save energy not needed for immediate reproduction: Norberg, 1981).
- Humans require much more brain growth after birth than do other primates. This is a required side effect of a large brain, but possibly allows more post-natal programming of the central nervous system as part of the process.
- Human beings experience a long and intense juvenile learning period that may determine (rather than body growth) the optimal age of reproductive maturity. Since the marked adolescent growth spurt subsidized by others suggests that juvenile growth could be higher at an earlier age, perhaps human children have evolved delayed sexual maturity until proportional gains in Vx from learning (rather than body size) are matched by the proportional losses due to mortality.
- Human age at sexual maturity and first reproduction are only slightly later than chimpanzees, with some population means almost overlapping (Hiwi menarche at 12.6 years, Pume first birth at 15.5 years vs. chimpanzee female sexual maturity at ~10 years, and first birth >14 years for some populations of wild Chimpanzees: Walker et al., 2006; Kramer et al., 2009 vs. Emery Thompson and Sabbi, this volume) because primaparous human mothers receive high levels of help (advice, caretaking, etc.) and provisioning. Age at first reproduction in humans is determined by when a female can reproduce with extensive help from others, something not possible for other primates.
- Much higher early adult fertility by human females (Kaplan et al., 2000; Emery Thompson and Ellison, 2018) that is highly subsidized through provisioning of both reproductive aged females and their offspring. Mean inter-birth intervals in hunter-gatherers are around 3.3 years, vs. 5–6 years for wild chimpanzees (Marlowe, 2005; Emery Thompson and Sabbi, this volume).
- Declining fecundability of human females by the mid-twenties, despite that fact that, at that age, females have an additional life expectancy of nearly forty years (fertility begins to decline when only ⅕–⅛ of the mean adult lifespan is over). Chimpanzee females maintain high fertility into their forties if they are healthy (Emery Thompson et al., 2007).
- Cessation of ovulatory cycles in human females (menopause) by their mid-forties, and a post reproductive period constituting a large fraction of adult life (Levitis et al., 2013).
- Dichotomy of human male reproductive trajectories with the end of reproduction in the early fifties for most males who remain monogamously pair-bonded to post-menopausal females (e.g., Hill and Hurtado, 1996, figure 9.9; Kaplan et al., 2010), but significant fertility for a smaller fraction of males from their fifties to their seventies. While few chimpanzee males produce offspring after their mid-thirties and no chimpanzee male has been observed to father an offspring after age forty-three (Emery Thompson and Sabbi, this volume) human males in traditional societies often reproduce after age fifty because of late adult income peak (Kaplan et al., 2000; Koster et al., 2018), and the ability to accumulate resources and political alliances over the lifespan. Gurven (personal communication) has found a wide range of variation in the fraction of expected male fertility achieved after age fifty in small scale societies, ranging from only 1.8 % in the Piro to 3.6% in the !Kung and Tsimane, to 14.3% in Forest Ache, 14.8% in Yanamamo, and then 31.4% in Gambia. Clearly, there are socioecological conditions that allow human male reproduction far later than is ever achieved in apes (even when lifespan is considered).
- Onset of significant physical and cognitive senescence ten to fifteen years after reproductive cessation for most human males and all females (Salthouse, 2009; Chan et al., 2014).
- Steep physical deterioration and senescence in humans in conjunction with dependence on kin provisioning and care by the early seventies.
- Rapid mental and physical senescence and high yearly probability of death (>20% per year) after age seventy (e.g., Hill and Hurtado, 1996).
The derived human life history emerged over the past 5 million years although there are indications that the Hominini tribe (chimpanzee-bonobo-human) may already have some traits in common that are more humanlike than the other Hominids (orangutan, gorilla) — smaller size, less sexual dimorphism, greater relative brain size (Emery Thompson and Sabbi, this volume). In general brain growth and early tooth eruption patterns of early hominins suggest that the derived human life history began to change significantly well after origins of genus Homo (Schwartz, 2012; Rosas et al., 2017), but hominin tooth eruption patterns are complicated to interpret because of early weaning and cultural food processing for human infants (Dean & Cole, 2013).
This derived human life history evolved due to a series of constraints and conditions that sometimes resulted in contradicting adaptive challenges, and ended with a spectacular cooperative breeding ape. Key among these was:
- increased early hominin mortality due to a terrestrial activity and sleeping niche with greater predator exposure;
- lowered later hominin mortality due to weapons, fire and frequent provisioning of sick and injured individuals;
- increased later hominin mortality due to conspecific violence including high rates of infanticide/juvenile homicide because of provisioner conflicts with juvenile recipients, and higher levels of adult homicide from quick-kill weaponry;
- numerous bursts of rapid hominin population growth due to worldwide expansion and colonization of “empty niches” with initial high food abundance;
- lowered seasonal variance in food supply in hominins because of opportunistic ominvory, food transfers to buffer shortfalls and unique inter-group visiting allowing resource access to distant regions;
- extensive juvenile food dependence among later hominins due to the skill- (and learning-) intensive extractive/predatory feeding niche;
- an overall greater energy consumption/expenditure budget per gram of body weight than apes, presumably to pay for large brains and greater somatic maintenance and repair to facilitate longer lifespans (Pontzer, 2016);
- multiple overlapping dependent juveniles in hominins and obligate alloparental provisioning (Hill and Hurtado, 2009), as dependent juveniles accumulate.
Finally, it is important to note that the human life history has extensively modified human social structure from that of other great apes, and has been uniquely influenced by cultural norms and social learning. Life history traits related to pair bonding and paternal investment along with extensive juvenile dependency resulted in a social structure that includes bisexual dispersal and/or philopatry, bilocal coresidence, extensive peaceful visiting across social groups in order to provide or receive kin assistance and unique cooperative relations with affinal kin not seen in any other species of life on earth (Chapais, 2009, 2011; Hill et al., 2011). As hominins became more extensively dependent on cultural adaptations, and generalized their social learning proclivities from food procurement and tool-making techniques to other aspects of life, they began to socially acquire mortality-reducing (or increasing) behaviours and fertility-modifying patterns, including social norms regulating traits like age at weaning, age of first mating and pair bonding, typical parental investment patterns and control over offspring’s reproductive behaviour. The population trends in some LH traits in Homo sapiens is based partially on imitation and social norms rather than independent individual “decisions” about optimal life history. Fitness-maximizing life histories are constrained by cultural norms, and non-adaptive life histories can become prevalent via social learning (e.g. the demographic transition in worldwide fertility). This means that human life histories are exceptionally influenced by cultural transmission and social learning.
Three Major Areas of Research
Life history research in humans is particularly useful and scientifically significant (with broad implications) when it integrates age-specific mortality, fertility and developmental patterns with other key phenotypic adaptations and behaviours that make humans an exceptionally successful mammal. The summary of the derived human life history given above logically leads to a recognition of three major research topics. The first of these is about adaptive origins: how can we explain the evolution of the derived human life history and its special features that ultimately make humans a spectacularly successful life form on earth? The second of these is about population differences across time and space: how can we explain observed variation in life history traits across different socio-ecological conditions, and to what extent is the variation due to local genetic evolution, adaptive phenotypic plasticity or a mismatch between evolved LH mechanisms and current environments experienced by modern humans? We particularly want to know the underlying physiological mechanisms and ontogeny of the key life history traits (e.g. Flatt and Heyland, 2011; Ellison, 2016), as well as the range of variation regularly produced via evolved reaction norms and phenotypic plasticity that evolved during hominin history. This will require a far more sophisticated understanding of the physiological mechanisms responsible for the expression of life history traits, and the ability to conditionally adjust life history over a single lifespan. The study of mechanisms should be fully integrated with theoretical models of optimal life history in order to provide a complete understanding of evolution. Finally, we need to understand the evolutionary process of genetic and phenotypic frequency change. How are optimal life histories selected over time when current reproductive value determines optimal future mortality and fertility and vice versa? Some researchers have suggested that this optimality problem requires dynamic programming (Mangel and Satterthwaite, 2016).
Ten Interesting Issues in Evolutionary Demography
Below I present a list of ten interesting, important and unsolved issues in anthropological demography. This list is not meant to be exhaustive; it simply represents my own interests and observations during the past twenty years of life history research (two of these were also discussed as “human life history puzzles” in Mace, 2000). My identification of the questions below is meant to stimulate future research, and occasionally provide hypotheses, not to provide definitive answers to any of the questions listed.
1) The Hunter-Gatherer Demographic Paradox
Howell’s (1979) monograph Demography of the Dobe !Kung was a highly influential early publication in anthropological demography. Since then, almost a dozen detailed demographic studies of hunter-gatherer populations have provided quantitative estimates of fertility and mortality rates under socio-ecological conditions that are presumed similar to those in which our human ancestors existed for hundreds of thousands of years (see Hewlett, 1991; Pennington, 2001; Marlowe, 2005; Gurven & Kaplan, 2007; Ramirez Rozzi, 2018). Almost all these studies, however, show substantial positive population growth rates, leading us to wonder whether we really know what the ancestral human life history looked like. Put bluntly, until we can discover empirically a real ethnographic life history that results in zero population growth and consists of reasonable (not pathological) fertility and mortality levels adapted to commonly experienced ecological constraints, we may not fully understand how the human life history diverged from other apes.
Modern hunter-gatherer demographic parameters that result in significantly positive population growth cannot directly reflect the human life history trait values through most of ancestral history. Even with progressive worldwide colonization, our species must have shown very close to zero population growth for almost all of the past three-hundred-thousand years. Malthus would be shocked at the measured population growth rates for most modern hunter-gatherers. Life tables from nearly a dozen hunter-gatherer populations, and median life history parameters from many more, all imply population growth of more than one half percent per year (ibid., see Table 1). Median hunter-gatherer values (Marlowe, 2005) of 55% juvenile survival to adulthood, Completed Family Size of 7.1 and presumed 1.5% adult mortality rate lead to population growth rates of > 2% per year. Modest tweaking of measured demographic parameters within the range observed in careful ethnographic studies still does not achieve zero growth. There are only a few ethnographic exceptions. For example, the Onge of the Andaman Islands were reported to have high mortality, high rates of childlessness and a female age at first birth of twenty-eight years, far below zero growth, but no supporting data are provided to back that claim (Cipriani, 1961).
A population growth rate of only one tenth of one percent per year means that a founding population of ten individuals will grow to over 700 billion people in only 25,000 years! Clearly, the life tables we have observed in modern hunter-gatherer groups cannot represent most of the history of Homo sapiens. This problem was first overtly discussed by Hill and Hurtado (1996: Chapter 14), but more completely explored by Boone (2002), Gurven and Kaplan (2007), and recently by Blurton-Jones (2016: Chapter 11). All authors carried out subsequent simulations to see how low the fertility and survival rates would have to be in order to achieve zero population growth. Assuming that adult mortality rates, prior to senescence, in ancestral human populations were usually about 1.5% per year (74% of women who reach sexual maturity survive to the end of a twenty-year reproductive career) and a 105 sex ratio, we can examine what Completed Family Size (CFS) and juvenile survivorship rate is required to get zero growth. Probing low fertility options, the simple answer is that a CFS of about ~4.5 live births with 50% juvenile mortality will lead to zero growth. But a CFS of 4.5 with a female reproductive span of twenty years (twenty to forty years old from first to last birth) implies a 5.7 year inter-birth interval. This is much longer than the IBI ever measured in any traditional human population and would require physiological birth-spacing mechanisms that probably do not exist in humans (lactational anovulation combined with nutritional stress leading to a birth interval almost twice as long as that typically observed in extant hunter-gatherers (Marlowe, 2005)).
Table 1. Median forager from Marlowe, 2005. Data on each group from Hewlett, 1991; Pennington, 2001; Gurven & Kaplan, 2007; Ramirez Rozzi, 2018.
|
Group |
Juv surv |
age fbirth |
age lbirth |
CFS |
Adult mort |
females per gen |
gen time |
increase per year |
|
|
Med. Forager |
0.55 |
0.49 |
19.25 |
39 |
7.1 |
0.015 |
1.90 |
39 |
2.32% |
|
Efe |
0.78 |
0.49 |
19 |
39 |
2.7 |
0.015 |
1.03 |
39 |
0.07% |
|
Hiwi |
0.51 |
0.49 |
20.5 |
37.8 |
5.1 |
0.015 |
1.28 |
37.8 |
0.73% |
|
Kung |
0.6 |
0.49 |
19 |
37 |
4.7 |
0.015 |
1.38 |
37 |
1.02% |
|
Agta |
0.42 |
0.49 |
19.5 |
41 |
7.6 |
0.02 |
1.56 |
41 |
1.36% |
|
Hadza |
0.56 |
0.49 |
19 |
39 |
6.1 |
0.015 |
1.67 |
39 |
1.71% |
|
Ache |
0.61 |
0.49 |
19.5 |
42.1 |
8.2 |
0.01 |
2.43 |
42.1 |
3.39% |
|
Baka |
0.66 |
0.49 |
18 |
39 |
7.3 |
0.015 |
2.35 |
39 |
3.46% |
Hunter-gatherer children are typically weaned by age 2.5, and even with later weaning natural suckling rarely results in anovulation after about age 2.5 (because human children naturally begin to eat pre-processed adult foods by that age). Normally, nourished forager women will conceive within half a dozen cycles of ovulatory resumption (Bentley, 1985). Hence a mean IBI of >5 years is probably not possible in human societies unless they are undergoing catastrophic starvation or stress. Of course, we could allow the population mean CFS to be low due to high levels of primary and secondary sterility, but only populations with extremely high STD infection rates ever show such a pattern in modern ethnographic studies. Alternatively, we can assume more realistic fertility (mean reproductive span nineteen to forty-one years old, mean IBI = 4.5 years, mean TFR = 6.1) and estimate levels of juvenile mortality required to produce zero growth. This modification is more in line with Charnov’s (1986) observation that juvenile mortality is the life history variable that shows the greatest change when ecological conditions become good or poor. Based on a CFS of 6.1, we estimate that only 34% of children born could survive to the age of first reproduction if the population is stationary. This is again doubtful, because even the highest-mortality hunter-gatherer populations documented show much higher juvenile survival (Marlowe, 2005) than our simulation requires. Also, this mortality level would imply that human juvenile survival is worse than that of wild chimpanzees (unlikely, given observed levels of alloparental care in humans).
Our conclusion is that only a combination of both the lowest natural fertility rates and the highest juvenile mortality rates ever ethnographically observed can come close to producing zero population growth. Such a life history probably implies resource limitations (food intake is related to both fertility and juvenile mortality in all mammals) much more severe than ever observed in any modern group of hunter-gatherers (so much for the “original affluent society” label). Because of this, both Hill and Hurtado, and Blurton-Jones explored other solutions to the hunter-gatherer paradox based on frequent population crashes (a few generations of growth followed by a serious crash repeatedly), or higher adult mortality due to warfare, or very high infanticide rates. Hill’s student Keckler (1997) did simulations showing that frequent and severe population crashes (including exterminations caused by warfare, climate variations or disease epidemics) could result in long-term zero population growth, but this would require us to revise our basic understanding of human history. Blurton-Jones’ conclusions were similar. The most important lesson at this point is that the hunter-gatherer demographic paradox reminds us that we still cannot state with confidence that we know the typical life history parameters that characterized much of human ancestral history. Modern hunter-gatherer studies do not yet give us the answer.
2) Body Size Variation Around the World
The standard mammalian LH model developed by Charnov (1991) presumes that energetic throughput is reflected by the empirically derived allometric growth law such that change in mass is a decelerating function of achieved body weight (dw/dt = Aw^.75). Charnov simplified female mammalian life history to assume that some energy harvested goes into growth during the juvenile period, and that energy is converted to reproductive effort at adulthood. This means that energy for reproduction is a direct function of body size, and, for a given species of mammal with a species-typical offspring size at birth, fertility increases monotonically with female body size. However, the proportional gains in fertility with each extra gram of body growth are lower as mammals get larger. Eventually, growing one more time unit before sexual maturity will lead to a greater proportional loss in probability of reaching reproductive age than will be the proportional gain in fertility from growing during that additional time unit. This defines the optimal age at which to stop growing and start reproducing. According to this model, then, mortality rates are the main determinant of the optimal female adult body size for any particular feeding niche and the corresponding growth rate around the age of sexual maturity. Higher mortality should lead to earlier cessation of growth and smaller adult body size. This model was adopted by Hill and Hurtado (1996: Chapter 11) in an attempt to “explain” typical adult female body size and age at first reproduction for female Ache hunter-gatherers of Paraguay. Hill and Hurtado then employed the same model to “predict” both smaller body size and later age at reproduction for !Kung Bushmen using the published growth and mortality parameters for that group. A decade later the same model was explored by Walker et al (2006) to account for body-size variation (due to variation in both growth and mortality rates) in a sample of twenty-two small-scale traditional societies. Walker et al concluded that some populations were small because of nutrition and slow childhood growth, and others because high mortality favored early sexual maturity. Finally, the model was adopted by Migliano and colleagues (2007), using data from South East Asian Negritos and African Pygmies to suggest that short stature in general (“pygmy phenotype”) was mainly due to high-mortality environments and early cessation of growth. However, the Migliano et al model was questioned by demographers working with African pygmies who found no evidence of high African pygmy mortality (Becker et al., 2010), and recently the Migliano et al model was shown to be incorrect for Baka pygmies, who have high juvenile and adult survival but are small because of genetically determined slow growth during early childhood (Ramirez Rozzi 2018). Miglano and Guillon (2012) extended their original argument and provided important cross-cultural analyses suggesting that differential mortality rates are indeed associated with much of the variance in height across a sample of small-scale populations around the world. However, their analyses are confounded by the fact that mortality and height are both strongly affected by nutrition, disease and economic well-being in all human societies (see Steckel, 2009 for review) such that a positive relationship between survival and height is expected, even when mortality rate has no direct causal impact on adult height. In their paper, Migliano and Gullion present only one result that cannot be parsimoniously explained by the association between better nutrition, better survival and higher childhood growth. That result is an apparent positive relationship between adult survival and age at menarche (ibid: table 3). However, that result seems extremely improbable and should be examined carefully. It is well known from observation and food intervention studies that greater food intake increases survival and decreases age at first reproduction (see Hill and Hurtado, 1996; tables 1.1 and 1.2 for review). The Migliano and Guillon result, if true, would contradict hundreds of studies in human and mammalian nutrition and biology that show that poorly nourished mammals show higher mortality and later ages of maturity. While Migliano and Guillon interpret their result to be consistent with a life history prediction that lower mortality should lead to later age at maturity, that prediction is only valid when nutritional intake is approximately constant (Charnov, 1991). In the real world, with tremendous differences in food intake across and within societies, there is no reason to expect that those who reach menarche at a later age will also have better survivorship — quite the opposite.
It is unclear whether the traditional mortality rates among South East Asian Negritos have been high enough over evolutionary time to produce the small body size of those groups. There is no year-by-year survival curve in the original Agta study, and various calculations have placed the forest-period survival rates to age of reproduction anywhere between 42% to 50% — not particularly low for a H-G population (Hewlett, 1991; Gurven & Kaplan, 2007; Migliano et al., 2007; Ramirez Rozzi, 2018; Early & Headland, 1998, Headland, personal communication). Very similar survival rate (51%) is reported for the Batak (another Asian Negrito group, see Migliano et al., 2007). One obvious alternative possibility is that South East Asian Negrito body size is an example of “insular dwarfism” acting on humans much like small body size has evolved in many other mammals living on islands. Importantly, early island populations of hominins in this region already show very small body size, as do recent non-descendant native populations (see Mijares et al., 2010 for ancient Philippines; and Brown et al., 2004; Bromham and Cardillo, 2007; Tucci et al., 2018 for discussion of H. floresienses and primate island dwarfism). Ironically, however, models of mammalian insular dwarfism generally assume that mortality on islands is low due to lack of predators, and that small body size is mainly an adaptation to both feeding competition and lack of need for large body size to escape predation (Lomolino, 1985). In any case, no popular theory of insular dwarfism assumes higher extrinsic mortality as the cause (Lomolino, 2005; Meiri and Raia, 2007). This leads us to wonder about human body size variation in general across time and space. To what extent are large and small body size due to advantages of longer or shorter growth periods driven by mortality rates, and to what extent is body size an adaptation to other ecological constraints (such as climate, feeding niche, frequency of violent contests)? It is also important to note that Charnov (2001) himself stated that the power-function growth pattern across phylogenic groups was not relevant to within-species growth, and that other life history models to explain body size, assuming sigmoid growth models (growth stops when the daily energy harvested in that niche is equaled by the increasing metabolic costs of the growing body) were more appropriate. The theoretical basis for the mortality-rate-driven body size variation within species should be carefully examined anew. In this light it is important to consider adaptive explanations for small body size that are not derived from LHT at all, but may be due to factors like mobility constraints in tropical forests (Venkataraman et al., 2018). Casual inspection does suggest that the largest hunter-gatherers in the ethnographic record live in open country and the smallest often inhabit tropical forests. How much is body size variation simply due to better or worse nutrition, and how much is genetically determined adaptation to other long term ecological constraints? Why does isolation on islands lead to both notably small (e.g. South East Asian Negritos) and notably large (e.g. Maori, Somoan: Swinburn et al., 1999) mean body size? Finally, how do population differences in body size correlate with life history variables in human populations around the world?
3) The Demographic Transition
Probably the most investigated and written about topic in recent human demography is the transition to lower mortality and lower fertility that swept through many human populations beginning around the end of the eighteenth century, and which is still in progress in much of the developing world (Caldwell, 1976; Coale, 1989; Lee, 2003; Goodman et al., 2012; Sear et al., 2016; Colleran, 2016). Good evolutionary analyses have clearly demonstrated that lowered fertility, greater survival and greater investment in offspring could hypothetically maximize fitness under the right conditions, but, empirically, recent widespread fertility reduction does not maximize fitness in human populations where relevant parameters have been measured (e.g. Borgerhoff Mulder, 1998; Kaplan et al., 1995, Kaplan, 1996; Kaplan and Lancaster, 2000; Goodman et al., 2012; Bolund and Lumaa, 2017). Nevertheless, the question of whether the fertility transition is adaptive is complicated, because: (1) the fertility transition has proceeded through phases that might have been adaptive in some times and places (Hruschka and Burger, 2015); and (2) the fertility transition may not be permanent (Burger and DeLong, 2016). However, it seems clear that the demographic transition is not simply an adaptive reaction norm that maximizes fitness in modern times through low fertility. This realization forces us to examine the proximate mechanisms of fertility outcomes that might have been adaptive under past conditions but would lead to less than maximum fitness under recent conditions (mismatch). Whatever those mechanisms, they may be under strong negative selection currently. While a clear understanding of the demographic transition will require considerably more work on the evolved phenotypic plasticity of fertility decision mechanisms (adaptive reaction norms that produce fertility variation in the range of ancestral ecological conditions), some important considerations can be identified.
Firstly, the trend to not convert increased resource access into increased fertility (or offspring survival) has existed in humans for a long time before its manifestation in the demographic transition. In other mammals, resource availability directly determines fertility or survivorship and subsequent equilibrium population density (e.g. Robinson and Redford, 1986; Boutin, 1990). In other words, if we double the resource availability, we find a short period of population growth followed by an approximate doubling of the population density on the landscape. This has not been true in humans for a long time. Instead, in historic times, when the resource base was doubled, we find only a slight increase in human population density and instead an increase in standard of living (and per capita income) of the population. People use extra resource availability to improve shelter quality (housing), clothing, adornment, quality and quantity of utility goods and status display to other people. These forms of “extra-somatic” investment do not generally lead to linear increases in the reproductive value of individuals and hence probably do not maximize inclusive fitness. In short, the human tendency to engage in extra-somatic investment, storage and display (wealth accumulation) rather than fitness maximization had already begun thousands of years before the demographic transition in recent times. How did such psychological mechanisms, producing wealth accumulation, status display and an increase in standards of living at the expense of fitness maximization, evolve in our ancestral past?
Secondly, early theoretical models of the demographic transition focused on examining the conditions leading to increasing investment per offspring as the cause of associated low fertility. Some models do show that very low fertility and high parental investment could be an adaptive response (e.g. Kaplan, 1994, 1996), but the conditions required for fitness maximization with very low fertility are never seen empirically. That theory shows that parents should decrease fertility when the proportional increases in offspring reproductive value from greater PI are greater than the proportional fertility loss due to that investment. While most parental investment models assume diminishing returns to PI, there are hypothetical conditions where greater investment might give accelerating returns across feasible levels of investment. For example, if, by investing a little bit more than other parents, the offspring with the highest PI get all the best jobs (winner-take-all payoff structure), or all the mates, etc., then maximum investment in a single offspring (or very few offspring) might maximize summed offspring reproductive value across all offspring (fitness). In other words, if returns to PI are positively accelerated across the entire range of feasible options, then lifetime production of just one or very few offspring might maximize fitness. But, in reality, nobody has ever shown such a payoff structure for PI in any real ecological conditions experienced by humans. Therefore, how could humans evolve such a reaction norm if the conditions have never existed?
Thirdly, the conceptualization of the demographic transition as a problem of quantity vs. quality of offspring does not jibe with observed parental behavior. Instead of parents investing more and more per offspring when they have fewer offspring, parents also invest more and more in themselves as they decrease family size. Additionally, the signalling benefits of some public “parental investment” are unclear. Opting for a higher standard of living, rather than fitness maximization, appears an old human pattern. What really happens during the modern demographic transition? Parents have fewer children, invest more in each of them (especially education), but also buy nicer houses, clothes, fancy cars, go on expensive vacations and purchase a myriad of status enhancing and display items. Why does the human psychology prioritize such things, and under what ancestral conditions might that human psychology have arisen?
Finally, the role of cultural norms and social learning must be integrated into the biological and mechanistic view of fertility. There is overwhelming evidence that copying low fertility patterns from higher status groups is the single strongest proximate determinant of the demographic transition (Colleran, 2016). Demographers must confront cultural evolution head on if we are going to fully understand the trend to lower fertility.
4) Menopause and Cooperative Breeding
Adult human females cease ovulation at an age long before they are expected to die. While most mammals show some reproductive senescence and a short post-fertile life span, the complete termination of fertility function among females long before the typical age of adult death, and the apparent significance of post-reproductive helpers, makes human menopause a rather important life history problem. Indeed, even in relatively high-mortality hunter-gatherer societies, more than 40% of adult female years lived are experienced as post-reproductive (Levitus et al., 2013). Since fitness in mammals is strongly related to offspring production, how can the early termination of reproduction maximize fitness? This leads to two possible answers. Firstly, perhaps menopause does not maximize fitness relative to the alternative of continued reproduction. If so, then we need to determine why continued reproduction late into the lifespan is not observed in human females. Are there constraints that make this impossible even though it is typical of most mammals? Secondly, if menopause does maximize inclusive fitness, it must do so via the positive impact of post-reproductive women on their close kin.
The most popularly considered and discussed evolutionary explanation of menopause has become known as the grandmother hypothesis (Hawkes et al., 1998). However, there are half a dozen different versions of the grandmother hypothesis now which all borrow that label (and more variants are likely to be proposed). An evaluation, therefore, requires that we specify which “grandmother hypothesis” we have in mind. Evolutionary demographers working on the puzzle of menopause in the 1980s first proposed that menopause might be favoured because the probability of an older adult woman dying before her offspring were raised to “independence” was high in ancestral societies. Selection would favour women who stopped reproducing in middle age and instead invested in helping daughters and grand-offspring (cf. Williams, 1957). However, empirical mortality data quickly showed that idea was wrong. Survival is high for middle-aged hunter-gatherer women and menopause takes place when women still have a good chance of surviving another fifteen years, to the age of “independence” of their offspring. The second alternative considered was that the inclusive fitness effects of grandmother support in the human extractive foraging economy might be so high that women could maximize fitness by ceasing reproduction and focusing on helping their grandchildren once they had enough grandchildren available to help (Hawkes et al., 1989, 1998).
There are at least three problems associated with this “grandmother-helper” hypothesis. Firstly, if the reproductive value of a woman’s offspring can be greatly improved by helping, why not evolve a life history of older sibling helpers, rather than grandmother helpers? After all, siblings are twice as related to their younger siblings as is a grandmother, and should be more willing to forgo reproduction for a while to help younger siblings who share as many of their genes as their own offspring would. Kaplan et al. (2010) have proposed that the answer to this problem lies in the unique increases of energy production over adulthood found in the human economic niche. Grandmothers (and grandfathers) are far more capable of provisioning grand-offspring than are older adolescent siblings. The second problem is that the adaptive grandmother-helper hypothesis should predict that menopause is facultative: that women with many grandchildren experience menopause, and perhaps earlier, but women with few or no living grandchildren should keep reproducing directly. No such pattern is seen around the world; instead, age at menopause is surprisingly invariant across human populations. The third problem is that empirical analyses have always appeared to show that higher fitness could be achieved via direct reproduction (if it were to continue at the level that younger women achieve), than could be achieved via the helping effects of grandmothers. Grandmother help is indeed significant, but appears not to be sufficient to justify adaptive cessation of direct reproduction (Hill and Hurtado, 1991, 1996; Rogers, 1993; Sear and Mace, 2008). This suggests that menopause might be partially due to a physiological constraint of declining fertility as women age.
After the two general adaptive hypotheses for menopause were proposed, a myriad of interesting specific adaptive models have been put forward. Firstly, some researchers have suggested that the exponential nature and rate of atresia and follicular exhaustion in mammalian ovaries is a phylogenetic constraint that makes it difficult for mammals to extend fertility much beyond age forty-five (e.g., Ellison and Ottinger 2014; Jones et al., 2007; but see Cloutier et al., 2015). Since few mammals ever live to age forty-five, the steep follicular exhaustion of mammalian females is not a problem for most species. This might mean that the evolutionary puzzle for humans is not so much cessation of ovulation in middle age, but, instead, how humans evolved a long post-reproductive lifespan after follicular depletion. However, Cloutier et al. (2015) show follicular exhaustion accelerates with age in humans but not chimpanzees, suggesting that perhaps early high fertility in humans is traded off against the extension of follicular viability, and that the timing of menopause could represent an adaptation despite the constraint of slow follicular atresia. If post-reproductive lifespan, rather than timing of menopause itself, is the adaptive puzzle, one simple answer might be that the long lifespan is found in both sexes because it is selected for in males due to late life fertility (see the section on derived human life history above, and Tuljapukar et al., 2007). However, most researchers seem more inclined to point to evidence of grandmaternal assistance to descendant kin as the most likely explanation for the long female post-reproductive period (e.g., Hawkes et al., 1989). Since virtually all ethnographers report that post-menopausal women are helpful to younger kin, a simple proposition is that the inclusive fitness effects of grandmother helping (daughters, sons, grand-offspring) might be sufficient to explain the delay in senescence (and long lifespan) after reproductive function has ceased.
In fact, the grandmother-helper hypothesis can also be combined with the “fertility decline” hypothesis to incorporate cooperative breeding into the menopause model explicitly. In the Hill and Hurtado (1991, 1996: Chapter 13) menopause model, we assumed that older women could achieve about half the fertility of women at their peak fertility years, because that is approximately the decline with age seen in other primates that do not have menopause. We then asked if higher fitness would be possible via grandmother helping or by continued direct reproduction at that rate. The answer was clear: direct reproduction would still contribute more genes than helping. However, what if we, instead, assume that natural fertility declines more steeply over time due to physiological senescence. Perhaps if fertility typically declines to 1/3 the maximum rate via follicular atresia by the mid forties, women might then achieve higher inclusive fitness by ceasing ovulation altogether and investing in grandmother helping. Indeed, Hill and Hurtado (1996: Chapter 13) develop a model showing that when Ache fertility drops to 1/6 of peak female fertility, women would indeed gain higher fitness by helping kin rather than using resources to continue direct reproduction.
An even more attractive variant of the grandmother-helper hypothesis might be termed the “inclusive fitness helper hypothesis”. This idea considers that human women receive high levels of kin support for their own reproduction. What if kin helpers were generally to stop helping older women reproduce over time because of follicular atresia and decreasing fecundability with age? Investing kin members should assess how best to use their own resources and which one of their available kin to help reproduce. If younger women have high natural fecundability and older women are inefficient (due to fertility senescence) in their conversion of resources into gene copies, then most relatives should stop subsidizing the reproduction of older female relatives and instead help younger related females. This might force older females to evolve menopause as their best option, given that they would not obtain much outside kin provisioning any longer. Just such a pattern appears to have evolved in some ant reproductive queens whose declining fertility with age can be “smelled” in the hydrocarbons on the exoskeleton, and as fecundability declines they are ultimately no longer fed by workers; instead, they reabsorb their ovaries and become workers themselves (Hill & Hurtado, 2012).
The realization that the high fertility of younger women in human societies is already due to help they are receiving from their mothers and others has also led to modifications of the “reproduce vs. help” calculations. Instead of asking what fitness a woman could achieve if she continued to reproduce (at observed population fertility levels) or helped other kin, we need to think in terms of kin-group selection. Can lineages with grandmother helpers achieve higher fitness than lineages without post-reproductive helpers? Once again, we need to estimate the survival and fertility effects of grandmother-helping achieved by lineages of females without post-reproductive helpers compared to those with helpers. Which type of kin group leaves more gene copies: one with low fertility and offspring survival due to absence of helpers, but in which all females reproduce through the whole lifespan; or one with higher early fertility and offspring survival (and higher fertility of sons as well?), but in which older women all cease reproduction and become helpers at some age?
Evolutionary modelling of menopause brings focus on one of the most important issues in evolutionary demography. Can we measure the true impact of kin help on demographic parameters and document the nature of human cooperative breeding? Indeed, more data would be helpful in deciding how the human socio-reproductive system should be described. I have sometimes used the term “assisted breeding” in order to avoid confusion with strict biological definitions of “cooperative breeding” that require reproductive suppression by the dominant female (see Clutton Brock, 2002). Likewise, Kramer and Ellison (2009) have referred to the helping socio-reproductive system as a “pooled energy budget” for small-scale societies. In the past twenty years, however, dozens of evolutionary demographers have described the human reproductive system as “cooperative breeding” (Hrdy, 2000, 2005, 2017; Burkhardt et al., 2009; Mace and Sear, 2005; Kramer, 2005, 2010; Hill and Hurtado, 2009; Van Schaik and Burkhardt, 2010; Burkhardt and Van Schaik, 2016; Sear and Coali, 2011; Smaldino et al, 2013, Meehan et al., 2013). While reproductive suppression among females is not typical in humans, provisioning and helping (by kin and non-kin) of offspring and their mothers is extensive and probably necessary for long-term population growth. How can we measure the fitness impacts of helping by kin and non-kin in cooperatively breeding social units? To examine evolutionary origins of helping, we probably want to measure the impact of kin help alone, but later human societies, including all foraging societies studied, show extensive non-kin helping as well. We need to measure the fitness impacts of non-kin cooperation (e.g. dyadic reciprocity arrangements) vs. the fitness that would be experienced without that cooperation. In the measurement of kin-helper effects, we (Hill & Hurtado, 1996, 2009) and others (e.g., Blurton-Jones, 2016; Sear and Mace, 2008) have pointed out that substitution by other kin seriously complicates the measurements. In theory, one could measure the impact of a grandmother’s help on her grand-offspring’s survival simply by comparing the survival of children who do or do not have a living grandmother. However, in reality, because of marginal inclusive fitness benefits, we expect that other kin will opportunistically provide substitute help when grandmother’s help is missing. So, by comparing the survival of children with and without a grandmother, we may grossly underestimate the impact of a/the grandmother’s help on average. This is a problem to be faced in all life history research on cooperative breeding and alloparental helping in humans.
5) Age at Sexual Maturity and First Reproduction
In most mammals, age of female sexual maturity and first reproduction are tightly coupled. As mentioned earlier, the standard mammalian LH model is that juvenile females grow until the proportional gain in reproductive value is matched by the proportional loss due to mortality. At that age females become sexually mature. Hence, fast-growing mammals and those living in high-mortality landscapes are expected to reach sexual maturity at young ages: very soon after those females conceive offspring and give birth. In humans, this model may be inappropriate for two reasons. Firstly, adult females do not reproduce using only their own energy capture and allocation, so the effects of growth and body size on future energy capture are less clear. Instead, female reproduction is highly subsidized, and marginal increases in female reproductive value are probably derived from social networks built to gain kin help rather than from growth and increase in body size. Secondly, sexual maturity in humans often takes place long before first reproduction, because of cultural patterns and social norms that regulate marriage and hence copulation frequency for young adult females. This is a prime example of how social learning and enforced social norms can interact with other ecological constraints to produce life history variation.
Age at first marriage for females (and hence regular copulation) is not highly variable in hunter-gatherer populations (always near the age of menarche — Marlowe, 2005), but varies considerably among other types of societies (e.g., Dixon, 1971; Blanc and Rutenberg, 1990; Jones, 2010). Among males, culturally determined variation in allowable age at first marriage is much greater than among females, even in hunter-gatherer societies. Hence, age at first reproduction becomes a research problem in cultural evolution and social norms, rather than simply a fitness optimization problem in biology.
6) Variation in Age of Peak Fertility
In most female mammals, peak fertility and fecundability is observed soon after sexual maturity. Male fertility is more complex, because acquiring mates often requires both strength, achieved dominance and social alliances, and hence takes place at a later age closer to peak strength. Humans do not seem to fit the pattern for either mammalian sex. First, peak female fertility in hunter-gatherers and small-scale societies varies surprisingly. Combining yearly data into five-year intervals, we find peak female fertility ranging all the way from the first adult five-year interval (15–19 years), to middle age (30–34 years, Ramirez Rozzi, 2018: figure 3). How can we explain a fifteen-year age difference in peak female fertility across small-scale natural fertility societies where almost all women are married by their mid-teens? These differences are probably not artifacts of small samples, since there are more than two hundred women years at risk in both the samples for the societies with youngest (Aka) and oldest (Ache) peak fertility. What cultural or environmental variables might underlie such variation? Thus far there is little theory or even speculation to address this. Secondly, human males often show high fertility in middle age and well beyond the age of peak body strength (Tuljapurkar et al., 2007; Walker et al., 2002). Indeed, in some polygynous societies, male fertility peaks in their fifties even though male strength peaks uniformly in the early twenties. This is probably due to wealth accumulation and political power patterns, and a male age-specific resource production profile that generally peaks in middle age, across economies as disparate as hunter-gatherers and modern America (Koster et al., 2018; US Bureau of Labor Statistics 2017). How and why late male fertility is achieved in some societies but not others, and why male fertility does not peak at later ages for the most wealth-stratified societies in human history (Ross et al., 2018) is an interesting problem in evolutionary demography.
7) Sex Differences in Reproductive Skew (Monogamy vs. Polygyny)
In most mammals, male fertility variance is much higher than female fertility variance. This is due to the investment asymmetry in mammals whereby females obligatorily invest substantially more resources (due to internal gestation and lactation) in each offspring produced than do males, who instead compete fiercely to gain access to fertile females. Larger, stronger, healthier males with better territories or more resources to offer female mates are chosen more often by females, and win more often in direct physical competition for access to fertile females. Because the energy content of an ejaculation is minimal and no paternal investment is obligate in mammals, male fertility is limited only by access to females. In contrast, female mammals show limited differences in fertility, mainly due to body condition, energy balance and size effects on fecundity. The probability density distribution of achieved lifetime fertility is right-skewed for both males and females, but with some very successful individual males, and many more males who completely fail to reproduce. Hence, the skew in this LRS distribution is much greater in males than in females.
How does the sex difference in reproductive skew look in humans compared to other mammals, and how much does it vary across societies? And why is it that extremely high variance in male income is empirically associated with lower polygyny rather than higher polygyny rates as for most mammals (Ross et al., 2018)? Reproductive skew measures provide a good indication of potential for sexual selection. Humans are believed to be generally monogamous with exceptionally high levels of paternal investment in offspring. Is this view congruent with the measured sex differences in reproductive variance? Differences in that skew are usually a good metric of the level of polygyny typical of the species. In a perfectly monogamous species with lifelong pair bonding, reproductive skew in both sexes should be identical. In a highly polygynous species, reproductive skew is much greater in males. By calculating the ratio of male to female reproductive skew, evolutionary demographers are uniquely positioned to examine actual mating patterns, rather than reported cultural ideals in marriage practices. For example, a recent research project examining sex differences in reproductive skew in ninety-seven human societies and seventy-six mammalian species discovered some interesting patterns (Ross et al., 2023). Firstly, humans do indeed show higher reproductive skew in males than in females. Secondly, humans have much lower sex differences in reproductive skew than most other mammals, but they fall within the mammalian range. Thirdly, polygynous human societies show larger sex differences in reproductive skew than those that practice monogamous social norms. Fourthly, polygynous human societies show lower sex differences in reproductive skew than do most polygynous mammal species. This appears to imply that paternal investment is still quite important even in polygynous human societies.
Finally, the low sex difference in reproductive skew found in humans is partially due to higher female reproductive variance than is usually true in mammals. This surprising result might be explained by the large reproductive subsidies that are required by human women during their reproductive career. Just as in other cooperative breeding species, some females can obtain a lot of help and others little or no help (maybe even mild reproductive suppression due to social stress). Hence, variation between females is not due only to health and their own resources, but also to differences in the resources and labour offered by helpers and provisioners. This is a bit like ants, where the reproductive variance between queens can be greater than the reproductive variance between drones when queens mate polyandrously and there are significant differences in colony size (and number of workers). The high female variance result seems to confirm that human societies do practice a mild form of “cooperative breeding”. Further work should examine conditions under which both males and females experience high reproductive variance across human societies.
8) Extreme Longevity (Kin Assistance or Kin Parasitism)
In almost all human societies examined, a small percentage of older adults survive for several years after their productive net energy production (daily harvest minus daily consumption) rates drop below zero. For example, using data from three foraging societies, Kaplan et al. (2000) show that net energy productivity in males drops below zero (they produce less energy than they consume) by age sixty and in females by age sixty-nine. Also, individual senescence and a steep upturn in age-specific mortality rate is generally obvious by the mid-sixties to early seventies. Yet demographic data from the same groups (Ache, Hiwi, Hadza) show that 35% (Ache), 27% (Hiwi), 43% (Hadza) who survive to age fifteen will also survive to age sixty-five. And some individuals, even in traditional small-scale societies, will survive to age eighty, a full fifteen to twenty years after their net resource productivity drops below zero. Clearly this is only possible because of social provisioning of the elderly.
The widespread provisioning of elderly individuals leads to some interesting speculation about “functional” old age in human societies compared to other species, where a post-productive survival period does not exist (Rose, 1994). Do unproductive older individuals “pay back” those who feed them by providing important services (e.g. tool making, wisdom and experience in decisions, useful social and political alliances, etc.). Or are old people essentially subsidized by kin (and others) even though their inclusive fitness contributions are negative? Presumably, if this is the case, emotional bonding mechanisms are responsible for this maladaptive pattern. In simple fitness currency, their kin, and the elderly individuals themselves should both be motivated to terminate investment in their survival when the resources being used to keep them alive could be used to generate greater impact on the reproductive value of younger kin.
Geronticide is common in human history, and the highest suicide rates for both sexes are for people in their sixties and seventies (Bertolote and Fleischmann, 2002). Variation across societies, however, is substantial. In places where aged individuals are revered (often lineage-based societies with ancestor worship), the signal value of an actual living apical lineage ancestor, who has memories of even older ancestors, might be symbolically important if it helped to legitimize property claims and rights vis à vis other competing lineage groups. Hence post-productive provisioning and care for older kin might make adaptive sense if we knew all the details of what exactly elderly kin members provide. In other places, however, it is hard to see that the non-productive elderly serve an fitness-enhancing function to those who care for them. Their knowledge base might be limited, or even out of date (with generational change in relevant environments), and they may undergo cognitive senescence to the point that information and advice they provide is of limited value. Nonetheless such people are sometimes kept alive for years. I, myself, for example, have lived in a house in one traditional society where I observed an elderly woman (aged eighty-six) who was unable to walk, feed herself, or even get out of bed for more than four years, was incontinent for two of those years, and yet is still bathed, fed and cared for like an infant (she is still alive at the time of writing). In such cases, extreme old age looks like a stage that diminishes the fitness of close kin, and yet, care of the aged is quite common. More data on the frequency of this life stage should lead us to examine the proximate emotional bonding mechanisms between humans that lead to cooperation, caretaking and provisioning and may result in a human life history strikingly different from other mammals.
9) Sex Differences in Lifespan
In perhaps all human societies, and among most mammal species, females have longer lifespans than do males. This difference is one of the strongest and most consistent sex differences in nature. For example, in 2018 there were only seven males among the oldest 100 people living on earth (Wikipedia 2018). The 2009 Guinness Book of World Records listed seventy-six people in the world known to be over 110 years of age. Seventy-two of those listed were female, as was the oldest chimpanzee ever known in captivity, and the ten oldest people to have lived documented since birth records have been available. Women live longer than men on average. LHT should provide guidance on this pattern. For example, it is generally accepted that higher reproductive effort per unit time is the main cause of higher mortality in males. This is expected if the payoff structure for male reproductive effort is highly skewed with ever increasing payoffs for the most successful males (see reproductive skew above). Winning in male mating competition requires very high reproductive effort per year (fighting, territorial display, signaling phenotypic quality, mate-guarding females, etc.). That high male reproductive effort must come at the expense of investing in somatic maintenance, repair and immune function, for example; furthermore, some forms of mating competition lead directly to higher mortality (such as male-male combat for females or territories). Likewise, cooperatively breeding females often live especially long lives because of the outside assistance they receive during adulthood. In cooperative breeding birds, adult workloads are lessened, and load lightening appears to improve survival and fertility (Meade et al., 2010) and may slow rates of physiological ageing (Guindre-Parker and Rubenstein, 2017).
However, early male death in humans also seems unexpected from an evolutionary perspective because a significant proportion of human males, but not females, continue to reproduce into old age (see “the derived human life history”, and Tuljapurkar et al., 2007). Human males accumulate resources and political power over the lifespan, such that older males can attain considerable reproductive success. This means that there should possibly be stronger selection on male survival than on female survival after the age of menopause. These two contradictory predictions from LHT invite researchers to provide a clear and consistent picture of the sex difference in the human lifespan. Why do males die at younger ages, despite the fact that in traditional societies it is not uncommon for men to expect more than 10% of their offspring to be conceived after they are aged fifty? How much variation is there in human societies in the sex difference in lifespan, and do the cross-cultural differences in sex differential survival to old age co-vary with the fraction of male reproduction that takes place after female menopause? Can physiological differences in disease resistance be overridden by cultural practices? For example, in most societies, female children also show slightly higher survival than male children, probably because of subtle differences in immune function. However, that small difference is easily overridden to produce excess female juvenile mortality in many societies where female infanticide and neglect are adopted in order to bias lineage sex ratios to contain more males.
10) Sex Ratio Manipulation and Non-Reproductive Adults
Humans in large modern societies often show a slightly male-biased sex ratio at birth (around 106 males/females) and that ratio usually decreases over the lifespan because males die at higher rates than females at all ages (Pongou, 2013). This pattern is not universal, however, particularly when we examine small-scale traditional societies. Male-biased sex ratios at birth and at later ages are commonly found in hunter-gatherer demographic studies. For example, 7/7 hunter-gatherer societies with measured sex ratio at birth showed male-biased ratios in one review, and 17/29 still showed a male-biased ratio in adulthood (Hewlett, 1991). In modern states such as India and China, male-biased sex ratios are extreme and related to cultural preferences for males (Hesketh and Wei Xing, 2006) who will carry on a lineage name, inherit patrilineally transmitted property and social rights, and live patrilocally with the parents who are supported in older age by co-resident children. However, the male-biased sex ratio is also common in groups and subpopulations that may not be patrilineal, have little property to transmit (e.g. Jacobson et al., 1999) and especially among societies that experience high rates of warfare (Divale and Harris, 1976). This leads us to wonder whether a coherent evolutionary model can explain an adaptive preference for sons among a majority of human societies in world history.
Fisher (1930) and others (e.g., Charnov, 1982) have outlined a basic theory of parental investment (PI) in offspring by sex. Fisher pointed out that, since all offspring have one mother and one father, populations should tend to evolve 50:50 sex ratios because when one sex was in short supply, parents producing that sex could expect higher fitness per offspring. These theoreticians noted that parental investment in male and female offspring should be equal when the population sex ratio is 50:50. If offspring of one sex is cheaper to raise to sexual maturity, then parents should simply produce more of that sex since total investment in each sex offspring should be equal. This logic led early human evolutionary demographers to assume that the 106 male to 100 female sex ratio bias at birth might simply be due to the higher juvenile male mortality rates around the world. If boys die at slightly higher rates, then average required PI per male born will be slightly lower and therefore according to this theory parents would produce slightly more sons.
The fact that so many human societies not only show male-biased sex ratio at birth, but also show forms of neglect of female children, and often male-biased adult sex ratios, suggests that we might explore another possible explanation. Perhaps humans, as cooperative breeders, have evolved male-biased sex ratios, via lineage selection, because excess males are intended statistically to function as helpers for close kin (much like the female-sex-ratio bias of workers among ants and wasps, or the male-biased sex ratio of termite colony workers). In other words, human lineages may have evolved non-reproductive castes. This is precisely the pattern found in other cooperatively breeding mammals (McNutt and Silk, 2008; Silk and Brown, 2008). Non-reproductive males are more common than non-reproductive females in most human societies. This might be due to the many activities in which males have a slight comparative advantage, such as work effort (males have higher work capacity than females because of greater lean muscle mass), food provisioning (males produce more food than females in general), or support during conflict (males are active participants in coalitionary violence). This theory of male-biased sex ratio may link the common prevalence of a male non-reproductive “caste” to sex-ratio manipulation in traditional human societies. Children born male that later adopt female-gender socio-economic roles (childcare, weaving and manufacture of clothing and female implements, plant collection rather than hunting, etc.) are reported at significant frequencies in a large number of small-scale ethnographic studies (e.g. Jacobs, 1968; Lang, 1998). In modern societies, between 5–10% of the adult male population is exclusively homosexual (Sell et al., 1995). The fact that this phenotype has a strong genetic component (Ganna el al 2019) and must have arisen via natural selection on specific alleles, suggests that this might possibly represent an adaptive non-reproductive caste.
The term “homosexual” is not a good fit for the cooperative breeding hypothesis, which is more about socio-economic gender roles than sexuality. Such males adopt female body language, adornments, styles of speech and engage in female-specific sex roles overtly, but their actual sexual behavior is not always well-known or studied. Terms like “berdache” or “wo-spirit” (in North American ethnographic studies) and similar native terms for males who adopt a female socio-economic role exist in almost every small-scale, traditional society that is well described (in my own tribal fieldwork such males were described using local terms like “panegi” (Ache), “omegit” (Kuna), “bayot” (Binisaya), etc.). Some aspects of gender orientation in modern societies may be related to this ancestral history, but also appear to show some significant differences not discussed here.
The frequency of male-biased sex ratio and the prevalence of individuals born male who adopt female socioeconomic roles (MTF) in adulthood (e.g. Jacobs, 1968; Lang, 1998) suggests that perhaps humans as cooperative breeders have evolved to overproduce male helpers (Vasey et al 2007; Vasey and Vanderlan 2010; Vanderlaan et al., 2013). One might hypothesize that lineages producing more male offspring outcompete lineages with an unbiased sex ratio under many socioecological contexts typical of small-scale human societies. Several other observations seem to provide some support for this possibility. First, female children who later adopt male socioeconomic roles (FTM) are much more rarely described in traditional societies, and in modern societies their prevalence is still less than the prevalence of male children who adopt female identities in adulthood (MTF), although the difference in frequency has diminished considerably (eg. Sell et al., 1995; Leinung and Joseph 2020). Second, both the male-biased sex ratio and the prevalence of MTF individuals seems absent in closely related great apes (Emery Thompson, personal communication). Third, MTF individuals are more likely in large families (that could use more helpers), they are usually later in birth order, and they are often small in body size (and hence less likely to be competitive male breeders) (see Vanderlaan et al., 2011). Fourth, a few studies have found that in traditional societies, females with MTF siblings have higher fertility (e.g., Iemmola and Ciani, 2009; Vanderlaan et al., 2011), or receive other fitness-enhancing benefits (Vasey et al., 2007). Theoretical modelling suggests that such an effect could maintain the genes required for a non-reproductive caste of MTF over time (e.g., Chaladze, 2016). However, because of their apparent increasing frequency in modern societies (Leinung and Joseph 2020), important future life history research should also examine the role and possibly adaptive nature of FTM individuals in human cooperative breeding kin groups.
Conclusion
Nancy Howell, an early pioneer and highly influential anthropological demographer, once characterized the field as “devilishly difficult but uncommonly interesting” (1986). Howell continued to lead as she made the transition from the “demography” label to the “life history” label, incorporating evolutionary biology to describe the timing and development of !Kung bushman mortality, fertility and development (Howell, 2010). I agree with Howell’s assessment: human demography and life history theory are far more complex than we had originally and naively presumed back in the 1980s when I and others began to explicitly incorporate LHT into the field. This is because biology and life itself are more complicated than many of us once realized. The relationship between genotypes and phenotypes remains a complex black box for the most part, and the constraints on what is possible (rather than optimal) among imaginary phenotypic alternatives depend on a complex biochemical and developmental recipe that is only understood in the most general terms. Furthermore, the relationship between individual phenotypes and the filtered selective transmission of information (both DNA, and epigenic, including culture) renders selection to be a statistical set of patterns that are sometimes obscure with respect to the actual phenotypes of interest. I make these observations not to draw pessimistic conclusions, but simply to remind demographers of the future, that empirical studies of real populations and their actual life histories often constitute pathbreaking contributions as we struggle to determine what aspects of LHT really provide major insights in the construction of life cycles, which are dead ends, and which seemingly logical propositions later turn out to have rested on flawed assumptions or faulty measurements. Theoretical guidance is crucial, but ultimately the lessons of how human life cycles are constructed will have to be validated empirically.
References1
Austad, S., and J. Hoffman, 2018. ‘Is antagonistic pleiotrophy ubiquitous in aging biology?’, Evolution, Medicine, and Public Health: 2018.1: pp. 287–94. https://doi.org/10.1093/emph/eoy033
Baumard, N., and C. Chevallier, 2015. ‘The nature and dynamics of world religions: A life-history approach’, Proc. R. Soc. B, 282.1818: p. 20151593. https://doi.org/10.1098/rspb.2015.1593
Baumard, N., 2018. ‘Increased affluence, life history theory, and the decline of shamanism’, Behavioral and Brain Sciences, 41. https://doi.org/10.1017/s0140525x17001984
Becker, N. S., P. Verdu, B. Hewlett, and S. Pavard, 2010. ‘Can Life History Trade-Offs Explain the Evolution of Short Stature in Human Pygmies? A Response to Migliano et al’, Human Biology, 82.1: pp. 17–27. https://doi.org/10.3378/027.082.0101
Bell, G., and V. Koufopanou, 1986. ‘The cost of reproduction’, in Oxford Surveys in Evolutionary Biology, III, ed. by R. Dawkins & M. Ridley (Oxford: Oxford University Press), pp. 83–131.
Bentley, Gillian R., 1985. ‘Hunter-gatherer energetics and fertility: a reassessment of the !Kung San’, Human Ecology 13: pp. 79–109. https://doi.org/10.1007/BF01531090
Bernardi, L. 2007. ‘An introduction to anthropological demography’, Max Planck Institute for Demographic Research, https://www.demogr.mpg.de/papers/working/wp-2007-031.pdf
Bertolote, J. M., and A. Fleischmann, 2002. ‘A global perspective in the epidemiology of suicide’, Suicidologi, 7.2. https://doi.org/10.5617/suicidologi.2330
Blanc, A. K., and N. Rutenberg, 1990. ‘Assessment of the quality of data on age at first sexual intercourse age at first marriage and age at first birth in the Demographic and Health Surveys’, An assessment of DHS-I data quality, 1990:1.
Bolund, E., and V. Lummaa, 2017. ‘The effects of resource availability and the demographic transition on the genetic correlation between number of children and grandchildren in humans’, Heredity, 118: pp. 186. https://doi.org/10.1038/hdy.2016.81
Boone, J. L. 2002. ‘Subsistence strategies and early human population history: An evolutionary ecological perspective’, World Archaeology, 34.1: pp. 6–25. https://doi.org/10.1080/00438240220134232
Borgerhoff Mulder, M. 1998. ‘The demographic transition: are we any closer to an evolutionary explanation?’, Trends in ecology & evolution, 13.7: pp. 266–70. https://doi.org/10.1016/S0169-5347(98)01357-3
Boutin, S. 1990. ‘Food supplementation experiments with terrestrial vertebrates: patterns, problems, and the future’, Canadian Journal of Zoology, 68.2: pp. 203–20. https://doi.org/10.1139/z90-031
Bromham, L., and M. Cardillo, 2007. ‘Primates follow the “island rule”: implications for interpreting Homo floresiensis’, Biology letters, 3.4: pp. 398–400. https://doi.org/10.1098/rsbl.2007.0113
Brown, P., T. Sutikna, M. J., Morwood, R. P. Soejono, E. W. Saptomo, and R. A. Due, 2004. ‘A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia’, Nature, 431: p. 1055–61. https://doi.org/10.1038/nature02999
Brumbach, B. H., A. J. Figueredo, and B. J. Ellis. 2009. ‘Effects of harsh and unpredictable environments in adolescence on development of life history strategies’, Human Nature, 20.1: pp. 25–51. https://doi.org/10.1007/s12110-009-9059-3
Burkart, J. M., S. B. Hrdy, and C. P. van Schaik. 2009. ‘Cooperative breeding and human cognitive evolution’, Evolutionary Anthropology: Issues, News, and Reviews, 18.5: pp. 175–86. https://doi.org/10.1002/evan.20222
Burkart, J. M., and C. P. van Schaik. 2016. ‘Revisiting the consequences of cooperative breeding’, Journal of Zoology, 299.2: pp. 77–83. https://doi.org/10.1111/jzo.12322
Burger O., J. P. DeLong. 2016. ‘What if fertility decline is not permanent? The need for an evolutionarily informed approach to understanding low fertility’, Phil. Trans. R. Soc. B371.1692: 20150157, http://dx.doi.org/10.1098/rstb.2015.0157
Caldwell, J. C., 1976. ‘Toward a restatement of demographic transition theory’, Population and development review: 2.3/4: pp. 321–66. https://doi.org/10.2307/1971615
Campbell, K. L., and J. W. Wood. 1988. ‘Fertility in traditional societies’, in Natural Human Fertility (London: Palgrave Macmillan), pp. 39–69. https://doi.org/10.1007/978-1-349-09961-0_4
Case, T. J. 1978. ‘On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates’, The Quarterly Review of Biology, 53.3: pp. 243–82. https://doi.org/10.1086/410622
Chaladze, G. 2016. ‘Heterosexual Male Carriers Could Explain Persistence of Homosexuality in Men: Individual-Based Simulations of an X-Linked Inheritance Model’, Archives of sexual behavior, 45.7: pp. 1705–11. https://doi.org/10.1007/s10508-016-0742-2
Chan, M. Y., D.C. Park, N. K. Savalia, S. E. Petersen, and G. S. Wig. 2014. ‘Decreased segregation of brain systems across the healthy adult lifespan’, Proceedings of the National Academy of Sciences, 111.46: pp. E4997-E5006. https://doi.org/10.1073/pnas.1415122111
Chapais, B. 2009. Primeval kinship: How pair-bonding gave birth to human society (Cambridge, Mass.: Harvard University Press).
Chapais, B. 2011. ‘The deep social structure of humankind’, Science, 331.6022: pp. 1276–77. https://doi.org/10.1126/science.1203281
Charlesworth, B. 1994. Evolution in Age-Structured Populations (Cambridge: Cambridge University Press), II.
Charnov, E. L. 1982. The theory of sex allocation (Princeton, NJ: Princeton University Press), XVIII.
Charnov, E. L. 1986. ‘Life history evolution in a “recruitment population”: why are adult mortality rates constant?’, Oikos, 47.2: pp. 129–34. https://doi.org/10.2307/3566037
Charnov, E. L. 1991. ‘Evolution of life history variation among female mammals’, Proceedings of the National Academy of Sciences, 88.4, pp. 1134–37. https://doi.org/10.1073/pnas.88.4.1134
Charnov, E. L. 1993. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (Oxford: Oxford University Press), VI.
Charnov, E. L. and D. Berrigan. 1993. ‘Why do female primates have such long lifespans and so few babies? Or life in the slow lane’, Evolutionary Anthropology: Issues, News, and Reviews, 1.6: pp. 191–94. https://doi.org/10.1002/evan.1360010604
Charnov, E. L. 2001. ‘Evolution of mammal life histories’, Evolutionary Ecology Research, 3: pp. 521–35, https://digitalrepository.unm.edu/cgi/viewcontent.cgi?article=1077&context=biol_fsp
Charnov, E. L. 2011. ‘Body size is the history of life: Growth confronting landscapes of death’, Evolutionary ecology research, 13: pp. 553–55, https://digitalrepository.unm.edu/cgi/viewcontent.cgi?article=1086&context=biol_fsp
Cipriani, Lidio. 1961. ‘Hygiene and medical practices among the Onge (Little Andaman)’, Anthropos 3.4: pp. 481–500.
Cloutier, C. T., J. E. Coxworth, & K. Hawkes. 2015. ‘Age-related decline in ovarian follicle stocks differ between chimpanzees (Pan troglodytes) and humans’, Age, 37.1: p. 10. https://doi.org/10.1007/s11357-015-9746-4
Clutton-Brock, T. 2002. ‘Breeding together: kin selection and mutualism in cooperative vertebrates’, Science, 296.5565: pp. 69–72. https://doi.org/10.1126/science.296.5565.69
Coale, A. J. 1989. ‘Demographic transition’, in Social Economics (London: Palgrave Macmillan), pp. 16–23.
Colleran, H. 2016. ‘The cultural evolution of fertility decline’, Phil. Trans. R. Soc. B, 371.1692: p. 20150152. https://doi.org/10.1098/rstb.2015.0152
Dean, M. C., and T. J. Cole. 2013. ‘Human life history evolution explains dissociation between the timing of tooth eruption and peak rates of root growth’, PLoS One, 8.1: p. e54534. https://doi.org/10.1371/journal.pone.0054534
Divale, W. T., and M. Harris. 1976. ‘Population, warfare, and the male supremacist complex’, American Anthropologist, 78.3: pp. 521–38. https://doi.org/10.1525/aa.1976.78.3.02a00020
Dixon, R. B. 1971. ‘Explaining cross-cultural variations in age at marriage and proportions never marrying’, Population Studies, 25.2: pp. 215–33. https://doi.org/10.2307/2173211
Early, J. D., and T. N. Headland. 1998. Population dynamics of a Philippine rain forest people: The San Ildefonso Agta (Gainesville, FL: University Press of Florida).
Ellison, P. T., 2017. ‘Endocrinology, energetics, and human life history: A synthetic model’, Hormones and Behaviour, 91: pp. 97–106. https://doi.org/10.1016/j.yhbeh.2016.09.006
Ellison, P. T., and M. A. Ottinger. 2014. ‘A comparative perspective on reproductive aging, reproductive cessation, post-reproductive life, and social behavior’, in Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers (Washington DC: The National Academies Press). Pp. 315–38.
Emery Thompson, M., J. H. Jones, A. E. Pusey, S. Brewer-Marsden, J. Goodall, D. Marsden, T. Matsuzawa, T. Nishida, V. Reynolds, Y. Sugiyama, and R. W Wrangham. 2007. ‘Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause’, Current Biology, 17.24: pp. 2150–56. https://doi.org/10.1016/j.cub.2007.11.033
Emery Thompson, Melissa, and Peter T. Ellison, 2018. ‘Fertility and fecundity’, in Chimpanzees and Human Evolution, ed. by Martin N. Muller, Richard W. Wrangham and David Pilbeam (Harvard University Press: Cambridge, MA), in press.
Fisher, R. A. 1930. The Genetical Theory of Natural Selection: A Complete Variorum Edition (Oxford: Oxford University Press).
Flatt, T., and A. Heyland. 2011. Mechanisms of Life History Evolution (New York: Oxford University Press).
Gadgil, M., and W. H. Bossert. 1970. ‘Life historical consequences of natural selection’, The American Naturalist, 104.935, pp. 1–24.
Ganna, A., Verweij, K.J., Nivard, M.G., Maier, R., Wedow, R., Busch, A.S., Abdellaoui, A., Guo, S., Sathirapongsasuti, J.F., 23andMe Research Team 16 and Lichtenstein, P., 2019. ‘Large-scale GWAS reveals insights into the genetic architecture of same-sex sexual behavior’. Science, 365.6456: pp. eaat7693. https://doi.org/10.1126/science.aat7693
Goodman, A., I. Koupil, and D. W. Lawson. 2012. ‘Low fertility increases descendant socioeconomic position but reduces long-term fitness in a modern post-industrial society’, Proceedings of the Royal Society of London B: Biological Sciences, 279.1746: pp. 4342–51. https://doi.org/10.1098/rspb.2012.1415
Guindre-Parker, S., and D. R. Rubenstein. 2017. ‘Cooperative breeding reduces the oxidative costs of reproduction’, in Integrative and Comparative Biology, LVII (New York: USA: Oxford University Press), pp. E280-E280.
Gurven, M., and H. Kaplan. 2007. ‘Longevity among hunter‐gatherers: a cross‐cultural examination’, Population and Development Review, 33.2: pp. 321–65. https://doi.org/10.1111/j.1728-4457.2007.00171.x
Gurven, M., J. Stieglitz, P. L. Hooper, C. Gomes, and H. Kaplan., 2012. ‘From the womb to the tomb: The role of transfers in shaping the evolved human life history’, Experimental Gerontology, 47.10: pp. 807–13. https://doi.org/10.1016/j.exger.2012.05.006
Harshman, L. G., and A. J. Zera. 2007. ‘The cost of reproduction: the devil in the details’, Trends in ecology & evolution, 22.2: pp. 80–86. https://doi.org/10.1016/j.tree.2006.10.008
Harvey, P. H., and R. M. Zammuto. 1985. ‘Patterns of mortality and age at first reproduction in natural populations of mammals’, Nature, 315.6017: pp. 319–20. https://doi.org/10.1038/315319a0
Hart, R. W., and R. B. Setlow. 2001. ‘Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species’, Science’s SAGE KE, 2001.1: pp. 4. https://doi.org/10.1126/sageke.2001.1.cp4
Hawkes, K., J. F. O’Connell, and N. G. Blurton-Jones. 1989. ‘Hardworking hadza grandmothers’, In (V. Standen & R. Foley, Eds) Comparative Socioecology of Mammals and Man, pp. 341–66.
Hawkes, K., J. F. O’Connell, N. B. Jones, H. Alvarez, and E. L. Charnov. 1998. ‘Grandmothering, menopause, and the evolution of human life histories’, Proceedings of the National Academy of Sciences, 95.3: pp. 1336–39. https://doi.org/10.1073/pnas.95.3.1336
Hesketh, T., and Z.W. Xing. 2006. ‘Abnormal sex ratios in human populations: causes and consequences’, Proceedings of the National Academy of Sciences, 103.36: pp. 13271–13275. https://doi.org/10.1073/pnas.0602203103
Hewlett, B. S. 1991. ‘Demography and childcare in preindustrial societies’, Journal of Anthropological Research, 47.1: pp. 1–37. https://doi.org/10.1086/jar.47.1.3630579
Hill, K. R., and A. M. Hurtado. 1991. ‘The evolution of premature reproductive senescence and menopause in human females’, Human Nature, 2.4: pp. 313–50. https://doi.org/10.1007/bf02692196
Hill, K. R. 1993. ‘Life history theory and evolutionary anthropology’, Evolutionary Anthropology: Issues, News, and Reviews, 2.3: pp. 78–88. https://doi.org/10.1002/evan.1360020303
Hill, K. R., and A. M. Hurtado. 1996. Ache life history: The ecology and demography of a foraging people (Abingdon: Routledge).
Hill, K. R., and A. M. Hurtado. 2009. ‘Cooperative breeding in South American hunter–gatherers’, Proceedings of the Royal Society of London B: Biological Sciences. 276.1674: pp. 3863–70. https://doi.org/10.1098/rspb.2009.1061
Hill, K. R., R. S. Walker, M. Božičević, J. Eder, T. Headland, B. Hewlett, A. M. Hurtado, F. Marlowe, P. Wiessner, and B. Wood. 2011. ‘Co-residence patterns in hunter-gatherer societies show unique human social structure’, Science, 331.6022: pp. 1286–89. https://doi.org/10.1126/science.1199071
Hill, K. R., and A. M. Hurtado, 2012. ‘Social science: Human reproductive assistance’, Nature, 483.7388: pp. 160–61. https://doi.org/10.1038/483160a
Hill, K. R., B. M. Wood, J. Baggio, A. M. Hurtado, and R. T. Boyd, 2014. ‘Hunter-gatherer inter-band interaction rates: Implications for cumulative culture’, PLoS One, 9.7: p. e102806. https://doi.org/10.1371/journal.pone.0102806
Howell, N. 1979. Demography of the Dobe !Kung (Aldine: Transaction Publishers).
—. 1986. ‘Demographic anthropology’, Annual Review of Anthropology, 15.1: pp. 219–46. https://doi.org/10.1146/annurev.an.15.100186.001251
Hrdy, S. B. 2000. Mother nature: Maternal instincts and how they shape the human species (New York: Ballantine Books).
—. 2005. Cooperative breeders with an ace in the hole. In: Voland E, Chasiotis A, Schiefenhovel W, editors. Grandmotherhood: The Evolutionary Origin of the Second Half of Female Life. (New Brunswick: Rutgers University Press) pp. 295–317.
—. 2017. ‘Comes the child before man: how cooperative breeding and prolonged postweaning dependence shaped human potential’, In Hunter-gatherer childhoods, Routledge, pp. 65–91.
Hruschka D. J., O. Burger. 2016. ‘How does variance in fertility change over the demographic transition?’, Phil. Trans. R. Soc. B371.1692: pp. 20150155, http://dx.doi.org/10.1098/rstb.2015.0155
Iemmola, F., and A. C. Ciani. 2009. ‘New evidence of genetic factors influencing sexual orientation in men: Female fecundity increase in the maternal line’, Archives of sexual behavior, 38.3: pp. 393–99. https://doi.org/10.1007/s10508-008-9381-6
Isler, K., and C. P. van Schaik. 2012. ‘How our ancestors broke through the gray ceiling: Comparative evidence for cooperative breeding in early homo’, Current Anthropology, 53.S6: pp. S453-S465. https://doi.org/10.1086/667623
Jacobs, S. E. 1968. ‘Berdache: A brief review of the literature’, Colorado Anthropologist, 1.1: pp. 25–40.
Jacobsen, R., H. Møller, and A. Mouritsen. 1999. ‘Natural variation in the human sex ratio’, Human Reproduction, 14.12: pp. 3120–25. https://doi.org/10.1093/humrep/14.12.3120
Jones Gavin, W., 2010. Changing Marriage Patterns in Asia. Singapore: Asia Research Institute, National University of Singapore Working Paper.
Jones, K.P., L. C. Walker, D. Anderson, A. Lacreuse, S. L. Robson, and K. Hawkes. 2007. ‘Depletion of ovarian follicles with age in chimpanzees: similarities to humans’, Biology of Reproduction, 77.2: pp. 247–51. https://doi.org/10.1095/biolreprod.106.059634
Jones, N. B. 2016. Demography and Evolutionary Ecology of Hadza Hunter-Gatherers (Cambridge: Cambridge University Press), LXXI.
Kaplan, H. 1994. ‘Evolutionary and wealth flows theories of fertility: Empirical tests and new models’, Population and Development Review, 20.4: pp. 753–91. https://doi.org/10.2307/2137661
Kaplan, H. S., J. B. Lancaster, S. E. Johnson, and J. A. Bock. 1995. ‘Does observed fertility maximize fitness among New Mexican men?’, Human Nature, 6.4: pp. 325–60. https://doi.org/10.1007/bf02734205
Kaplan, H. 1996. ‘A theory of fertility and parental investment in traditional and modern human societies’, American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists, 101.S23: pp. 91–135. https://doi.org/10.1002/(sici)1096-8644(1996)23+%3C91::aid-ajpa4%3E3.0.co;2-c
Kaplan, H. 1997. ‘The evolution of the human life course’ In Kenneth W. Wachter and Caleb E. Finch, editors. Between Zeus and the salmon: The biodemography of longevity, Washington D.C., The National Academies Press. pp. 175–211. https://doi.org/10.17226/5740
Kaplan, H., K. Hill, J. Lancaster, and A. M. Hurtado. 2000. ‘A theory of human life history evolution: diet, intelligence, and longevity’, Evolutionary Anthropology: Issues, News, and Reviews, 9.4: pp. 156–85. https://doi.org/10.1002/1520-6505(2000)9:4%3C156::aid-evan5%3E3.0.co;2-7
Kaplan, H., and J. B. Lancaster. 2000. ‘The evolutionary economics and psychology of the demographic transition to low fertility’, In L. Cronk, N. Chagnon, W. Irons, editors, Adaptation and human behavior: An anthropological perspective, pp. 283–322. https://doi.org/10.4324/9781351329200
Kaplan, H. S., and A. J. Robson. 2002. ‘The emergence of humans: The coevolution of intelligence and longevity with intergenerational transfers’, Proceedings of the National Academy of Sciences, 99.15: pp. 10221–10226. https://doi.org/10.1073/pnas.152502899
Kaplan, H. S., and S. W. Gangestad. 2005. ‘Life history theory and evolutionary psychology’, In D. Buss editor, The handbook of evolutionary psychology, pp. 68–95.
Kaplan, H. S., M. Gurven, J. Winking, P. L. Hooper, and J. Stieglitz. 2010. ‘Learning, menopause, and the human adaptive complex’, Annals of the New York Academy of Sciences, 1204.1, pp. 30–42. https://doi.org/10.1111/j.1749-6632.2010.05528.x
Keckler, C. N. 1997. ‘Catastrophic mortality in simulations of forager age-at-death: where did all the humans go’, Integrating Archaeological Demography: Multidisciplinary Approaches to Prehistoric Populations. Center for Archaeological Investigations, Occasional Papers, 24: pp. 205–28.
Kertzer, D. I., T.E. Fricke, and T. Fricke, (eds.). 1997. Anthropological demography: Toward a new synthesis (Chicago, IL: University of Chicago Press).
Ketterson, E. D., and V. Nolan Jr. 1992. ‘Hormones and life histories: an integrative approach’, The American Naturalist, 140: pp. S33-S62. https://doi.org/10.1086/285396
Kirkwood, T. B. and S.N. Austad. 2000. ‘Why do we age?’, Nature, 408.6809: pp. 233–38. https://doi.org/10.1038/35041682
Kleiber, M. 1932. ‘Body size and metabolism’, ENE, 1.9, Hilgardia, 6: pp. 315–51. https://doi.org/10.1152/physrev.1947.27.4.511
Koster, J. et al. (39 coauthors) 2020. ‘The life history of human foraging: Cross-cultural and individual variation’. Science advances, 6.26: pp. 100–20. https://doi.org/10.1126/sciadv.aax9070
Kramer, K. L. 2005. ‘Children’s help and the pace of reproduction: cooperative breeding in humans’, Evolutionary Anthropology: Issues, News, and Reviews, 14.6: pp. 224–37. https://doi.org/10.1002/evan.20082
Kramer, K. L., R. D. Greaves, and P. T. Ellison, 2009. ‘Early reproductive maturity among Pumé foragers: Implications of a pooled energy model to fast life histories’, American Journal of Human Biology: The Official Journal of the Human Biology Association, 21.4: pp. 430–37. https://doi.org/10.1002/ajhb.20930
Kramer, K. L. 2010. ‘Cooperative breeding and its significance to the demographic success of humans’, Annual Review of Anthropology, 39.1: pp. 417–36. https://doi.org/10.1146/annurev.anthro.012809.105054
Kuzawa, C. W., H. T. Chugani, L. I. Grossman, L. Lipovich, O. Muzik, P. R. Hof, D. E. Wildman, C. C. Sherwood, W. R. Leonard, and N. Lange. 2014. ‘Metabolic costs and evolutionary implications of human brain development’, Proceedings of the National Academy of Sciences, 111.36: pp. 13010–13015. https://doi.org/10.1073/pnas.1323099111
Lance, V.A. 2003. ‘Alligator physiology and life history’, Experimental Gerentology 38.7: pp. 801–05. https://doi.org/10.1016/s0531-5565(03)00112-8
Lang, S. 1998. Men as women, women as men: Changing gender in Native American cultures (Austin: University of Texas Press).
Lee, R. 2003. ‘The demographic transition: three centuries of fundamental change’, Journal of Economic Perspectives, 17.4: pp. 167–90. https://doi.org/10.1257/089533003772034943
Leinung, M.C. and Joseph, J. 2020. ‘Changing demographics in transgender individuals seeking hormonal therapy: Are trans women more common than trans men?’. Transgender Health, 5.4: pp. 241–45. https://doi.org/10.1089/trgh.2019.0070
Levitis, D.A., O. Burger, and L. B. Lackey. 2013. ‘The human post‐fertile lifespan in comparative evolutionary context’, Evolutionary Anthropology: Issues, News, and Reviews, 22.2: pp. 66–79. https://doi.org/10.1002/evan.21332
Lomolino, M. V. 1985. ‘Body size of mammals on islands: the island rule reexamined’, The American Naturalist, 125.2: pp. 310–16. https://doi.org/10.1086/284343
Lomolino, M. V. 2005. ‘Body size evolution in insular vertebrates: generality of the island rule’, Journal of Biogeography, 32.10: pp. 1683–99. https://doi.org/10.1111/j.1365-2699.2005.01314.x
Mace, R. 2000. ‘Evolutionary ecology of human life history’, Animal Behaviour, 59.1: pp. 1–10. https://doi.org/10.1006/anbe.1999.1287
Mace, R., and R. Sear. 2005. ‘Are humans cooperative breeders’, in E. Voland, A. Chasiotis & W. Schiefenhoevel editors, Rutgers Univ. Press. pp. 143–59.
MacRae, S.L., M. M. Croken, R. B. Calder, A. Aliper, B. Milholland, R. R. White, A. Zhavoronkov, V. N. Gladyshev, A. Seluanov, V. Gorbunova, and Z. D. Zhang. 2015. ‘DNA repair in species with extreme lifespan differences’, Aging, 7.12: pp. 1171–82. https://doi.org/10.18632/aging.100866
Mangel, M., and W. H. Satterthwaite. 2016. ‘Modelling anadromous salmonid life-history’, in Evolutionary biology of the Atlantic salmon, ed. by T. Vladic and E. Petersson (Boca Raton: CRC Press), pp. 221–47.
Marlowe, F. W. 2005. ‘Hunter‐gatherers and human evolution’, Evolutionary Anthropology: Issues, News, and Reviews, 14.2: pp. 54–67. https://doi.org/10.1002/evan.20046
McNutt, J. W., and J. B. Silk. 2008. ‘Pup production, sex ratios, and survivorship in African wild dogs, Lycaon pictus’, Behavioural ecology and Sociobiology, 62.7: pp. 1061–67. https://doi.org/10.1007/s00265-007-0533-9
Meade, J., K. B. Nam, A. P. Beckerman, and B. J. Hatchwell. 2010. ‘Consequences of “load‐lightening” for future indirect fitness gains by helpers in a cooperatively breeding bird’, Journal of Animal Ecology, 79.3: pp. 529–37. https://doi.org/10.1111/j.1365-2656.2009.01656.x
Meehan, C. L., R. Quinlan, and C. D. Malcom. 2013. ‘Cooperative breeding and maternal energy expenditure among Aka foragers’, American Journal of Human Biology, 25.1: pp. 42–57. https://doi.org/10.1002/ajhb.22336
Meiri, S., P. Raia, and A. B. Phillimore. 2011. ‘Slaying dragons: limited evidence for unusual body size evolution on islands’, Journal of Biogeography, 38.1: pp. 89–100. https://doi.org/10.1111/j.1365-2699.2010.02390.x
Migliano, A. B., L. Vinicius, and M. M. Lahr. 2007. ‘Life history trade-offs explain the evolution of human pygmies’, Proceedings of the National Academy of Sciences, 104.51, pp. 20216–20219. https://doi.org/10.1073/pnas.0708024105
Migliano, A. B., and M. Guillon. 2012. ‘The effects of mortality, subsistence, and ecology on human adult height and implications for Homo evolution’, Current Anthropology, 53.S6: pp. S359-S368. https://doi.org/10.1086/667694
Mijares, A. S., F. Détroit, P. Piper, R. Grün, P. Bellwood, M. Aubert, G. Champion, N. Cuevas, A. de Leon, and E. Dizon. 2010. ‘New evidence for a 67,000-year-old human presence at Callao Cave, Luzon, Philippines’, Journal of Human Evolution, 59.1: pp. 123–32. https://doi.org/10.1016/j.jhevol.2010.04.008
Norberg, R. A. 1981. ‘Temporary weight decrease in breeding birds may result in more fledged young’, The American Naturalist, 118.6: pp. 838–50. https://doi.org/10.1086/283874
Oli, M. K. 2004. ‘The fast–slow continuum and mammalian life-history patterns: an empirical evaluation’, Basic and Applied Ecology, 5.5: pp. 449–63. https://doi.org/10.1016/j.baae.2004.06.002
Parker, G. A., and J. M. Smith, 1990. ‘Optimality theory in evolutionary biology’, Nature, 348.6296: pp. 27–33. https://doi.org/10.1038/348027a0
Pennington, R. 2001. ‘Hunter-gatherer demography’, In C. Panter-Brick, R.H. Layton, and P. Rowly-Conwy editors, Hunter-gatherers: An interdisciplinary perspective. Cambridge Univ. Press. pp. 170–204.
Pontzer, H., M. H. Brown, D. A. Raichlen, H. Dunsworth, B. Hare, K. Walker, A. Luke, L. R. Dugas, R. Durazo-Arvizu, D. Schoeller, and J. Plange-Rhule. 2016. ‘Metabolic acceleration and the evolution of human brain size and life history’, Nature, 533.7603: pp. 390–92. https://doi.org/10.1038/nature17654
Promislow, D. E., and P. H. Harvey. 1990. ‘Living fast and dying young: a comparative analysis of life‐history variation among mammals’, Journal of Zoology, 220.3: pp. 417–37. https://doi.org/10.1111/j.1469-7998.1990.tb04316.x
Pope E. C., G. C. Hays, T. M. Thys, T. K. Doyle, D. W. Sims, N. Queiroz, V. J. Hobson, L. Kubicek, and D. R. Houghton. 2010. ‘The biology and ecology of the ocean sunfish Mola mola: a review of current knowledge and future research perspectives’, Reviews in fish biology and fisheries, 20.4: pp. 471–87. https://doi.org/10.1007/s11160-009-9155-9
Purvis, A., and P. H. Harvey, 1995. ‘Mammal life‐history evolution: a comparative test of Charnov’s model’, Journal of Zoology, 237.2: pp. 259–83. https://doi.org/10.1111/j.1469-7998.1995.tb02762.x
Rozzi, F. V. R. 2018. ‘Reproduction in the Baka pygmies and drop in their fertility with the arrival of alcohol’, Proceedings of the National Academy of Sciences: 115.27: pp. E6126-E6134. https://doi.org/10.1073/pnas.1719637115
Ricklefs, R. E., and M. Wikelski. 2002. ‘The physiology/life-history nexus’, Trends in Ecology & Evolution, 17.10: pp. 462–68. https://doi.org/10.1016/s0169-5347(02)02578-8
Robinson, J. G., and K. H. Redford. 1986. ‘Body size, diet, and population density of Neotropical forest mammals’, The American Naturalist, 128.5: pp. 665–80. https://doi.org/10.1086/284596
Rogers, A. R. 1993. ‘Why menopause?’, Evolutionary Ecology, 7.4: pp. 406–20. https://doi.org/10.1007/bf01237872
Rosas, A., L. Ríos, A. Estalrrich, H. Liversidge, A. García-Tabernero, R. Huguet, H. Cardoso, M. Bastir, C. Lalueza-Fox, M. de la Rasilla, and C. Dean. 2017. ‘The growth pattern of Neandertals, reconstructed from a juvenile skeleton from El Sidrón (Spain)’, Science, 357.6357: pp. 1282–87. https://doi.org/10.1126/science.aan6463
Rose, M. R. 1994. Evolutionary Biology of Aging (Oxford: Oxford University Press).
Ross C. T. et al. (33 co-authors). 2018. ‘Greater wealth inequality, less polygyny: rethinking the polygyny threshold model’, J. R. Soc. Interface, 15.144: pp. 20180035. https://doi.org/10.1098/rsif.2018.0035
Ross C. T. et al. (68 co-authors). 2023. ‘Reproductive inequality in humans and other mammals’. Revised and submitted for publication.
Salthouse, T. A. 2009. ‘When does age-related cognitive decline begin?’, Neurobiology of Aging, 30.4: pp. 507–14. https://doi.org/10.1016/j.neurobiolaging.2008.09.023
Schwartz, G. T. 2012. ‘Growth, development, and life history throughout the evolution of Homo’, Current Anthropology, 53.S6: pp. S395-S408. https://doi.org/10.1086/667591
Sear, R., and R. Mace. 2008. ‘Who keeps children alive? A review of the effects of kin on child survival’, Evolution and Human Behavior, 29.1: pp. 1–18. https://doi.org/10.1016/j.evolhumbehav.2007.10.001
Sear, R., and D. Coall. 2011. ‘How much does family matter? Cooperative breeding and the demographic transition’, Population and Development Review, 37: pp. 81–112. https://doi.org/10.1111/j.1728-4457.2011.00379.x
Sear, R., D. W. Lawson, H. Kaplan, and M. K. Shenk. 2016. ‘Understanding variation in human fertility: what can we learn from evolutionary demography?’ Philosophical Transactions of the Royal Society B: Biological Sciences, 371.1692: pp.20150144. https://doi.org/10.1098/rstb.2015.0144
Sell, R. L., J. A. Wells, and D. Wypij. 1995. ‘The prevalence of homosexual behavior and attraction in the United States, the United Kingdom and France: Results of national population-based samples’, Archives of Sexual Behavior, 24.3: pp. 235–48. https://doi.org/10.1007/bf01541598
Silk, J. B., and G. R. Brown. 2008. ‘Local resource competition and local resource enhancement shape primate birth sex ratios’, Proceedings of the Royal Society of London B: Biological Sciences, 275.1644: pp. 1761–65. https://doi.org/10.1098/rspb.2008.0340
Smaldino, P. E., L. Newson, J. C. Schank, and P. J. Richerson. 2013. ‘Simulating the evolution of the human family: Cooperative breeding increases in harsh environments’, PLoS One, 8.11: pp. e80753. https://doi.org/10.1371/journal.pone.0080753
Stearns, S. C. 1976. ‘Life-history tactics: a review of the ideas’, The Quarterly Review of Biology, 51.1: pp. 3–47. https://doi.org/10.1086/409052
Stearns, Stephen C. 1992. The Evolution of Life Histories (Oxford: Oxford University Press).
Steckel, R. H. 2009. ‘Heights and human welfare: Recent developments and new directions’, Explorations in Economic History, 46.1: pp. 1–23. https://doi.org/10.1016/j.eeh.2008.12.001
Strassmann, B. I., and B. Gillespie, 2002. ‘Life–history theory, fertility and reproductive success in humans’, Proceedings of the Royal Society of London B: Biological Sciences, 269.1491: pp. 553–62. https://doi.org/10.1098/rspb.2001.1912
Swinburn, B. A., S. J. Ley, H. E. Carmichael, and L. D. Plank, 1999. ‘Body size and composition in Polynesians’, International Journal of Obesity, 23.11: pp. 1178–83. https://doi.org/10.1038/sj.ijo.0801053
Thomas, F., F. Renaud, E. Benefice, T. De Meeüs, and J. F. Guegan. 2001. ‘International variability of ages at menarche and menopause: patterns and main determinants’, Human Biology: 73.2: pp. 271–90. https://doi.org/10.1353/hub.2001.0029
Thompson, M. E., M. N. Muller, K. Sabbi, Z. P. Machanda, E. Otali, and R. W. Wrangham. 2016. ‘Faster reproductive rates trade off against offspring growth in wild chimpanzees’, Proceedings of the National Academy of Sciences: 113.28: pp. 7780–85. https://doi.org/10.1073/pnas.1522168113
Tucci, S., S. H. Vohr, R. C. McCoy, B. Vernot, M. R. Robinson, C. Barbieri, B. J. Nelson, W. Fu, G. A. Purnomo, H. Sudoyo, and E. E. Eichler. 2018. ‘Evolutionary history and adaptation of a human pygmy population of Flores Island, Indonesia’, Science, 361.6401: pp. 511–16. https://doi.org/10.1126/science.aar8486
Tuljapurkar, S. D., C. O. Puleston, and M. D. Gurven. 2007. ‘Why men matter: mating patterns drive evolution of human lifespan’, PloS One, 2.8: pp. e785. https://doi.org/10.1371/journal.pone.0000785
Urlacher, S. S., and K. L. Kramer. 2018. ‘Evidence for energetic tradeoffs between physical activity and childhood growth across the nutritional transition’, Nature.com Scientific reports, 8.1, pp. 369. https://doi.org/10.1038/s41598-017-18738-4
Urlacher, S. S., P. T. Ellison, L. S. Sugiyama, H. Pontzer, G. Eick, M. A. Liebert, T. J. Cepon-Robins, T. E. Gildner, and J. J. Snodgrass. 2018. ‘Tradeoffs between immune function and childhood growth among Amazonian forager-horticulturalists’, Proceedings of the National Academy of Sciences, 115.17: pp. E3914-E3921. https://doi.org/10.1073/pnas.1717522115
Van Schaik, C. P., and J. M. Burkart, 2010. ‘Mind the gap: Cooperative breeding and the evolution of our unique features’, in Mind the Gap (Berlin, Heidelberg: Springer), pp. 477–96.
VanderLaan, D. P., and P. L. Vasey. 2011. ‘Male sexual orientation in Independent Samoa: Evidence for fraternal birth order and maternal fecundity effects’, Archives of Sexual Behavior, 40.3: pp. 495–503. https://doi.org/10.1007/s10508-009-9576-5
VanderLaan, D. P., Z. Ren, and P. L. Vasey. 2013. ‘Male androphilia in the ancestral environment’, Human Nature, 24.4: pp. 375–401. https://doi.org/10.1007/s12110-013-9182-z
Vasey, P. L., D. S. Pocock, and D. P. VanderLaan. 2007. ‘Kin selection and male androphilia in Samoan fa’afafine’, Evolution and Human Behavior, 28.3: pp. 159–67. https://doi.org/10.1016/j.evolhumbehav.2006.08.004
Vasey, P.L. and VanderLaan, D.P. 2010. ‘An adaptive cognitive dissociation between willingness to help kin and nonkin in Samoan fa’afafine’. Psychological Science, 21.2: pp. 292–97. https://doi.org/10.1177/0956797609359623
Vaupel, J. W. 2010. ‘Biodemography of human ageing’, Nature, 464.7288: pp. 536. https://doi.org/10.1038/nature08984
Venkataraman, V. V., A. K. Yegian, I. J. Wallace, N. B. Holowka, I. Tacey, M. Gurven, and T. S. Kraft. 2018. ‘Locomotor constraints favour the evolution of the human pygmy phenotype in tropical rainforests’, Proceedings of the Royal Society B, 285.1890: p. 20181492. https://doi.org/10.1098/rspb.2018.1492
Walker, R., K. Hill, H. Kaplan, and G. McMillan. 2002. ‘Age-dependency in hunting ability among the Ache of Eastern Paraguay’, Journal of Human Evolution, 42.6: pp. 639–57. https://doi.org/10.1006/jhev.2001.0541
Walker, R., M. Gurven, K. Hill, A. Migliano, N. Chagnon, R. De Souza, G. Djurovic, R. Hames, A. M. Hurtado, H. Kaplan, and K. Kramer. 2006. ‘Growth rates and life histories in twenty‐two small‐scale societies’, American Journal of Human Biology: The Official Journal of the Human Biology Association, 18.3: pp. 295–311. https://doi.org/10.1002/ajhb.20510
West, G. B., J. H. Brown, and B. J. Enquist. 1997. ‘A general model for the origin of allometric scaling laws in biology’, Science, 276.5309: pp. 122–26. https://doi.org/10.1126/science.276.5309.122
Western, D. 1979. ‘Size, life history and ecology in mammals’, African Journal of Ecology, 17.4: pp. 185–204. https://doi.org/10.1111/j.1365-2028.1979.tb00256.x
Williams, G. C. 1957. ‘Pleiotropy, natural selection, and the evolution of senescence’, Evolution, 11.4: pp. 398–411. https://doi.org/10.2307/2406060
Wikipedia. 2018. https://en.wikipedia.org/wiki/List_of_oldest_living_people
US Bureau of Labor Statistics. 2017. https://www.bls.gov/news.release/wkyeng.t03.htm
1 Note this chapter has been posted on the Open Science Framework website since 18/10/2019, after it was accepted for publication, so the references will reflect when the chapter was written and not the OBP publication date
