6. Ecological Evolutionary Demography: Understanding Variation in Demographic Behaviour
© 2024 Siobhán M. Cully & Mary K. Shenk, CC BY 4.0 https://doi.org/10.11647/OBP.0251.06
Ecological evolutionary demography is the branch of evolutionary demography that focuses on the potential adaptive value of demographic behaviour at the level of the individual. First defined by Low and colleagues some twenty-five years ago, ecological evolutionary demography has gained important ground in developing our understanding of the ultimate evolutionary ecological drivers of fertility and mortality, often in combination with more proximate determinants of these demographic outcomes. In doing so, the field has provided solutions for apparent paradoxes associated with human fertility — how humans sustain high fertility despite highly dependent young and slow development of offspring, as well as the demographic transition — and has led to an improved understanding of the basic pattern of human mortality. A third core area in mainstream demography — migration — has received less attention from an ecological evolutionary perspective, but work on dispersal generates insights into how various “push” and “pull” factors affect the costs and benefits of leaving the natal community, and how such strategies vary across individuals, households and societies. Given the broad framework underlying ecological evolutionary demography investigations of demographic behaviour, the field has outstanding potential for integration across demography and the evolutionary social sciences. We offer several potential pathways for immediate pursuit and anticipate that this will invigorate further the impact of the field on understanding human demographic behaviour.
Introduction
Demography lies at the heart of every statement about selection.
— Jones (2010, p. 74)
Biological, anthropological and formal demographers have long pursued a set of overlapping interests in parallel and with limited interchange. This is despite clear overlap in goals and methods: demography’s core concepts of fertility and mortality are central to the definition of biological fitness that serves as the foundation of the evolutionary sciences (Jones, 2010) and evolution has provided much-needed theory for the primarily descriptive discipline of population demography (Kaplan and Gurven, 2008; Sear, 2016).
“Ecological evolutionary demography” (EED) (sensu Low, Clarke, and Lockridge, 1992) represents a marriage of these interests. It is the study of contemporary human demographic behaviour from an evolutionary and ecological perspective. With its origins in the fields of evolutionary and behavioural ecology, ecological evolutionary demography focuses equally (1) on how individual demographic behaviours adjust to particular socio-ecological contexts both historically and cross-culturally, and (2) how individual-level constraints affect decision-making within a given socio-ecological context (Smith and Winterhalder, 1992a). In particular, ecological evolutionary demography anticipates that individuals will adjust their (demographic) behaviour in the pursuit of maximizing lifetime reproductive success1 (LRS) such that, consciously or unconsciously, an individual makes decisions that attempt to maximize fitness.2 EED is distinct from the broader discipline of human behavioural/evolutionary ecology in its explicit interest in demographic outcomes: fertility, mortality and migration. EED overlaps with other areas of evolutionary demography, but is distinct from mainstream evolutionary demography due to its strong empirical focus on using data from (relatively) contemporary populations to: (1) understand the evolution of species-typical traits (e.g. the human mortality profile); (2) test evolutionary hypotheses about demographic traits; and (3) to understand variation in contemporary demographic patterns.
The evolutionary ecological view of human demography thus has been largely divorced not only from mainstream, medical and anthropological demography, but also from much research in the field of evolutionary demography as practised by evolutionary biologists whose work generally focuses on non-human species. For example, although both sets of scholars refer to themselves as “evolutionary demographers”, ecological evolutionary demographers studying human demographic behaviour — generally from interview, survey or historical data — make up a small fraction of the evolutionary demography society,3 the primary academic society supporting scholarship in evolutionary demography. Instead, many evolutionary demographers focus more heavily on the evolutionary biology of life history trade-offs, with a particularly strong emphasis on understanding the limits to lifespan (e.g. Carey, 2003; Zuo and others, 2018; Colchero and others, 2016; Dong and others, 2016), and how longevity trades off with fertility (e.g. Kirkwood and Rose, 1991; Gagnon and others, 2009; Bolund and others, 2016), generally with a stronger focus on animal and plant models and experimental — as opposed to observational — methods.
Chapter Outline and Objectives
In the remainder of this chapter, we hope to clarify both the particular contributions made by ecological evolutionary demographers to the broader field of evolutionary demography, and the scope for increasing integration of the EED perspective within core areas of evolutionary demography. This chapter is modelled on Low et al. (1992), and aims to provide a broad, if not comprehensive, overview of EED as it has informed understanding of core demographic concerns: fertility, mortality and migration. In each area, we synthesize recent and seminal theories and case studies and show how these provide new and important insights into the ultimate drivers of demographic behaviour. These sections are flanked by an expanded discussion of the theoretical and methodological toolkits used by EED and a conclusion that notes how EED is poised to contribute to our understanding of complex demographic behaviour within and across societies. While obviously relevant, ecological drivers of demographic behaviour, per se, are not central to this chapter; these are reviewed usefully by Uggla (this volume).
Core Frameworks, Methods and Datasets
Ecological evolutionary demography uses core principles from the field of human evolutionary and behavioural ecology, notably life history theory, to understand the ultimate causes of demographic behaviour. The goal of life history theory is to explain the evolution and development of strategies that optimize the usage of resources across the life course and across varying ecological conditions (Stearns 1992). Life history strategies exist at the species level as responses to past ecological conditions and at the individual level as responses to variable ecological and developmental conditions (Ellis and others, 2009). According to this framework, demographic behaviour is the outcome of allocation decisions whereby an individual chooses how to invest energy and resources across a number of competing biological demands, including somatic effort (growth, maintenance of the body, immune function) and reproductive effort (mating and parenting). Variation in life history traits such as age at sexual maturity, age at first birth, birth spacing, age at last birth, and number of offspring born, results from trade-offs in the distribution of resources or energy to these competing life functions (Stearns, 1992; Charnov, 1993; Roff, 1993). The “principle of allocation” contends that greater investment in one domain — growth, maintenance, mating, gestation, parenting — occurs at the expense of others. The costs and benefits of different strategies and trade-offs vary as a function of individual characteristics (e.g. age, sex, health status) and local circumstances (e.g. resource distribution, level of competition for mates or resources), meaning that strategies that are optimal for an individual in one environment are not optimal for a different individual in a different environment (Ellis et al., 2009; Bogin, 2009; Chisholm, 1999; Hill, this volume).
There are several aspects of the ecological evolutionary approach that complement mainstream demographic approaches. First, whereas mainstream demography is primarily “bottom-up” — building theory from observed associations — ecological evolutionary demography is primarily “top-down” — testing well-developed theories with demographic data (Kaplan and Gurven, 2008). In essence, ecological evolutionary demography tends to pursue what Ernst Mayr and then Niko Tinbergen (Mayr, 1961; Tinbergen, 1963) referred to as “ultimate” questions, surrounding the fitness value of traits in contemporary environments, whereas mainstream demographers are typically more interested in “proximate” questions, examining the correlates and predictors of patterns of demographic behaviour, often without asking why or how the behaviours benefit or disadvantage the individuals who perform them (Low and others, 1992: 5). Importantly, proximate responses to environmental factors that affect demographic behaviour will not be maintained if they are not favoured by selection. Thus, in our view, a complete understanding of demographic behaviour requires an evolutionary perspective, as this perspective is the most likely to provide information about the stability of observed associations over time and across contexts.
Second, ecological evolutionary demography focuses strongly on individual decision-making within specific contexts — employing “methodological individualism”4 (Weber, 1978; Smith and Winterhalder, 1992a) to make inferences about how an individual’s characteristics lead to optimal behaviour that is specific to that individual. Mainstream demography has historically made greater use of data aggregated at larger levels (e.g. cities, countries or other populations) to make inferences about how social and economic variables affect demographic behaviour at regional scales. A focus on the individual level is well represented in recent work in demography (a tradition known in the field as “microdemography”), but has tended to emphasize quantification of, proximate causes for, and/or policy-relevant aspects of demographic events. This difference in approach affects how the costs and benefits of demographic behaviour are understood (Low and others, 1992: 11). In particular, benefits at the societal level may be directly contradicted by individual-level benefits. For example, encouraging fertility reduction (e.g. see Bulatao, 1985) is very unlikely to be successful if such behaviour is promoted to “benefit society” and more likely to be successful if it is accompanied by tangible benefits to parents of fewer children. Daughter-neglect is similarly resistant to “public good” incentives; a variety of examples suggest that the valuation of daughters arises in relation to the perceived usefulness of those daughters to individual families (e.g. Das Gupta and others, 2003; Fraser Schoen, 2014). Indeed, EED is explicitly interested in how variation in demographic behaviour arises and is sceptical of inferences drawn from pooled data that compare central tendencies due to the problem of overextending inferences caused by the ecological fallacy5 (Pollet and others, 2014), and the potential to obscure underlying causes of demographic behaviour that are driven by individual-, not population-level considerations (e.g. Alvergne and Lummaa, 2014; Low, 2000).
Third, ecological evolutionary demography is especially concerned with the ways in which the specific socio-ecological contexts in which individuals are embedded modify individual demographic behaviour (see also Uggla, this volume). EED employs “ecological selectionism”6 — under the assumption that different ecologies are likely to produce different behavioural optima. For example, different types of subsistence systems correlate with different demographic behaviours in terms of age of marriage, number of marriage partners and level of fertility both across and often within societies. Across populations, horticultural societies, which are limited in terms of the labour needed to work the land (“labour-limited”), are commonly polygynous with relatively early ages at first marriage (Goody, 1976; Harrell, 1997), while intensive agricultural societies, where resources are limited by the amount of land available (“land-limited”), are more likely to be monogamous and focus investment on a smaller number of offspring. Within-society variation is leveraged by Daniel Nettle to explore how environmental harshness in contemporary England maps onto reproductive behaviour. He finds that individuals residing in deprived neighbourhoods have faster life histories, reproduce earlier, more often and with lesser apparent investment in each child (Nettle, 2010). There is also increasing attention being paid to understanding the effects of ecological context at multiple levels within and across communities: Mattison et al. (2022), for example, show that indicators of market integration differ across individual, household and community levels, each with different influences on reproductive and health outcomes.
Finally, while a key strength of ecological evolutionary demography is the focus on empirical work in contemporary or recent historical populations, researchers have also borrowed formal models from economics (e.g. Kaplan, 1996), population genetics (e.g. Coulson and others, 2010), and formal demography (e.g. Jones and Bliege Bird, 2014; Rogers, 1990) to draw conclusions about demographic behaviour. An exciting recent development has been the increasing incorporation of models from cultural evolutionary theory (e.g. Mattison et al., 2018; Kolodny, Feldman, and Creanza, 2018), such that demographic behaviour is predicted not solely on the basis of what behaviours are predicted to be optimal, but also on the basis of how behaviours are socially transmitted. Although the attempt to integrate these disciplines is in its early stages (Creanza and others, 2017), demographic behaviour (as opposed to demographic intent) is readily observed and may provide one of the more straightforward routes forward for refined synthesis. This line of thinking should also address with much more clarity the extent to which cultural processes may be ultimately responsible for demographic behaviour, as commonly assumed by demographic models (Low and others, 1992: 8), versus the extent to which “materialist” incentives drive demographic behaviour (Sheehan and others, 2018; Shenk and others, 2013) in line with much thinking in human behavioural ecology, versus how these two “forces” interact to drive demographic behaviour (Henrich, 2004).
Methods & Data
Congruent with its focus on the individual, ecological evolutionary demography relies primarily on datasets that include details of individuals’ demographic behaviour as the behaviour manifests within particular contexts (i.e. the household and the local community). The earliest examples derive from first-hand data collection in small-scale communities, whose demographic behaviour, by “ethnographic analogy”,7 could provide unique insights into presumed behaviour of prehistoric human ancestors. James Woodburn was an early pioneer of such work with the Hadza (Woodburn, 1968; Konner, 2017); his work on the Hadza subsequently inspired numerous demographic inquiries from a “neo-Darwinian” perspective focusing on small-scale societies, including Lee and DeVore’s seminal work Man the Hunter (1968). Nancy Howell’s Demography of the Dobe !Kung (1979) “set the standard for hunter-gatherer demography” (Konner, 2017). This tradition has continued in more recent examples, including Frank Marlowe’s The Hadza (2010), and Nicholas Blurton-Jones’ Demography and Evolutionary Ecology of Hadza Hunter-Gatherers (2016). Other important works in the EED tradition include Pennington and Harpending’s Structure of an African Pastoralist Community (1993) and Hill and Hurtado’s Ache Life History (1996). In each case, the authors have painstakingly gathered data on the demographic statuses and events experienced by individuals, including births, deaths, marriages and divorces, as well as genealogies that allow these individuals to be linked together in families and lineages. Unlike much of the data gathered in mainstream demographic work, ecological evolutionary demographers often spend years residing within their study communities, so that the data provided is of exceptionally high quality. Indeed, many contemporary methods to circumvent problems of estimating demographic events that arise in non-literate populations were pioneered by ecological evolutionary demographers (e.g. see Konigsberg and Frankenberg, 1992; Quinlan and Hagen, 2008).
A variety of secondary data sets have also propelled ecological evolutionary demography into arguably more complex social realms. These data sets are collected by individuals and groups for different purposes (Smith and others, 2011), but contain data that may be used to reconstruct individual life histories and demographic behaviour. Increasingly used by human behavioural ecologists and evolutionary demographers (Nettle and others, 2013), such data sets provide a number of specific challenges and opportunities that both expand and constrain their use in tests of evolutionarily informed hypotheses.
Common sources for secondary datasets analysed by ecological evolutionary demographers include parish records, household registers and research-driven demographic and public health data sets. Firstly, historical demographic records have been employed successfully by several ecological evolutionary demographers. Such records are invaluable for linking families across multiple generations, within specific, known historical, demographic and ecological contexts, and for detailing the variation in demographic decision-making as it relates to individual constraints and opportunities. Indeed, many of the topics of interest to human behavioural ecologists, such as choice of marriage partner, fertility and mortality schedules, evidence of parental investment and reproductive success (Smith, 2000; Smith and Winterhalder, 1992a), can be examined using data contained in parish registers, allowing for sophisticated evolutionary analysis of pre-existing data in well-described historical contexts (Boone, 1986, 1988; Voland, 2000; Lummaa, 2004; Clarke and Low, 2001).
Secondly, large, statistically robust data sets, including high-quality data on many variables of interest to evolutionary demographers, and derived from large-scale populations, are readily available and often financially cost-free to analyse. These data sets have a number of advantages compared to small primary data sets historically of interest to ecological evolutionary demographers, including large sample sizes, rich data and often longitudinal designs (Mattison and Sear, 2016) that facilitate in-depth analysis of individual life histories. They also point to significant variability within so-called WEIRD (Western Educated Industrialized Rich and Democratic) societies (Henrich and others, 2010) — variability that may be usefully mined to explore the context-specific nature of demographic behaviour in contemporary, industrialized settings (see Stulp and others, 2016).
Yet secondary data sets are subject to a number of important methodological challenges. Firstly, demographic events are often recorded long after they occurred and are subject to errors, including those due to systematic biases in recall (e.g. a propensity to forget deaths of certain classes of individuals, such as the unbaptized). There are techniques to estimate the level of under-registration that is produced by such problems (e.g. Eriksson and others, 2018) and to infer missing data (e.g. Langkamp and others, 2010). Nonetheless, care must be taken to ensure that data are reliable for analysis (see e.g. Wrigley, 1997). Secondly, the population that is able to be registered (i.e. that is “under observation”) may differ systematically from the populations about which the dataset serves to generalize. The characteristics and behaviours of migrants may differ systematically from those of individuals who remain in the study area, for example. This can make it difficult to characterize the constraints affecting different classes of individuals and, in turn, how these affect reproductive and demographic decision-making (e.g. Strassman and Clarke, 1998) and the timing of demographic events (Voland, 2000). Thirdly, because secondary data sets are compiled for a variety of different reasons, the ability to use them to examine the complex causal factors affecting individual decision-making can be limited. Reliable socio-economic information is often lacking from parish and household registers, for example, confounding attempts to describe resource-based differences that are often thought to play key roles in demographic behaviour. Large-scale secondary datasets will only have the variables deemed of interest by previous researchers, regardless of whether these are the most relevant variables for any particular analysis (e.g. Shenk and others, 2013). Fourthly, large-scale secondary data sets leave researchers with many “degrees of freedom” (Stulp and others, 2016) that affect how they operationalize variables and conduct analyses and hence draw their conclusions (e.g. Silberzahn and Uhlmann, 2015). Pre-registering protocols may decrease unintentional researcher biases (Munafò and others, 2017), but caution must be exercised assiduously to maintain objectivity. Finally, cross-cultural comparative data analysis has produced exciting results that underscore both the general and context-specific nature of demographic behaviour (e.g. Borgerhoff Mulder and others, 2009; Hill and others, 2011), but poses special difficulties due to the differences in how data were collected or studies deployed across populations. None of these difficulties applies only in relation to work in EED, and all, in our view, are outweighed by the usefulness of inferences that can generally be drawn from appropriate analyses of rich datasets (see also Stulp and others, 2016).
Fertility
Fertility, survivorship and population growth rates together define an individual’s fitness. Thus, it is not surprising that fertility has been a key focus of ecological evolutionary demography at least since the 1980s (Sear and others, 2016). In that time, ecological evolutionary demographers have shed light on two key paradoxes: how humans sustain high fertility despite the high costs of childbearing, and why fertility has dropped in industrialized settings in association with the so-called “demographic transition”. In addition, the field has contributed theoretical and empirical advances for every component of fertility, from understanding the variation in age at first reproduction, to understanding the predictors of fertility, to predicting interbirth intervals and parity progression, to characterizing variation in age at last birth and explaining menopause. We focus here on some of the key contributions of ecological evolutionary demographers to illustrate the breadth and promise of the field.
The Paradox of “High” Fertility
Evolutionary scholars consider the species-typical fertility of humans to be paradoxically high.8 Despite the high costs of fertility to human females given extreme altriciality of human infants, human women have faster rates of reproduction than predicted based on non-human primate models (including those of the great apes), and the duration of breastfeeding for our highly dependent offspring is correspondingly short (e.g. Kramer, 2005; Sellen, 2001) (Figure 1). The highest population fertility on record belongs to the Hutterites, a North American Anabaptist sect, whose population reached a total fertility rate of eleven children (Eaton and Mayer, 1953) while the record fertility for an individual woman was set in the eighteenth century by a woman who reportedly gave birth to sixty-nine children (Glenday, 1988). Such figures are remarkable given how dependent human infants are — with brains three times larger than that of a chimpanzee (Navarrete and others, 2011), the energetic demands of human infants are superlative (Walker and others, 2008; Foley and Lee, 1991; Kuzawa and others, 2014). Early in the infant’s life, the vast majority of calories provided to feed these demands derives from breastmilk (Sellen, 2007), seriously constraining a woman’s ability to meet her own energetic demands alongside those of her young infant, not to mention other dependent offspring at older ages (Gurven and Walker, 2006).
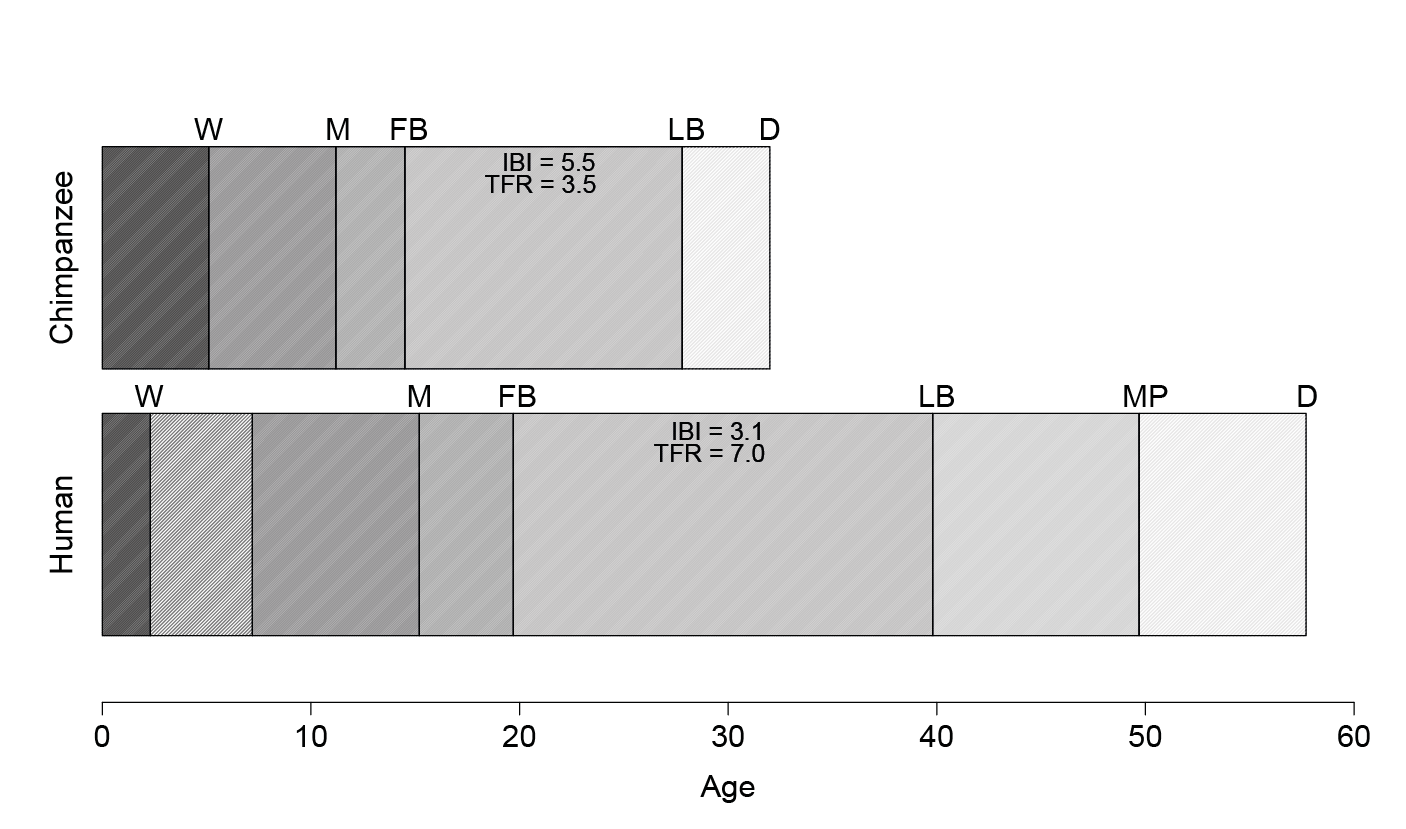
Figure 1. Life histories in chimpanzees (top) and humans (bottom). Human life histories are longer for virtually every distinct phase therein. However, human weaning occurs earlier than expected based on non-human primate models; inter-birth intervals are correspondingly short. W=weaning; M=1st menses; FB=1st birth; LB=last birth; D=death; IBI=inter-birth interval; TFR=total fertility rate. Adapted from Kramer, 2005.
The solution to this, of course, is that human mothers receive significant assistance from others (“allocaregivers”), who subsidize the high costs of child-rearing. Indeed, humans are often considered “cooperative breeders” (e.g. Kramer, 2005, 2010; Mace and Sear, 2005; Sear and Coall, 2011; Hrdy, 2005), which implies that assistance in child-rearing is a key feature of the human life history. Who provides the most such assistance is debated. The longest-held view is that men form pair bonds with women and, in exchange for female fidelity, take up a provisioning role for their mutual children (e.g. Lancaster and Lancaster, 1987; Kaplan and others, 2000; see Mattison, 2016). This view has been challenged by proponents of the “grandmother hypothesis” (Hawkes, 2004), which posits a larger role taken by maternal grandmothers in caring for dependent offspring, together with evidence suggesting that a variety of other caretakers, including siblings (Kramer, 2005; Turke,1988; Mattison and Neill, 2013), step in at different times and places (Sear and Mace, 2008). We follow Sear (2016a) in emphasizing that while the solution to this paradox involves a universal tendency to assist mothers, flexibility in humans allows specific caretakers to assist in different contexts.
The Paradox of ‘Low’ Fertility
Evolutionarily high fertility in humans gives rise to the second paradox addressed usefully by ecological evolutionary demography: the demographic transition, a global phenomenon in which high fertility and mortality rates declined to low levels beginning in late-eighteenth-century Europe followed eventually by much of the remaining world. While most scholars link demographic transitions to economic, social and technological changes associated with industrialization and economic development, the specific causal mechanisms most important in transitions remain the subject of debate. Vining (1986) famously argued that “the” demographic transition contradicted evolutionary explanations for fertility given that (a) individuals voluntarily limited their fertility significantly despite increasing access to resources, and (b) wealthy and high-status people often lowered their fertility to a greater degree than people with fewer resources. Since Vining’s paper was published, numerous human evolutionary demographers taking an ecological approach have tackled the question of why the demographic transition has occurred — especially why fertility has declined — and how fertility decline is consistent with evolutionary explanations.
In the broadest sense, evolutionary models of the demographic transition fall into three categories (Borgerhoff Mulder 1998): (i) some argue that the transition is optimal with respect to fitness; (ii) some that lower fertility is the consequence of Darwinian but non-genetic means of inheritance (e.g. cultural evolution); and finally, (iii) some argue that such behaviour is maladaptive.
Causal explanations in ecological evolutionary demography (i.e. (i) above) fall into several categories, which are not mutually exclusive (despite occasional claims to the contrary) — many of which align closely with approaches taken by non-evolutionary demographers (see Shenk and others, 2013 for review). Many researchers taking a life history approach have argued that reductions in rates of risk and mortality — particularly infant and child mortality — change levels of optimal fertility, motivating parents to have fewer children and invest more in each given the greater likelihood that their children will survive and reproduce (e.g. Chisholm and others, 1993; Leslie and Winterhalder, 2002; Quinlan, 2006). Other researchers examine the costs and benefits of investing in self and children. Specifically, when the costs of children are low (for instance when children’s agricultural labour helps to subsidize the costs of their upbringing), fertility should be higher than when the costs of children are high (for example, where land saturation tightly limits inheritance or in modern market economies where children are not economically productive but are costly to raise) (e.g. Kramer, 2005; Mace, 1998; Sear and Coall, 2011; Luttbeg and others, 2000). Kaplan (1996), following Becker (1993), has argued that fertility declines with increasing payoffs to investment in human capital (primarily education) in modern labour markets; these effects may be complemented by increases in adult life span and child survival rates, which also result in greater payoffs to investments in self and in children given the length of time over which benefits accrue (Cervellati and Sunde, 2005; Galor, 2012). The opportunity costs of raising children also increase in modern labour markets, especially for women (Low and others, 1992; Turke, 1989), who may reduce fertility to pursue career opportunities or otherwise delay reproduction to an age when infertility becomes more likely (Kaplan and others, 2000).
Cultural evolutionary theory (ii, above) focuses on the social processes that lead to fertility decline, arguing that humans have evolved learning biases that may lead to (or at least intensify) low fertility through emulation of high-status individuals with few children (e.g. Boyd and Richerson, 1995; Richerson and Boyd, 2005). Related models suggest that in modern societies, the decreasing density of pronatalist kin leads to increasing transmission of low-fertility norms (Newson and others, 2005). Cultural transmission models can be seen either as mechanisms of how fertility decline spreads or as causal models that posit why individuals adopt low fertility — in the former sense they are not “ecological” approaches, but in the latter sense they are.
Increased wealth does not imply a quality-quantity trade-off: Although a quality-quantity trade-off is one way to explain the demographic transition, wealth does not automatically give rise to such a trade-off. In other words, greater wealth (or maternal quality; see Emery Thompson and others, 2016; Ellison, 2003) should not, on its own, produce a fitness advantage through a reduction in childbearing. As encapsulated by Kaplan’s (1996) embodied capital theory, wealth, per se, is not what drives investments into child quality over quantity. Rather, socio-ecological contexts that provide sufficient benefits to skills acquisition or other investments in child quality are what set the stage for steeper quantity-quality trade-offs. Because the wealthy tend to inhabit contexts that reward investments in child quality (i.e. wealth and perceived returns to parental investment often covary (Mace, 2008; Lawson and Mace, 2011)), it often appears as though humans violate the more general expectation that wealth alleviates the quantity-quality trade-off (Low and others, 2002; Hopcroft, 2006). If so, looking at the relationship between wealth and fertility within groups experiencing the same strength of fertility trade-offs should unmask a positive association between wealth and fertility that is not apparent when one does not control for the socio-ecological context producing this trade-off (Mace, 2008) (Figure 2). Few studies have attempted such a multi-level approach, but Alvergne and Lummaa (2014) found evidence both for and against an ecological fallacy applied to wealth and fertility in Mongolia — on the one hand, once context (here, urban versus rural) was accounted for, wealth showed a positive relationship with lifetime reproductive success; on the other, women’s education traded off steeply with childbearing, suggesting that status acquisition could drive fertility to below-optimal levels (see also Shenk and others, 2016). Future work assessing fertility trade-offs must therefore be attentive to how the context establishes returns on investments in child quality and how individual attempts to secure status trade off with investments in posterity.

Figure 2. The ecological fallacy and the demographic transition. Data aggregated across contexts may obscure or reverse positive relationships between wealth and fertility that arise within wealth strata.
Timing of Fertility
The historical focus on overall fertility is complemented by a more recent focus on the timing of fertility, including the timing of age at first birth, interbirth intervals and, even more recently, the age at last birth. Timing of reproduction is increasingly recognized as an important contribution to fitness, particularly because fertility poorly predicts fitness in non-stationary populations (Jones and Bliege Bird, 2014). All else being equal, in growing or stationary populations, earlier reproduction is favoured (Voland, 1998): earlier-born offspring represent a greater marginal benefit to parental reproductive success than later-born offspring; and earlier reproduction shortens generation times, increasing fitness over many generations (Lewontin, 1965; Jones, 2011). At the same time, earlier reproduction reflects a key transition in a woman’s life history from investments in growth to investments in reproduction (Stearns, 1992; Allal and others, 2004). The timing of this shift is important to future reproductive success, as a woman draws from the reserves built up during the pre-reproductive period to support reproduction after growth has halted (e.g. Hill and Hurtado, 1996). Accordingly, reproduction that occurs too early is associated with poor consequences for mothers and children, including low birthweight (Koniak-Griffin and Turner-Pluta, 2001), whereas greater investments into growth are associated with better outcomes, such as reduced stillbirths and infant mortality (e.g. Sear and others, 2004). In general, organisms should benefit from earlier reproduction if there are no associated costs (see Brown and Sibly, 2006), but should delay reproduction when this improves future reproductive prospects.
Trade-offs in the timing of reproduction arise across the reproductive lifespan, affecting each bout of reproduction (see Sheppard and Coall, this volume). Thus, in addition to age at first birth, trade-offs have been invoked to explain the spacing of births (Blurton-Jones, 1986) and, more recently, the timing of age at last birth (Mattison et al., 2018; Towner, Nenko, and Walton, 2016). In general, longer interbirth intervals are interpreted to reflect increased parental investment in children (e.g, Blurton-Jones, 1986; Bereczkei and others, 2000) and as a means of protecting mothers from the physiological and energetic costs of overly rapid reproduction (e.g. Panter-Brick, 1991). Birth spacing is also a useful nexus for investigating parent-offspring conflict (Trivers, 1974) as children are wont to demand more investment from their parents than is optimal vis-à-vis parental fertility (McDade, 2001; Kushnick, 2009). As with timing of age at first birth, costs and benefits of early versus late reproduction vary according to individual circumstances, and increased availability of resources (e.g. energetic, temporal, financial) are anticipated to alleviate the costs of reproduction and to sustain faster rates of reproduction, all else being equal (Gurven and others, 2016). Unlike age at first birth, subsequent births may be less likely to reflect trade-offs in investments in self versus children, as major investments in self are theorized to occur prior to first reproduction (Stearns, 1992), and are more likely to reflect motivations to switch investment from one child to another. Similarly, earlier ages of last birth can be theorized to reflect shifts toward investments in child quality, as age at last birth is a primary means of reducing overall fertility, freeing parents to allocate resources to existing children (Towner, Nenko, and Walton, 2016; for a summary of theories on age at last birth, see Mattison et al., 2018).
In sum, fertility and the timing thereof are key drivers of fitness, affecting population growth and dynamics. Ecological evolutionary demography has provided theories addressing both why fertility is potentially so high in humans (due to our system of cooperative breeding), and why it may display, on aggregate, a negative relationship with wealth and economic development. In each case, the costs and benefits of reproduction must be weighed against competing costs and benefits of growth, maintenance, resource acquisition, and status maintenance and the likely effects of each on both current and future offspring. All else being equal, anything that acts to alleviate the costs of reproduction (e.g. presence of allocarers, wealth) can be expected to increase fertility, while anything that contributes to the costs of reproduction (e.g. physiological and energetic costs, high opportunity costs of children) can be expected to decrease it. More fundamentally, EED does not expect fertility to behave the same way in every context, but anticipates that “fertility schedules should respond to ecological conditions.” Indeed, although it is possible to describe a human pattern of fertility in relation to other species, it is probably more accurate to describe human fertility as exceptionally flexible, even under “natural fertility” contexts. Thus, a major impulse in evolutionary ecological demography has been to understand the ecological and individual predictors not only of number of children, but also the timing and cessation of childbearing, including strategies surrounding the timing of reproductive maturity, the timing of childbearing, and how these trade off with investments in oneself and in parenting other children.
Mortality
Mortality is relatively little studied by EED compared to other areas of evolutionary demography. This is despite evolutionary demography — and especially biodemography — making key early contributions to theories and descriptions of human mortality (see Wachter, 2008; Sear et al., 2016 for reviews of this literature). Perhaps because mortality is less readily observed (and more difficult to ask about) than fertility, EED with its emphasis on primary data collection in small-scale societies has engaged somewhat less with this core area of demography. Yet, mortality is central to understanding the evolution of human longevity (Hawkes, 2004; Kaplan and others, 2000) and more general patterns of life history (e.g. Charnov, 1991; Charnov and Berrigan, 1993; Ellis and others, 2009); thus, increasing research efforts in the evolutionary ecology of mortality would help to shed light on both general and site-specific causes and consequences of mortality (Burger and others, 2012). Here, we describe how EED has contributed to (1) understanding the basic pattern of human mortality, both in terms of contemporary variation and as it likely evolved over the last 200,000 years, (2) understanding how mortality reflects parental investment in children, and (3) describing how mortality can act as a predictor of variation in human life histories.
The Human Mortality Pattern
An early debate surrounding the human lifespan involved establishing a baseline, ancestral pattern of mortality. The Hobbesian view of a nasty, brutish and short human life had several proponents, including paleo-anthropologist Henri Vallois, who claimed that, among humans, “few individuals passed forty years, and it was only quite exceptionally that any passed fifty” (Vallois, 1961: 433; see also Weiss, 1981; Gurven and Kaplan, 2007). Indeed, evolutionary demographers previously believed that Paleolithic humans experienced life expectancies of only fifteen to twenty years (Cutler, 1975; Weiss, 1981). Such inferences were supported by prehistoric life tables built using osteological evidence recovered at sites such as the Libben site in Ohio (Lovejoy and others, 1977) and Indian Knoll in Kentucky (Herrmann and Konigsberg, 2002) where recovered remains revealed low infant mortality and high adult mortality. These mortality profiles were attributed to “immunological competence” acquired in childhood in small populations subjected to durable pathogenic environments (Lovejoy and others, 1977). Average life expectancies are also relatively short in chimpanzees under diverse ecological conditions (Hill and others, 2001; see also Muller and Wrangham, 2014; Emery Thompson and others, 2007; Wood and others, 2017), although it is reasonably common for individual chimpanzees to live beyond their reproductive years (Emery Thompson and others, 2007). Evidence from Neanderthals, the only other hominin to live contemporaneously with modern humans, lived for rather short durations on average (see Trinkaus, 1995), providing additional support for the idea that ancestral lifespans were significantly shorter in the human evolutionary past.
Yet life tables reconstructed based on data collected, sometimes prospectively, in diverse contemporary hunter-gatherer populations forced a revision of the foregoing view. Such data suggest that the mortality pattern that is characteristic of our species is well described by a Siler distribution (Gurven and Kaplan, 2007; Siler William, 1979; Gage, 1989; Wood and others, 2002) (Figure 3), in which mortality decreases sharply from infancy through childhood, remains more or less constant into middle age, and then rises steadily into old age in “Gompertz fashion” (Gurven and Kaplan, 2007: 322). Based on analysis of demographic data from foraging and foraging-horticulturalist communities, Gurven and Kaplan (2007) conclude that despite high mortality and significant variation across populations, a considerable fraction of humans would have lived to middle age and into post-reproductive periods even under the most stressed conditions. “For groups living without access to modern health care, public sanitation, immunizations, or adequate and predictable food supply, it seems that still at least one-fourth of the population is likely to live as grandparents for 15–20 years.” (p. 331) Indeed, Gurven and Kaplan (2007) helped to establish the slow rate of senescence in humans as a distinctive feature of human mortality profiles. Taken together, this evidence contributes to the EED view of longevity as a crucial evolved feature of the human life history (Gurven and Kaplan, 2007; Konigsberg and others, 2006), in which large-scale cooperation among individuals results in decreased mortality and frequent non-reproductive contributions to fitness (cf. Hamilton, 1966) that are focused instead on intra-familial transfers of resources and care (Lee, 2003; Kramer, 2010; Hawkes, 2004; Peccei, 2001; Kaplan and others, 2000: 200).

Figure 3. Characteristic mortality in humans and chimpanzees is described well by a Siler distribution and is similar in profile across these taxa, but humans have considerably lower mortality overall, and live for correspondingly longer.
Mortality Is a Proxy of Parental Investment
An obvious implication of the EED view of extended longevity in the context of cooperative breeding is that mortality is a reasonable proxy of inputs into child growth and development. Indeed, cooperative breeding is an enduring focus of work in human behavioural ecology, and probably the most common use of mortality data in EED is for testing hypotheses related to parental (and alloparental) investments in children. Parental investment theory (Trivers, 1972) stipulates that parents will invest in offspring to maximize parental reproductive success, and that such investments will be biased according to their children’s ability to convert a unit of parental investment into reproductive success. Thus, son-biased parental investment is thought to pay off when sons are better able to translate investments into reproduction (e.g. due to polygyny; see Sieff, 1990) and investments in a single heir to pay off when a more even distribution of resources leads to lineage failure (Hrdy and Judge, 1993; see also Johowa and others [n.d.]). Variation in mortality can serve as a marker of non-parental investment in children as well. Mattison and colleagues (Mattison et al., 2015; Mattison et al., 2018) explored differences in mortality in adopted versus biological children in colonial-era Taiwan as a test of kin-selection theory to see whether adopted daughters were neglected compared to biological daughters and therefore subjected to higher age-specific mortality (they weren’t). General tests of cooperative breeding hypotheses frequently use vital status to infer levels of allocare from different sources (Sear and Mace, 2008). Survivorship has further been used to evaluate quality-quantity trade-offs in human populations (see Lawson and others, 2012), again reflecting the assumption that increased investment into fewer children leads to higher rates of survivorship.
This also serves as a reminder that natural selection may often favour neglect of children. Such neglect can range from the extreme (e.g. infanticide, abandonment) to the subtle forms of neglect that most people with siblings will claim to have been subjected to during their childhoods (Hrdy, 1992, 2009). Indeed, while rarely beneficial to a given child, parental neglect may often be optimal for parents, especially in cases where children are insensitive to parental inputs (Caro and others, 2016).
Mortality Predicts Life History Variation
Mortality provides insights as both an outcome variable and as a predictor of demographic behaviour. Major sources of mortality for humans in the course of our evolutionary history and small-scale societies include malnutrition, infectious and parasitic diseases, and conflict with other humans (Gurven and Kaplan, 2007; Gurven and others, 2007; Hill and others, 2007). Yet, as described above, ecological evolutionary demographers have influentially argued that humans have achieved major reductions in mortality compared to apes via increased cooperation through food sharing and alloparenting/cooperative breeding (Kaplan and others, 2000; Kramer, 2010). This has implications for the timing of life history stages across the lifespan.
One influential hypothesis links variation in extrinsic mortality — sources of mortality that are relatively insensitive to adaptive decisions of organisms (Stearns, 1992:182) — to differences in the progression of life histories within and across populations. According to this argument, organisms in high-mortality environments discount the future and prioritize immediacy (Pepper and Nettle, 2013) to capitalize on fitness opportunities earlier in life because a high probability of death means that reproduction is likely to be curtailed (Daly and Wilson, 2005; Ellis et al., 2009; Charlesworth, 1994; Promislow and Harvey, 1990). Similarly, harsh environments generally favour offspring quantity over quality as a bet-hedging strategy to increase the probability that at least some will survive long enough to reproduce (see Einum and Fleming, 2004; Ellis and others, 2009 for a discussion of conservative versus diversified bet-hedging). By contrast, slower life histories are favoured in environments that are predictable, not harsh, but competitive (e.g., Kaplan, 1996), because the rewards of investing in growth and the accumulation of skills and resources are likely to pay off as the future appears more secure and as competition for resources and mates among conspecifics intensifies (Ellis and others, 2009). Cross-cultural evidence supports these general expectations — age at menarche and age at first birth occur approximately one year earlier for every 10% decline in child survivorship to age 15 (Walker and others, 2006; see also Wilson and Daly, 1997; Low and others, 2008) and small body size and early fertility peaks are observed in contexts with high mortality rates (Migliano and others, 2007).
An interesting corollary of hypotheses focused on extrinsic mortality are a group of “socialization” hypotheses that link the quality of parental investment and childhood environments to rates of development. The idea here is that the quality of parental investment serves as a mechanism by which children receive information about the levels of stress and support in local environments, including extrinsic mortality and morbidity (Belsky and others, 1991; Bereczkei and others, 2000; Chisholm and others, 1993; Ellis, 2004; Pepper and Nettle, 2017; reviewed in Ellis and others, 2009). Children reared in environments with low levels of parental investment are thought to cue in on these indicators during childhood to predict future environments and will adjust their life history strategies to accommodate harsh and/or unpredictable environments. Individuals reared in environments with cues of harshness and/or unpredictability — e.g. low socio-economic status, frequent residence or parental transitions — experience faster life histories, including earlier sexual debut, more sexual partners, and earlier age at first birth (see Ellis and others, 2009, for review, and Baldini, 2015, for a critique; see Pepper and Nettle, 2017, for a more recent review and theoretical treatment). This theory has major implications for understanding reproductive behaviour that is otherwise deemed “pathological” according to a public health perspective, and for the interventions employed to decrease the frequency of the early onset of reproduction (Draper and Harpending, 1988; Belsky and others,1991). For demography, it goes beyond standard demographic transition theory to link mortality to reproductive behaviour and attendant psychological mechanisms.
Future EED Work on Mortality
Several interesting questions remain to be addressed by an ecological evolutionary demographic perspective on mortality. Firstly, a question that continues to inspire significant interest in mainstream evolutionary demography involves whether there are limits to extensions of the lifespan (e.g. Oeppen and Vaupel, 2002; Tuljapurkar and others, 2000; Dong and others, 2016). Much of the answer to this question depends on the extent to which existing causes of mortality decline can be applied in forecasting future mortality decline. Burger et al. (2012) note that much of the exceptional decline in human mortality has arisen within only the last four generations and that the difference between contemporary mortality in industrialized populations and that of hunter-gatherers is much greater than the difference between hunter-gatherers and chimpanzees. This, in conjunction with significant contemporary variability in human mortality profiles between populations, may suggest that different factors are at work now compared to the factors operating to lower mortality in our evolutionary past. Indeed, whereas widespread sharing may have reduced deaths associated with famine and malnutrition, most deaths in contemporary hunter-gatherer populations are apparently due to infectious disease, especially post-contact, with additional mortality due to degenerative diseases and, in some groups, homicide. The contributions of modern healthcare and sanitation to declining mortality may extend the human lifespan much further than sharing (Burger and others, 2012); if such extensions facilitate ongoing downward inter-generational transfers, the implications for fitness are very different than if transfers to support longevity move in the other direction (Lee, 2013; Cyrus and Lee, 2013).
Secondly, an interesting question surrounds the extent to which fertility trades off with mortality and the types of evidence that may be used to evaluate such trade-offs. Studies exploring this issue are generally equivocal due to the difficulties associated with assessing the costs of reproduction (Gurven and others, 2016). An intriguing recent study provides evidence supporting such a trade-off in Utah where women’s lifespans were more strongly lengthened following demographic transition than were men’s, whose costs of reproduction were less affected (Bolund and others, 2016). More generally, if fertility is a determinant of mortality, then its effects must be controlled in analyses of mortality. If the costs of reproduction are easily borne by contemporary women (e.g. because their nutritional inputs are sufficient to sustain high fertility or because fertility is low in most modern contexts), then mortality may be relatively immune to the effects of fertility. More empirical data testing this association are needed. Finally, and much more generally, although evolutionary demography was best known early on for its work on mortality (Wachter, 2008; Sear and others, 2016), this area of scholarship has not kept pace with work on the evolutionary ecologies of fertility, which have dominated work in modern EED. Evolutionary ecological demography stands to contribute much to this core area of demographic study.
Migration
Migration is a fundamental driver of evolutionary and demographic change, and a key component of the balancing equation in demography. While the topic is extensively studied by mainstream demographers, it has more rarely been the focus of evolutionary analysis. Yet there has been important work in this area in ecological evolutionary demography. As discussed below, much work has modelled the decisions of adults to disperse from the natal community in terms of costs versus benefits of staying versus leaving. Other work focuses more closely on “post-marital residence”, i.e. the decisions made by couples over where to reside after establishing a reproductive union (Stone, 2014). Post-marital residence is highly variable in human societies (Mattison, 2019), from couples remaining in their natal communities (i.e. natalocality), to moving in with or close to the husband’s kin (virilocality), to moving in with or near to the wife’s kin (uxorilocality). Whether an individual disperses to a new area or stays in their natal community is relevant to key evolutionary questions of mating effort and parental investment, including access to and competition over mates and resources. Drawing from the perspectives of life history theory and the evolutionary study of territoriality, much research has examined the costs and benefits of remaining versus dispersing in different contexts with the goals of understanding when the balance is tipped in one direction or the other and how such decisions affect downstream health and demographic behaviour.
Ecological evolutionary demography provides models of dispersal decisions that unify many disparate costs and benefits (Emlen, 1995; Koenig and others, 1992). Fitness costs of dispersal range from energy, time and risk (of injury, disease, hunger, hostile people or dangerous animals in novel territories) to loss of access to nearby kin (Wood and Marlowe, 2011; Hill and others, 2011). Benefits to dispersal include the fitness benefits associated with control of new territories and associated resources (Hamilton and May, 1977) and mating opportunities (Clarke, 1993), and the reduction of inbreeding risk (Moore, 1993). Finally, scholars have recognized distinct benefits of remaining in the natal territory, including benefits derived from knowledge of local resources and risks as well as increased potential for kin investment and transmission of social information from known community members. As described below, the relative costs and benefits of staying versus leaving are predicted to differ systematically for males versus females, by age and by birth order. In humans, institutions that ratify inheritance can further constrain dispersal decisions (e.g. Clarke, 1993; Clarke and Low, 1992; Koenig, 1989; Strassman and Clarke, 1998; Towner, 2001, 2002). Access to resources has played a correspondingly large role in shaping human dispersal patterns.
Sex-Biased Dispersal
Ecological evolutionary demography has provided key insights into the role that subsistence plays in driving patterns of sex-biased dispersal. Whereas much of mainstream demography views sex-biased dispersal patterns as products of cultural institutions regulating marriage, EED pushes the causal arrow back to viewing these institutions as products of natural selection (Sear, Mattison, and Shenk 2024). For example, different ecologies are predicted to favour male versus female (or relatively egalitarian) control of resources, which, in turn, drives male versus female kin to reside together (e.g. Jordan and Mace, 2007; c.f. Alesina and others, 2013). In general, ecologies with economically defensible resources favour territoriality and group defence (Dyson‐Hudson and Smith, 1978; Cashdan and others, 1983; Mattison and others, 2016b). When the resource base becomes productive enough that male reproductive success is more significantly enhanced by resources than is female reproductive success, kinship systems become more male-oriented and virilocality can ensue. Thus, many human subsistence systems, especially those emphasizing the inheritance of land in intensive agricultural systems (e.g. Goody, 1976; Shenk and others, 2010), are characterized by resource defence (Alvard, 2003) and show patterns of either female dispersal (i.e. virilocality) or (typically male) unigeniture (e.g. Boone, 1986; Goody, 1976; Hrdy and Judge, 1993; Murdock, 1967). On the other hand, subsistence systems characterized by horticulture, expansive recourse bases or male absence (e.g. due to fishing) are often uxori- or nata-local, with female kin organizing subsistence efforts (e.g. Mattison, 2011; BenYishay and others, 2017; Alesina and others, 2013; Holden and Mace, 2003). Finally, hunting and gathering are often associated with flexibility in dispersal, with spouses moving between locations strategically over the life course in ways that maximize cooperation among kin as opposed to resource defence (e.g. Wood and Marlowe, 2011; Kramer and Greaves, 2011).
Ecological Constraints on Dispersal
Human dispersal decisions are contingent not just on the subsistence system and related inheritance practices, but also on individual resource-related conditions such as the wealth and status of both self and parents (Goody, 1976; Low and Clarke, 1991; Mace, 1996; Voland and Dunbar, 1995). Such considerations are formalized by the ecological constraints model of delayed dispersal (Emlen, 1995; Koenig and others, 1992; Strassman and Clarke, 1998), which suggests that when offspring have access to cooperative breeding opportunities or improved territories at home, they may delay dispersal either because (a) they will achieve greater fitness benefits (at least temporarily) from serving as helpers at the nest (i.e. by helping to improve parental fitness (Turke, 1988)) in a good breeding territory or agricultural estate, and/or (b) with the hope of inheriting the breeding territory or agricultural estate from their parents. Emlen (1995) has argued that many aspects of the organization of the family across species rest on the principles of inclusive fitness theory, ecological constraints theory and reproductive skew theory acting in concert, with the benefits of cooperation with relatives trading off with competition for resources and reproduction, in explaining the composition and longevity of family groups as well as the age and sex characteristics of dispersers. More specifically, ecological constraints on the resources needed for reproduction (e.g. Koenig and Mumme, 1987), in combination with the benefits of staying in the natal territory under such conditions (e.g., Stacey and Ligon, 1991), have been argued to lead both to reproductive delay and, as a consequence of such delay, to the formation of extended family units (Emlen, 1994, 1995).
Fundamental to the ecological constraints model is the lack of superior alternatives away from the natal household. Interestingly, if the opportunity costs of leaving are low, then many of the factors that are associated with delayed dispersal in the context of ecological constraints lead to dispersal in the absence of such constraints. For example, non-heirs — especially those born at higher birth orders, in larger families or in areas with harsher ecological conditions — are more likely to benefit from dispersal than children with lower birth orders, who reside within smaller families or who are formally appointed as heirs (Boone, 1986; Clarke and Low, 1992; Hrdy and Judge, 1993; Voland and Dunbar, 1995). The likelihood of dispersal among humans peaks in the late teens and twenties (Clarke and Low, 1992; Castro and Rogers, 1984) across cultures. Childless and unmarried people — the same categories of individuals predicted to have the lowest opportunity costs of caring for siblings (e.g., Kramer, 2005) — are more likely to disperse than married individuals or individuals who have children, because these categories of individual will benefit more from additional opportunities to secure mates or the resources necessary to start a family (Glover and Towner, 2009; Strassman and Clarke, 1998; Towner, 2002, 1999).
Push and Pull Factors
The ecological evolutionary perspective focusing on the costs and benefits of dispersal (and as a corollary the costs and benefits of natal philopatry) parallels the discussion of push and pull factors in the study of migration among demographers. Push factors are the reasons that motivate people to leave one community (e.g. poor job prospects, land saturation, high mortality rates) and pull factors are the reasons that motivate people to move to a new community (e.g. good job prospects, access to land and better health care) (e.g. Schoorl and others, 2000; Massey and others, 1994; Jedwab and others, 2015). Most of the work on dispersal in EED focuses on these motivations, providing a link to the literature in mainstream demography. Yet there has been less attention among human evolutionary demographers to recent and ongoing patterns of rural to urban migration and international migration from the developing world — a central focus of migration scholarship in mainstream demography (see Mace, 2008). This will inevitably affect demographic studies of small-scale populations (Neill, 2007; Mattison and Sear, 2016), however, and theoretical links between urbanization, risk, fertility and parental investment (Hrdy, 1992; Mace, 2008) suggest a productive nexus for theoretical and empirical work in ecological evolutionary demography. Gillian Bentley and colleagues, for example, have examined the impact of growing up in Bangladesh vs. in the UK on reproductive function among women of Bangladeshi origin through the lens of life history theory, arguing for a critical period of environmental sensitivity during childhood. They found that growing up in the more stressful environment of Bangladesh (in terms of nutritional stress and exposure to infectious disease) was associated with lower allocations to reproductive effort in terms of progesterone levels (Mora and others, 2007) and ovarian reserves (Begum and others, 2016), but not in terms of levels of estradiol (Núñez‐De La Mora and others, 2008) or age at menopause (Murphy and others, 2013). Such insights are important when considering how to extend the demography of small-scale societies within the contexts of migration, where characteristics of sending populations may suggest different interventions into health and well-being for migrants than those employed as standard in receiving populations.
Additional topics that are of central importance to understanding ecologies of migration, if somewhat peripheral to ecological evolutionary demography, include the genetic signatures of migration, which have been used to map the journey out of Africa onto humans’ contemporary global distribution. This work has taken many dimensions, including tracing the timing and route of the migrations through archaeological and genetic markers (e.g. the many articles in Crawford & Campbell, 2012 and Cavalli-Sforza et al., 1994), with some arguing that there has been selection among humans for alleles that favour expansion or migratory behaviour under conditions of resource surplus (e.g. Harpending and Cochran, 2002). While many mainstream demographers do not share the interest of anthropologists in the ancient human past, understanding these patterns creates a baseline for understanding policy-relevant types of human migration in the contemporary world and recent past. Another exciting perspective that will add to our broader understanding of human migration derives from cultural evolutionary theory, which has been particularly interested in the effects of migration on social learning processes. For example, unlike its effects on population structure, migration need not erode between-group cultural variation as acculturation to local norms and customs can serve to maintain barriers (Mesoudi, 2017). Similarly, social assortment prevents acculturation and has interesting implications for the maintenance versus erosion of cooperation within groups (Mesoudi, 2017; Boyd and Richerson, 2009) and the likelihood of large-scale demographic events such as warfare (Divale, 1974; Macfarlan and others, 2018; Mathew and Boyd, 2011; Richerson and Boyd, 1998). This work has direct relevance to mainstream demographers interested in diffusion models of behaviour, and also to the patterns and pace of the demographic and social assimilation of immigrants into host populations.
In sum, ecological evolutionary demography has much to contribute to understanding migration decisions. Although historically focused on specific decisions surrounding marriage and family-building, the ultimate rationale provided by evolutionary theory is poised to provide a unifying model of the push and pull factors that have elsewhere been described to affect migration decisions in other contexts (e.g. labour migration). Because migration affects access to resources and social support, it has important consequences for the key drivers of human decision-making, affecting all realms of interest for human behavioural ecologists (Winterhalder and Smith, 2000).
Concluding Thoughts: Key Insights, Limitations, and New Directions
In the quarter century since Low, Clarke & Lockridge (1992) published their article defining the field of ecological evolutionary demography, we have learned much about how individual-level constraints and differences in socio-ecologies affect fertility, mortality and migration. Key topics addressed by this work include resolving both why humans have, as a species, higher fertility than expected based on our long life histories, and why fertility has dropped in association with the demographic transition. The field has also described the basic pattern of human mortality and the reasons our mortality is so low, as well as its interlinkages with other core topics within demography (e.g. fertility and the lifespan). Ecological evolutionary demography has engaged somewhat less with migration studies, but ecological constraints theory and optimization approaches are poised to unify the disparate factors known to affect the decisions of whether, when and where to migrate. In our review of this material, we have touched on several limitations or fringe topics that we believe will be important to revisit as the field continues to grow and strengthen. We draw attention to those here and offer additional suggestions that aim to further integrate ecological evolutionary demography with other core areas of the wider field of evolutionary demography.
A fruitful pathway for integrating the various subfields of demography, including evolutionary demography, is to begin to bridge more systematically the proximate-ultimate division that characterizes much of the current scholarship. An emerging area with good potential to do this involves the study of psychological mechanisms underlying fertility decisions (McAllister and others, 2016; Pepper and others, 2016; McAllister and others [n.d.]). For example, desired family size, including both what happens when you surpass your desired fertility (Mcallister and others, 2012), as well as the “unmet need” or unfulfilled desire for children in post-demographic transition contexts (Testa, 2007), when mothers don’t have as many children as their stated fertility desires (Kaplan and others, 2003), are usefully studied from an evolutionary perspective and address key questions in the mainstream demography of fertility. Likewise, much of the work in cultural evolution of fertility describes the uptake of contraception through social networks and in relation to individual circumstances (Colleran and Mace, 2015; Alvergne and others, 2011; Colleran, 2016). Although there is significant debate about whether cultural evolutionary theory is better described as proximate or ultimate (e.g. Laland and others, 2013; Bateson and Laland, 2013), this may actually position it quite well for linking these two perspectives in relation to the mechanisms driving fertility decisions, as well as the adaptive value and long-run dynamics of demographic behaviour.
Various intersections between core areas of demography provide additional scope for extensions of traditional realms of inquiry into more complex understandings of human demographic behaviour. As alluded to above, the feedbacks between fertility and mortality create one nexus that will shed light on demographic behaviour in the past, present, and future. For example, the Neolithic transition, which was accompanied by global shifts toward agricultural and sedentary lifestyles some ten to twelve thousand years ago (Bentley and others, 2009; Bocquet-Appel and Bar-Yosef, 2008; BocquetAppel and others, 2006) is often considered paradoxical, in that increased fertility and mortality were simultaneously thought to have accompanied this transition. Recent scholarship testing key premises of this transition in contemporary small-scale populations transitioning to sedentism have revealed how sedentism can in fact produce the hypothesized effect, with overall increases in fertility despite increased mortality (Page and others, 2016). In particular, cooperative breeding has been key to sustaining high fertility despite increased infectious disease accompanying sedentary lifeways.
There is also interest in the interaction of fertility and mortality, both in our evolutionary past and in the modern world where the average life expectancy for humans has increased “linearly at almost three months per year over the past 160 years” (Gurven and Kaplan, 2007: 321) and women now live almost a third of their lives in a post-reproductive phase. Some findings have shown clear trade-offs between high fertility and mortality, a phenomenon known as maternal depletion, with high fertility being associated with higher mortality in some studies (Gagnon and others, 2009) but not in others, and with reviews of the evidence showing complex results consistent with maternal depletion in some settings, including modern settings (e.g. Hurt and others, 2006; Le Bourg, 2007). More recent work in contemporary high fertility populations suggests, however, that women may often be buffered against trade-offs between health and high fertility, even in high mortality settings (Gurven and others, 2016). An intriguing recent hypothesis suggests that low fertility is to blame for the uptick in female morbidity (especially auto-immune conditions) in many contemporary settings (Natri and others, 2019). Much remains to untangle about the relationship between ecology, fertility and longevity in this complex relationship.
Expanding methodologies provides further scope for integration across the subfields of demography. One means by which this is already occurring is via the use of new methods that provide information on proxies of health and demographic behaviour. Central among these are biomarkers that provide information on endocrine and immune function (e.g. McDade and others, 2007; Worthman and Costello, 2009; Valeggia, 2007). The adoption of the use of mobile phones and other devices such as motes (wireless sensing devices) in data collection facilitate tracking of complex social networks (e.g. Page and others, 2017), migratory patterns and other microdemographic data (e.g. disease transmission (Marcel Salathé and others, 2010) that can be challenging to collect via observation or survey. The use of such methods connects ecological evolutionary demographers with practitioners of applied health and demography, showcasing and calling for more work in applied evolutionary demography (Gibson and Lawson, 2014, 2015) and for demographically relevant work in evolutionary medicine and public health (Nesse and Stearns, 2008; Wells and others, 2017). Work in this area has included an explicit focus on population change (Gibson, 2014), family structure and health (Lawson and Uggla, 2014), social disparities in health (Pepper and Nettle, 2017) and nutritional transition (Wells, 2014). A parallel focus on gender and female autonomy has also provided counterintuitive reasons for undesirable social behaviour, including domestic violence (Jones and Ferguson, 2009; Stieglitz and others, 2018), crime and social violence (Schacht and others, 2014; Schacht and Kramer, 2016), dowry harassment (Shenk. 2007), biased sex ratios (Shenk and others, 2014), sex-biased parental investment (Mattison and others 2016a) and the effects of adoption on mortality and investment in children (Mattison et al., 2015; Mattison et al., 2018; Perry, Daly, and Macfarlan, 2014; Prall and Scelza, 2017), and even female genital cutting (Howard and Gibson, 2017). Such insights suggest different targets for intervention by focusing on the evolutionary benefits of socially undesirable behaviours (see also Hill, 1993). Many policy-relevant ideas brought forward by ecological evolutionary demographers simply would not be identified without an evolutionary perspective; such ideas are especially crucial in areas of policy where problems persist, and new thinking is sorely needed. For example, Gibson and colleagues’ work on how the installation of water taps affected women in a low-resource setting was informed by life history theory, which highlights how health and fertility are connected. They found an increase in fertility after the installation of this labour-saving technology, which would not have been predicted under standard public health models (Gibson 2014). Equally, EED should consider topics of core interest to mainstream demography, such as the end points of fertility transition and how best to support ageing populations. More generally, these methods and applied topics should open more integrated research, with the potential to reconnect work in ecological evolutionary demography with mainstream demography, as both increasingly emphasize health and improved forms of data collection and population monitoring.
Lastly, even a relatively lengthy overview of ecological evolutionary demography necessarily omits interesting work in areas that don’t quite fall within the core of the field. Given the breadth of work in life history theory and parental investment, ecological evolutionary demography provides theory for understanding patterns in many related areas, including the upstream regulators of fertility and spacing behaviour, such as marriage (e.g. Chagnon and others, 2017; Marlowe, 2000), conflicts of interest between the sexes (Leonetti and others, 2007; Moya and others, 2016) and downstream consequences of behaviour, such as social (Mattison and others, 2016b) and health inequality (Pepper and Nettle, 2014). While we have not dedicated the same attention to all of these and many more interesting areas of research, we hope that this review has demonstrated the importance of the ecological perspective to evolutionary demography and, conversely, the usefulness of demographic methods and practice to the ecological perspective. Integration of related methods and theory lies at the heart of the initial founding of the discipline of ecological evolutionary demography (Low et al., 1992). We reiterate here that such integration is critical for recognizing the causes and consequences of well-established demographic patterns, and for identifying new patterns and departures from established theories that may be in need of refinement. In other words, ecological evolutionary demography necessarily comprises threads of diverse disciplines. The task for future work is to interweave these for a fuller and more robust science of demography.
Acknowledgements
Thanks to the editors for inviting us to submit this chapter to this important volume and to two reviewers for comments and criticisms. Adam Reynolds coded the figures. The National Science Foundation provided writing support to Siobhán Cully during her rotation as a program officer in the cultural anthropology program.
References9
Alesina, Alberto, Paola Giuliano, and Nathan Nunn. 2013. ‘On the Origins of Gender Roles: Women and the Plough’, The Quarterly Journal of Economics, 128.2: pp. 469–530. https://doi.org/10.1093/qje/qjt005
Allal, N., R. Sear, A. M. Prentice, and R. Mace. 2004. ‘An Evolutionary Model of Stature, Age at First Birth and Reproductive Success in Gambian Women’, Proceedings of the Royal Society of London B: Biological Sciences, 271.1538: pp. 465–70. https://doi.org/10.1098/rspb.2003.2623
Alvard, Michael. 2003. ‘Kinship, Lineage Idenity, and an Evolutionary Perspective on the Structure of Cooperative Big Game Hunting Groups in Indonesia’, Human Nature, 142: pp. 129–63. https://doi.org/10.1007/s12110-003-1001-5
Alvergne, Alexandra, Mhairi A. Gibson, Eshetu Gurmu, and Ruth Mace. 2011. ‘Social Transmission and the Spread of Modern Contraception in Rural Ethiopia’, PLoS ONE, 6.7: e22515. https://doi.org/10.1371/journal.pone.0022515
Alvergne, Alexandra, and Virpi Lummaa. 2014. ‘Ecological Variation in Wealth–Fertility Relationships in Mongolia: The “Central Theoretical Problem of Sociobiology” Not a Problem after All?’, Proceedings of the Royal Society of London B: Biological Sciences, 281.1796: pp. 20141733. https://doi.org/10.1098/rspb.2014.1733
Baldini, Ryan. 2015. ‘Harsh Environments and “Fast” Human Life Histories: What Does the Theory Say?’, BioRxiv: pp. 014647. https://doi.org/10.1101/014647
Bateson, Patrick, and Kevin N. Laland. 2013. ‘Tinbergen’s Four Questions: An Appreciation and an Update’, Trends in Ecology & Evolution, 28.12: pp. 712–18. https://doi.org/10.1016/j.tree.2013.09.013
Becker, Gary S. 1993. Human Capital: A Theoretical and Empirical Analysis, with Special Reference to Education, 3rd Edition (Chicago: University of Chicago Press).
Begum, Khurshida, Shanthi Muttukrishna, Lynnette Leidy Sievert, Taniya Sharmeen, Lorna Murphy et al. 2016. ‘Ethnicity or Environment: Effects of Migration on Ovarian Reserve among Bangladeshi Women in the United Kingdom’, Fertility and Sterility, 105.3: pp. 744–54. https://doi.org/10.1016/j.fertnstert.2015.11.024
Belsky, J., L. Steinberg, and P. Draper. 1991. ‘Childhood Experience, Interpersonal Development, and Reproductive Strategy: An Evolutionary Theory of Socialization’, Child Development, 62.4: pp. 647–70. https://doi.org/10.2307/1131166
Bentley, R. Alexander, Robert H. Layton, and Jamshid Tehrani. 2009. ‘Kinship, Marriage, and the Genetics of Past Human Dispersals’, Human Biology, 81.3: pp. 159–79. https://doi.org/10.1353/hub.2009.a362933
BenYishay, Ariel, Pauline Grosjean, and Joe Vecci. 2017. ‘The Fish Is the Friend of Matriliny: Reef Density and Matrilineal Inheritance’, Journal of Development Economics, 127: pp. 234–49. https://doi.org/10.1016/j.jdeveco.2017.03.005
Bereczkei, T., A. Hofer, and Z. Ivan. 2000. ‘Low Birth Weight, Maternal Birth-Spacing Decisions, and Future Reproduction’, Human Nature, 11.2: pp. 183–205. https://doi.org/10.1007/s12110-000-1018-y
Blurton-Jones, Nicholas. 1986. ‘Bushman Birth Spacing: A Test for Optimal Interbirth Intervals’, Ethology and Sociobiology, 7.2: pp. 91–105. https://doi.org/10.1016/0162-3095(86)90002-6
—. 2016. Demography and Evolutionary Ecology of Hadza Hunter-Gatherers (Cambridge: Cambridge University Press).
Bocquet-Appel, Jean-Pierre, and Ofer Bar-Yosef. 2008. The Neolithic Demographic Transition and Its Consequences (New York: Springer Science & Business Media).
Bocquet-Appel, Jean-Pierre, Stephan Naji, George J. Armelagos, Kenneth C. Maes, Andrew T. Chamberlain et al. 2006. ‘Testing the Hypothesis of a Worldwide Neolithic Demographic Transition: Corroboration from American Cemeteries’, Current Anthropology, 47.2: pp. 341–65. https://doi.org/10.1086/498948
Bogin, Barry. 2009. ‘Childhood, adolescence, and longevity: A multilevel model of the evolution of reserve capacity in human life history’, American Journal of Human Biology, 21.4: pp. 567–77. https://doi.org/10.1002/ajhb.20895
Bolund, Elisabeth, Virpi Lummaa, Ken R. Smith, Heidi A. Hanson, and Alexei A. Maklakov. 2016. ‘Reduced Costs of Reproduction in Females Mediate a Shift from a Male-Biased to a Female-Biased Lifespan in Humans’, Scientific Reports, 6.1: pp. 24672. https://doi.org/10.1038/srep24672
Bongaarts, John. 1975. ‘Why High Birth Rates Are So Low’, Population and Development Review, 1.2: pp. 289–96. https://doi.org/10.2307/1972225
Boone, James L. 1986. ‘Parental Investment and Elite Family Structure in Preindustrial States: A Case Study of Late Medieval-Early Modern Portugese Genealogies’, American Anthropologist, 88.4: pp. 859–78. https://doi.org/10.1525/aa.1986.88.4.02a00050
—. 1988. ‘Parental Investment, Social Subordination and Population Processes among the 15th and 16th Century Portuguese Nobility’, in Human Reproductive Behaviour: A Darwinian Perspective, ed. by Laura L. Betzig, Monique Borgerhoff Mulder, and Paul W. Turke (Cambridge, UK: Cambridge University Press), pp. 201–19.
Borgerhoff Mulder, M. 1998. ‘The Demographic Transition: Are We Any Closer to an Evolutionary Explanation?’, Trends in Ecology & Evolution, 13.7: pp. 266–70. https://doi.org/10.1016/s0169-5347(98)01357-3
Borgerhoff Mulder, M., Samuel Bowles, T. Hertz, A. Bell, J. Beise et al. 2009. ‘Intergenerational Wealth Transmission and the Dynamics of Inequality in Small-Scale Societies’, Science, 326.5953: pp. 682–88. https://doi.org/10.1126/science.1178336
Boyd, Robert, and Peter J Richerson. 1995. ‘Why Does Culture Increase Human Adaptability?’, Ethology and Sociobiology, 16.2: pp. 125–43. https://doi.org/10.1016/0162-3095(94)00073-g
—. 2009. ‘Culture and the Evolution of Human Cooperation’, Philosophical Transactions of the Royal Society B: Biological Sciences, 364.1533: pp. 3281–88. https://doi.org/10.1098/rstb.2009.0134
Brown, James H., and Richard M. Sibly. 2006. ‘Life-History Evolution under a Production Constraint’, Proceedings of the National Academy of Sciences, 103.47: pp. 17595–99. https://doi.org/10.1073/pnas.0608522103
Bulatao, Rodolfo A. 1985. Reducing Fertility in Developing Countries: A Review of Determinants and Policy Levers (Washington, DC: World Bank).
Burger, Oskar, Annette Baudisch, and James W. Vaupel. 2012. ‘Human Mortality Improvement in Evolutionary Context’, Proceedings of the National Academy of Sciences, 109.44: pp. 18210–14. https://doi.org/10.1073/pnas.1215627109
Carey, J. R. 2003. Longevity: The Biology and Demography of Life Span (Princeton: Princeton University Press).
Caro, Shana M., Ashleigh S. Griffin, Camilla A. Hinde, and Stuart A. West. 2016. ‘Unpredictable Environments Lead to the Evolution of Parental Neglect in Birds’, Nature Communications, 7.1: pp. 10985. https://doi.org/10.1038/ncomms10985
Cashdan, Elizabeth, Alan Barnard, M. C. Bicchieri, Charles A. Bishop, Valda Blundell et al. 1983. ‘Territoriality among Human Foragers: Ecological Models and an Application to Four Bushman Groups [and Comments and Reply]’, Current Anthropology, 24.1: pp. 47–66. https://doi.org/10.1086/202934
Castro, Luis J., and Andrei Rogers. 1984. ‘What the Age Composition of Migrants Can Tell Us’, Population Bulletin of the United Nations 1983, 15: pp. 63–79. https://core.ac.uk/download/pdf/33893879.pdf
Cavalli-Sforza, Luigi Luca, Paolo Menozzi, Luca Cavalli-Sforza, Alberto Piazza, and Luigi Cavalli-Sforza. 1994. The History and Geography of Human Genes (Princeton: Princeton University Press).
Cervellati, Matteo, and Uwe Sunde. 2005. ‘Human Capital Formation, Life Expectancy, and the Process of Development’, American Economic Review, 95.5: pp. 1653–72. https://doi.org/10.1257/000282805775014380
Chagnon, Napoleon A., Robert F. Lynch, Mary K. Shenk, Raymond Hames, and Mark V. Flinn. 2017. ‘Cross-Cousin Marriage among the Yanomamö Shows Evidence of Parent–Offspring Conflict and Mate Competition between Brothers’, Proceedings of the National Academy of Sciences, 114.13: E2590–2607. https://doi.org/10.1073/pnas.1618655114
Charlesworth, Brian. 1994. Evolution in Age-Structured Populations (Cambridge: Cambridge University Press), ii.
Charnov, E. L. 1991. ‘Evolution of Life History Variation among Female Mammals’, Proceedings of the National Academy of Sciences, 88.4: pp. 1134–37. https://doi.org/10.1073/pnas.88.4.1134
Charnov, E. L., and Berrigan. D. 1993. ‘Why Do Female Primates Have Such Long Lifespans and so Few Babies? Or Life in the Slow Lane’, Evolutionary Anthropology: Issues, News, and Reviews, 1.6: pp. 191–94. https://doi.org/10.1002/evan.1360010604
Charnov, Eric L. 1993. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (New York: Oxford University Press).
Chisholm, James S., Peter T. Ellison, Jeremy Evans, P. C. Lee, Leslie Sue Lieberman et al. 1993. ‘Death, Hope, and Sex: Life-History Theory and the Development of Reproductive Strategies [and Comments and Reply]’, Current Anthropology, 34.1: pp. 1–24. https://doi.org/10.1086/204131
Clarke, A. L., and B. S. Low. 2001. ‘Testing Evolutionary Hypotheses with Demographic Data’, Population and Development Review, 27.4: pp. 633–60. https://doi.org/10.1111/j.1728-4457.2001.00633.x
Clarke, Alice L. 1993. ‘Women, Resources, and Dispersal in Nineteenth-Century Sweden’, Human Nature, 4.2: pp. 109–35. https://doi.org/10.1007/bf02734113
Clarke, Alice L., and Bobbi S. Low. 1992. ‘Ecological Correlates of Human Dispersal in 19th Century Sweden’, Animal Behaviour, 44.4: pp. 677–93. https://doi.org/10.1016/s0003-3472(05)80295-7
Colchero, Fernando, Roland Rau, Owen R. Jones, Julia A. Barthold, Dalia A. Conde et al. 2016. ‘The Emergence of Longevous Populations’, Proceedings of the National Academy of Sciences, 113.48: E7681–90. https://doi.org/10.1073/pnas.1612191113
Colleran, Heidi. 2016. ‘The Cultural Evolution of Fertility Decline’, Phil. Trans. R. Soc. B, 371.1692: pp. 20150152. https://doi.org/10.1098/rstb.2015.0152
Colleran, Heidi, and Ruth Mace. 2015. ‘Social Network- and Community-Level Influences on Contraceptive Use: Evidence from Rural Poland’, Proc. R. Soc. B, 282.1807: pp. 20150398. https://doi.org/10.1098/rspb.2015.0398
Coulson, Tim, hripad Tuljapurkar, and Dylan Z. Childs. 2010. ‘Using Evolutionary Demography to Link Life History Theory, Quantitative Genetics and Population Ecology’, Journal of Animal Ecology, 79.6: pp. 1226–40. https://doi.org/10.1111/j.1365-2656.2010.01734.x
Crawford, Michael H., and Benjamin C. Campbell. 2012. Causes and Consequences of Human Migration: An Evolutionary Perspective (Cambridge: Cambridge University Press).
Creanza, Nicole, Oren Kolodny, and Marcus W. Feldman. 2017. ‘Cultural Evolutionary Theory: How Culture Evolves and Why It Matters’, Proceedings of the National Academy of Sciences, 114.30: pp. 7782–89. https://doi.org/10.1073/pnas.1620732114
Cutler, R. G. 1975. ‘Evolution of Human Longevity and the Genetic Complexity Governing Aging Rate’, Proceedings of the National Academy of Sciences, 72.11: pp. 4664–68. https://doi.org/10.1073/pnas.72.11.4664
Cyrus, Chu C. Y., and Ronald D. Lee. 2013. ‘On the Evolution of Intergenerational Division of Labor, Menopause and Transfers among Adults and Offspring’, Journal of Theoretical Biology, 332: pp. 171–80. https://doi.org/10.1016/j.jtbi.2013.04.031
Daly, Martin, and Margo Wilson. 2005. ‘Carpe Diem: Adaptation and Devaluing the Future’, The Quarterly Review of Biology, 80.1: pp. 55–60. https://doi.org/10.1086/431025
Das Gupta, Monica, Zhenghua Jiang, Bohua Li, Zhenming Xie, Woojin Chung et al. 2003. ‘Why Is Son Preference so Persistent in East and South Asia? A Cross-Country Study of China, India and the Republic of Korea’, Journal of Development Studies, 40.2: pp. 153–87. https://doi.org/10.1080/00220380412331293807
Divale, William Tulio. 1974. ‘Migration, External Warfare and Matrilocal Residence’, Behavior Science Research, 9.2: pp. 75–133. https://doi.org/10.1177/106939717400900201
Dong, Xiao, Brandon Milholland, and Jan Vijg. 2016. ‘Evidence for a Limit to Human Lifespan’, Nature, 538.7624: pp. 257–59. https://doi.org/10.1038/nature19793
Draper, Pat, and Henry Harpending. 1988. ‘A Sociobiological Perspective on the Development of Human Reproductive Strategies’, in Sociobiological Perspectives on Human Development, ed. by K. B. MacDonald (New York: Springer-Verlag), pp. 340–72.
Dyson Hudson, R., and Eric Alden Smith. 1978. ‘Human Territoriality: An Ecological Reassessment’, American Anthropologist, 80.1: pp. 21–41. https://doi.org/10.1525/aa.1978.80.1.02a00020
Eaton, Joseph W., and Albert J. Mayer. 1953. ‘The Social Biology of Very High Fertility Among the Hutterites. The Demography of a Unique Population’, Human Biology; Baltimore, 25.3: pp. 206–64. https://pubmed.ncbi.nlm.nih.gov/13117490/
Einum, Sigurd, and Ian A. Fleming. 2004. ‘Environmental Unpredictability and Offspring Size: Conservative versus Diversified Bet-Hedging’, Evolutionary Ecology Research, 6.3: pp. 443–55. https://www.researchgate.net/publication/234081877_Environmental_unpredictability_and_offspring_size_Conservative_versus_diversified_bet-hedging
Ellis, Bruce J. 2004. ‘Timing of Pubertal Maturation in Girls: An Integrated Life History Approach’, Psychological Bulletin, 130.6: pp. 920–58. https://doi.org/10.1037/0033-2909.130.6.920
Ellis, Bruce J., Aurelio José Figueredo, Barbara H. Brumbach, and Gabriel L. Schlomer. 2009. ‘Fundamental Dimensions of Environmental Risk’, Human Nature, 20.2: pp. 204–68. https://doi.org/10.1007/s12110-009-9063-7
Ellison, P. T. 2003. ‘Energetics and Reproductive Effort’, American Journal of Human Biology, 15.3: pp. 342–51. https://doi.org/10.1002/ajhb.10152
Emery Thompson, Melissa, James H. Jones, Anne E. Pusey, Stella Brewer-Marsden, Jane Goodall et al. 2007. ‘Aging and Fertility Patterns in Wild Chimpanzees Provide Insights into the Evolution of Menopause’, Current Biology, 17.24: pp. 2150–56. https://doi.org/10.1016/j.cub.2007.11.033
Emery Thompson, Melissa, Martin N. Muller, Kris Sabbi, Zarin P. Machanda, Emily Otali et al. 2016. ‘Faster Reproductive Rates Trade off against Offspring Growth in Wild Chimpanzees’, Proceedings of the National Academy of Sciences, 113.28: pp. 7780–85. https://doi.org/10.1073/pnas.1522168113
Emlen, Stephen T. 1994. ‘Benefits, Constraints and the Evolution of the Family’, Trends in Ecology & Evolution, 9.8: pp. 282–85. https://doi.org/10.1016/0169-5347(94)90030-2
—. 1995. ‘An Evolutionary Theory of the Family’, Proceedings of the National Academy of Sciences, 92.18: pp. 8092–99. https://doi.org/10.1073/pnas.92.18.8092
Eriksson, Katherine, Gregory T. Niemesh, and Melissa Thomasson. 2018. ‘Revising Infant Mortality Rates for the Early Twentieth Century United States’, Demography, 55.6: pp. 2001–24. https://doi.org/10.1007/s13524-018-0723-2
Foley, Robert A., and Phyllis C. Lee. 1991. ‘Ecology and Energetics of Encephalization in Hominid Evolution’, Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 334.1270: pp. 223–32. https://doi.org/10.1098/rstb.1991.0111
Fraser Schoen, Roslyn. 2014. ‘The Social Complexity of Woman and Child Valuation in Rural Bangladesh’ (unpublished PhD Dissertation, Columbia, MO: University of Missouri).
Gage, Timothy B. 1989. ‘Bio-Mathematical Approaches to the Study of Human Variation in Mortality’, American Journal of Physical Anthropology, 32.S10: pp. 185–214. https://doi.org/10.1002/ajpa.1330320509
Gagnon, Alain, Ken R. Smith, Marc Tremblay, Hélène Vézina, Paul-Philippe Paré et al. 2009. ‘Is There a Trade-off between Fertility and Longevity? A Comparative Study of Women from Three Large Historical Databases Accounting for Mortality Selection’, American Journal of Human Biology, 21.4: pp. 533–40. https://doi.org/10.1002/ajhb.20893
Galor, Oded. 2012. ‘The Demographic Transition: Causes and Consequences’, Cliometrica, 6.1: pp. 1–28. https://doi.org/10.1007/s11698-011-0062-7
Gibson, Mhairi A. 2014. ‘How Development Intervention Drives Population Change in Rural Africa: A Case Study of Applied Evolutionary Anthropology’, in Applied Evolutionary Anthropology, ed. by Gibson, Mhairi A., and Lawson, David W. (New York: Springer), pp. 59–81.
Gibson, Mhairi A., and David W. Lawson. 2014. Applied Evolutionary Anthropology: Darwinian Approaches to Contemporary World Issues (New York: Springer Science & Business Media), i.
Gibson, Mhairi A., and David W. Lawson. 2015. ‘Applying Evolutionary Anthropology’, Evolutionary Anthropology: Issues, News, and Reviews, 24.1: pp. 3–14. https://doi.org/10.1002/evan.21432
Glenday, Craig, 1988. Guinness Book of World Records 2008 (New York: Sterling Publishing Company).
Glover, Susan M., and Mary C. Towner. 2009. ‘Long-Distance Dispersal to the Mining Frontier in Late 19th Century Colorado’, Behaviour, 146.4: pp. 677–700. https://doi.org/10.1163/156853908x395558
Goody, Jack. 1976. Production and Reproduction: A Comparative Study of the Domestic Domain (Cambridge and New York: Cambridge University Press).
Gurven, M., and H. Kaplan. 2007. ‘Longevity Among Hunter-Gatherers: A Cross-Cultural Examination’, Population and Development Review, 33.2: pp. 321–65. https://doi.org/10.1111/j.1728-4457.2007.00171.x
Gurven, M., H. Kaplan, and A. Z. Supa. 2007. ‘Mortality Experience of Tsimane Amerindians of Bolivia: Regional Variation and Temporal Trends’, American Journal of Human Biology, 19.3: pp. 376–98. https://doi.org/10.1002/ajhb.20600
Gurven, Michael, Megan Costa, Ben Trumble, Jonathan Stieglitz, Bret Beheim et al. 2016. ‘Health Costs of Reproduction Are Minimal despite High Fertility, Mortality and Subsistence Lifestyle’, Scientific Reports, 6.1: pp. 30056. https://doi.org/10.1038/srep30056
Gurven, Michael, and Robert S. Walker. 2006. ‘Energetic Demand of Multiple Dependents and the Evolution of Slow Human Growth’, Proceedings of the Royal Society B: Biological Sciences, 273.1588: pp. 835–41. https://doi.org/10.1098/rspb.2005.3380
Hamilton, W. D. 1966. ‘The Moulding of Senescence by Natural Selection’, Journal of Theoretical Biology, 12.1: pp. 12–45. https://doi.org/10.1016/0022-5193(66)90184-6
Hamilton, William D., and Robert M. May. 1977. ‘Dispersal in Stable Habitats’, Nature, 269.5629: pp. 578–81. https://doi.org/10.1038/269578a0
Hare, Darragh, Adam Z. Reynolds, Chun-Yi Sum, Mary K. Shenk, Tami Blumenfield et al. 2019. ‘Market Integration Is Not Monolithic: Different Indicators of Market Integration at Different Levels Predict Health and Reproductive Outcomes’. (Submitted to press; in revision).
Harpending, Henry, and Gregory Cochran. 2002. ‘In Our Genes’, Proceedings of the National Academy of Sciences, 99.1: pp. 10–12. https://doi.org/10.1073/pnas.012612799
Harrell, Stevan. 1997. Human Families (Boulder, CO: Westview Press).
Hawkes, Kristen. 2004. ‘Human Longevity: The Grandmother Effect’, Nature, 428.6979: pp. 128–29. https://doi.org/10.1038/428128a
Henrich, Joseph. 2004. ‘Demography and Cultural Evolution: How Adaptive Cultural Processes Can Produce Maladaptive Losses: The Tasmanian Case’, American Antiquity, 69.2: pp. 197–214. https://doi.org/10.2307/4128416
Henrich, Joseph, Steven J. Heine, and Ara Norenzayan. 2010. ‘Most People Are Not WEIRD’, Nature, 466.7302: pp. 29. https://doi.org/10.1038/466029a
Herrmann, Nicholas P., and Lyle W Konigsberg. 2002. ‘A Re-Examination of the Age-at-Death Distribution of Indian Knoll’, in Paleodemography: Age Distributions from Skeletal Samples, ed. by R. D. Hoppa and James W. Vaupel (Cambridge: Cambridge University Press), pp. 243–57.
Hill, Kim. 1993. ‘Life History Theory and Evolutionary Anthropology’, Evolutionary Anthropology, 2.3: pp. 78–88. https://doi.org/10.1002/evan.1360020303
Hill, Kim, Christophe Boesch, Jane Goodall, Anne Pusey, Jennifer Williams et al. 2001. ‘Mortality Rates among Wild Chimpanzees’, Journal of Human Evolution, 40.5: pp. 437–50. https://doi.org/10.1006/jhev.2001.0469
Hill, Kim, and A. Magdalena Hurtado. 1996. Ache Life History: The Ecology and Demography of a Foraging People (Hawthorne, NY: Aldine de Gruyter).
Hill, Kim, A. Magdalena Hurtado, and Robert S. Walker. 2007. ‘High Adult Mortality among Hiwi Hunter-Gatherers: Implications for Human Evolution’, Journal of Human Evolution, 52.4: pp. 443–54. https://doi.org/10.1016/j.jhevol.2006.11.003
Hill, Kim R., Robert S. Walker, Miran Božičević, James Eder, Thomas Headland et al. 2011. ‘Co-Residence Patterns in Hunter-Gatherer Societies Show Unique Human Social Structure’, Science, 331.6022: pp. 1286–89. https://doi.org/10.1126/science.1199071
Holden, Clare Janaki, and Ruth Mace. 2003. ‘Spread of Cattle Led to the Loss of Matrilineal Descent in Africa: A Coevolutionary Analysis’, Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 270.1532: pp. 2425–33. https://doi.org/10.1098/rspb.2003.2535
Hopcroft, Rosemary L. 2006. ‘Sex, Status, and Reproductive Success in the Contemporary United States’, Evolution and Human Behavior, 27.2: pp. 104–20. https://doi.org/10.1016/j.evolhumbehav.2005.07.004
Howard, Janet A., and Mhairi A. Gibson. 2017. ‘Frequency-Dependent Female Genital Cutting Behaviour Confers Evolutionary Fitness Benefits’, Nature Ecology & Evolution, 1: 0049. https://doi.org/10.1038/s41559-016-0049
Howell, N. 2000. Demography of the Dobe! Kung (Hawthorne, NY: Aldine de Gruyter).
Hrdy, Sarah Blaffer. 1992. ‘Fitness Tradeoffs in the History and Evolution of Delegated Mothering with Special Reference to Wet-Nursing, Abandonment, and Infanticide’, Ethology and Sociobiology, 13.5–6: pp. 409–42. https://doi.org/10.1016/0162-3095(92)90011-r
—. 2005. ‘Evolutionary Context of Human Development. The Cooperative Breeding Model’, in Family Relationships: An Evolutionary Perspective, ed. by Catherine A. Salmon and Todd K. Shackelford (New York: Oxford Academic), pp. 39–68.
—. 2009. Mothers and Others: The Evolutionary Origins of Mutual Understanding (Cambridge, MA: Belknap Press of Harvard University Press).
Hrdy, Sarah Blaffer, and Debra S. Judge. 1993. ‘Darwin and the Puzzle of Primogeniture’, Human Nature, 4.1: pp. 1–45. https://doi.org/10.1007/bf02734088
Hurt, Lisa S., Carine Ronsmans, and Suzanne L. Thomas. 2006. ‘The Effect of Number of Births on Women’s Mortality: Systematic Review of the Evidence for Women Who Have Completed Their Childbearing’, Population Studies, 60.1: pp. 55–71. https://doi.org/10.1080/00324720500436011
Jedwab, Remi, Luc Christiaensen, and Marina Gindelsky. 2015. ‘Demography, Urbanization and Development: Rural Push, Urban Pull And… Urban Push?’ (Washington DC: The World Bank)
Johowa, Johannes, Kai P. Willführ, and Eckart Volandd. [n.d.] ‘High Consanguinity Promotes Intergenerational Wealth Concentration in Socioeconomically Privileged Krummhörn Families of the 18th and 19th Centuries’, Evolution and Human Behavior, 40.2: 204–13. https://doi.org/10.1016/j.evolhumbehav.2018.11.005
Jones, James Holland. 2010. ‘Demography’, in Human Evolutionary Biology, ed. by M. P. Muehlenbein (Cambridge: Cambridge University Press).
—. 2011. ‘Primates and the Evolution of Long, Slow Life Histories’, Current Biology : CB, 21.18: R708–17. https://doi.org/10.1016/j.cub.2011.08.025
Jones, James Holland, and Rebecca Bliege Bird. 2014. ‘The Marginal Valuation of Fertility’, Evolution and Human Behavior, 35.1: pp. 65–71. https://doi.org/10.1016/j.evolhumbehav.2013.10.002
Jones, James Holland, and Brian Ferguson. 2009. ‘Demographic and Social Predictors of Intimate Partner Violence in Colombia’, Human Nature, 20.2: pp. 184–203. https://doi.org/10.1007/s12110-009-9064-6
Jordan, Fiona M., and Ruth Mace. 2007. ‘Changes in Post-Marital Residence Precede Changes in Descent Systems in Austronesian Societies’, conference proceeding. https://hdl.handle.net/1983/047674c4-8e14-4475-a271-d5bb60797202
Josephson, Steven C. 1993. ‘Status, Reproductive Success, and Marrying Polygynously’, Ethology and Sociobiology, 14.6: pp. 391–96. https://doi.org/10.1016/0162-3095(93)90027-f
Kaplan, Hillard. 1996. ‘A Theory of Fertility and Parental Investment in Traditional and Modern Human Societies’, American Journal of Physical Anthropology, 101.S23: pp. 91–135. https://doi.org/10.1002/(sici)1096-8644(1996)23+%3C91::aid-ajpa4%3E3.0.co;2-c
Kaplan, Hillard, Kim Hill, Jane Lancaster, and A. Magdalena Hurtado. 2000. ‘A Theory of Human Life History Evolution: Diet, Intelligence, and Longevity’, Evolutionary Anthropology, 9.4: pp. 156–85. https://doi.org/10.1002/1520-6505(2000)9:4%3C156::aid-evan5%3E3.0.co;2-7
Kaplan, Hillard, Jane B. Lancaster, T. W. Tucker, and Kermyt G. Anderson. 2002. ‘Evolutionary Approach to below Replacement Fertility’, American Journal of Human Biology, 14.2: pp. 233–56. https://doi.org/10.1002/ajhb.10041
Kaplan, Hillard S., and Michael Gurven. 2008. ‘Top-down and Bottom-up Research in Biodemography’, Demographic Research, 19: pp. 1587–1602. https://doi.org/10.4054/demres.2008.19.44
Kirkwood, T. B. L., and M. R. Rose. 1991. ‘Evolution of Senescence: Late Survival Sacrificed for Reproduction’, Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 332.1262: pp. 15–24. https://doi.org/10.1098/rstb.1991.0028
Koenig, Walter D. 1989. ‘Sex-Biased Dispersal in the Contemporary United States’, Evolution and Human Behavior, 10.4: pp. 263–77. https://doi.org/10.1016/0162-3095(89)90004-6
Koenig, Walter D., and Ronald L Mumme. 1987. Population Ecology of the Cooperatively Breeding Acorn Woodpecker (Princeton: Princeton University Press).
Koenig, Walter D., Frank A. Pitelka, William J. Carmen, Ronald L. Mumme, and Mark T. Stanback. 1992. ‘The Evolution of Delayed Dispersal in Cooperative Breeders’, The Quarterly Review of Biology, 67.2: pp. 111–50. https://doi.org/10.1086/417552
Kolodny, Oren, Marcus W. Feldman, and Nicole Creanza. 2018. ‘Integrative Studies of Cultural Evolution: Crossing Disciplinary Boundaries to Produce New Insights’, Phil. Trans. R. Soc. B, 373.1743: pp. 20170048. https://doi.org/10.1098/rstb.2017.0048
Koniak-Griffin, Deborah, and Carmen Turner-Pluta. 2001. ‘Health Risks and Psychosocial Outcomes of Early Childbearing: A Review of the Literature’, The Journal of Perinatal & Neonatal Nursing, 15.2: pp. 1–17. https://doi.org/10.1097/00005237-200109000-00002
Konigsberg, Lyle W., and Susan R. Frankenberg. 1992. ‘Estimation of Age Structure in Anthropological Demography’, American Journal of Physical Anthropology, 89.2: pp. 235–56. https://doi.org/10.1002/ajpa.1330890208
Konigsberg, Lyle W., Nicholas P. Herrmann, Kristen Hawkes, and Richard R. Paine. 2006. ‘The Osteological Evidence for Human Longevity in the Recent Past’, in The Evolution of Human Life History (Santa Fe: School of American Research Press), pp. 267–306.
Konner, Melvin J. 2011. ‘At the Cutting Edge of Human Adaptation’, American Scientist, 99.1: pp. 73. https://doi.org/10.1511/2011.88.73
Kramer, Karen L. 2005. ‘Children’s Help and the Pace of Reproduction: Cooperative Breeding in Humans’, Evolutionary Anthropology, 14.6: pp. 224–37. https://doi.org/10.1002/evan.20082
—. 2010. ‘Cooperative Breeding and Its Significance to the Demographic Success of Humans’, Annual Review of Anthropology, 39.1: pp. 417–36. https://doi.org/10.1146/annurev.anthro.012809.105054
Kramer, Karen L., and Russell D. Greaves. 2011. ‘Postmarital Residence and Bilateral Kin Associations among Hunter-Gatherers’, Human Nature, 22.1–2: pp. 41–63. https://doi.org/10.1007/s12110-011-9115-7
Kushnick, G. 2009. ‘Parental Supply and Offspring Demand amongst Karo Batak Mothers and Children’, Journal of Biosocial Science, 41.2: pp. 183. https://doi.org/10.1017/s0021932008002988
Kuzawa, Christopher W., Harry T. Chugani, Lawrence I. Grossman, Leonard Lipovich, Otto Muzik et al. 2014. ‘Metabolic Costs and Evolutionary Implications of Human Brain Development’, Proceedings of the National Academy of Sciences: 201323099. https://doi.org/10.1073/pnas.1323099111
Laland, Kevin N., John Odling-Smee, William Hoppitt, and Tobias Uller. 2013. ‘More on How and Why: Cause and Effect in Biology Revisited’, Biology & Philosophy, 28.5: pp. 719–45. https://doi.org/10.1007/s10539-012-9335-1
Lancaster, Jane B., and C. S. Lancaster. 1987. ‘The Watershed: Change in Parental-Investment and Family-Formation Strategies in the Course of Human Evolution’, in Parenting across the Life Span: Biosocial Dimensions, ed. by Jane B. Lancaster, Jeanne Altmann, S. Rossi, and L. R. Sherrod (Hawthorne, NY: Aldine Publishing Company), pp. 187–205.
Langkamp, Diane L., Amy Lehman, and Stanley Lemeshow. 2010. ‘Techniques for Handling Missing Data in Secondary Analyses of Large Surveys’, Academic Pediatrics, 10.3: pp. 205–10. https://doi.org/10.1016/j.acap.2010.01.005
Lawson, David, and Caroline Uggla. 2014. ‘Family Structure and Health in the Developing World: What Can Evolutionary Anthropology Contribute to Population Health Science?’, in Applied Evolutionary Anthropology: Darwinian Approaches to Contemporary World Issues (New York: Springer), pp. 85–118.
Lawson, David W., Alexandra Alvergne, and Mhairi A. Gibson. 2012. ‘The Life-History Trade-off between Fertility and Child Survival’, Proceedings of the Royal Society B: Biological Sciences, 279.1748: pp. 4755–64. https://doi.org/10.1098/rspb.2012.1635
Lawson, David W., and Ruth Mace. 2011. ‘Parental Investment and the Optimization of Human Family Size’, Philosophical Transactions of the Royal Society B: Biological Sciences, 366.1563: pp. 333–43. https://doi.org/10.1098/rstb.2010.0297
Le Bourg, Éric. 2007. ‘Does Reproduction Decrease Longevity in Human Beings?’, Ageing Research Reviews, 6.2: pp. 141–49. https://doi.org/10.1016/j.arr.2007.04.002
Lee, R. D. 2003. ‘Rethinking the Evolutionary Theory of Aging: Transfers, Not Births, Shape Senescence in Social Species’, Proceedings of the National Academy of Sciences of the United States of America, 100.16: pp. 9637. https://doi.org/10.1073/pnas.1530303100
Lee, Richard, and Ian DeVore. 1968. Man the Hunter (New York: Aldine De Gruyter).
Lee, Ronald. 2013. ‘Intergenerational Transfers, the Biological Life Cycle, and Human Society’, Population and Development Review, 38.s1: pp. 23–35. https://doi.org/10.1111/j.1728-4457.2013.00549.x
Leonetti, Donna L., Dilip C. Nath, and Natabar S. Hemam. 2007. ‘In-Law Conflict: Women’s Reproductive Lives and the Roles of Their Mothers and Husbands among the Matrilineal Khasi’, Current Anthropology, 48.6: pp. 861–90. https://doi.org/10.1086/520976
Leslie, Paul, and Bruce Winterhalder, 2002. ‘Demographic Consequences of Unpredictability in Fertility Outcomes’, American Journal of Human Biology, 14.2: pp. 168–83. https://doi.org/10.1002/ajhb.10044
Lewontin, R. C. 1965. ‘Selection for Colonizing Ability’, in The Genetics of Colonizing Species, ed. by H. G. Baker and G. L. Stebbins (New York: Academic Press), pp. 77–94.
Lovejoy, C. Owen, Richard S. Meindl, Thomas R. Pryzbeck, Thomas S. Barton, Kingsbury Heiple et al. 1977. ‘Paleodemography of the Libben Site, Ottawa County, Ohio’, Science, 198: pp. 291–93. https://doi.org/10.1126/science.198.4314.291
Low, Bobbi S. 2000. ‘Sex, Wealth, and Fertility: Old Rules, New Environments’, in Adaptation and Human Behavior: An Anthropological Perspective, ed. by Lee Cronk, Napoleon A Chagnon, and William Irons (Hawthorne, NY: Aldine De Gruyter), pp. 323–44.
Low, Bobbi S., and Alice L. Clarke. 1991. ‘Family Patterns in Nineteenth-Century Sweden: Impact of Occupational Status and Landownership’, Journal of Family History, 16.2: pp. 117–38. https://doi.org/10.1177/036319909101600202
Low, Bobbi S., Ashley Hazel, Nicholas Parker, and Kathleen B. Welch. 2008. ‘Influences on Women’s Reproductive Lives: Unexpected Ecological Underpinnings’, Cross-Cultural Research, 42.3: pp. 201–19. https://doi.org/10.1177/1069397108317669
Low, Bobbi S., Carl P. Simon, and Kermyt G. Anderson. 2002. ‘An Evolutionary Ecological Perspective on Demographic Transitions: Modeling Multiple Currencies’, American Journal of Human Biology, 14.2: pp. 149–67. https://doi.org/10.1002/ajhb.10043
Low, B. S., A. L. Clarke, and K. A. Lockridge. 1992. ‘Toward an Ecological Demography’, Population and Development Review, 18.1: pp. 1–31. https://doi.org/10.2307/1971857
Lummaa, Virpi. 2004. ‘Church Records Advancing Evolutionary Biology: Consanguinity, Inbreeding, and Genetic Drift in Italy by Luigi Luca Cavalli-Sforza, Antonio Moroni and Gianna Zei. Princeton University Press, 2004. £52.95 Pbk (320 Pages) ISBN 0691089914’, Trends in Ecology & Evolution, 19.6: pp. 286–87.
Luttbeg, Barney, Monique Borgerhoff Mulder, and Marc Mangel. 2000. ‘To Marry Again or Not: A Dynamic Model for Demographic Transition’, Adaptation and Human Behavior: An Anthropological Perspective, ed. by. Lee Cronk, Napoleon Chagnon, and William Irons (New York: Aldine de Gruyter), pp. 345–68.
Mace, Ruth, 1996. ‘Biased Parental Investment and Reproductive Success in Gabbra Pastoralists’, Behavioural ecology and Sociobiology, 38.2: pp. 75–81. https://doi.org/10.1007/s002650050219
—. 1998. ‘The Coevolution of Human Fertility and Wealth Inheritance Strategies’, Philosophical Transactions of the Royal Society of London, Series B, 353.1367: pp. 389–97. https://doi.org/10.1098/rstb.1998.0217
—. 2008. ‘Reproducing in Cities’, Science (New York, N.Y.), 319.5864: pp. 764–66. https://doi.org/10.1126/science.1153960
Mace, Ruth, and Rebecca Sear, 2005. ‘Are Humans Cooperative Breeders?’, in Grandmotherhood: The Evolutionary Significance of the Second Half of Female Life, ed. by Voland, Eckart, Chasiotis, Athanasios and Schiefenhoevel, Wulf (New Brunswick, NJ: Rutgers University Press), pp. 143–59.
Macfarlan, Shane J., Pamela I. Erickson, James Yost, Jhanira Regalado, Lilia Jaramillo et al. 2018. ‘Bands of Brothers and In-Laws: Waorani Warfare, Marriage and Alliance Formation’, Proceedings of the Royal Society B: Biological Sciences, 285.1890. https://doi.org/10.1098/rspb.2018.1859
Marcel Salathé, M. Kazandjieva, J. W. Lee, P. Levis, Marcus W. Feldman et al. 2010. ‘A High-Resolution Human Contact Network for Infectious Disease Transmission’, Proceedings of the National Academy of Sciences, USA, 107.51: pp. 22020–25. https://doi.org/10.1073/pnas.1009094108
Marlowe, Frank, 2000. ‘Paternal Investment and the Human Mating System’, Behavioral Processes, 51.1–3: pp. 45–61. https://doi.org/10.1016/s0376-6357(00)00118-2
—. 2010. The Hadza: Hunter-Gatherers of Tanzania (Berkeley, CA: University of California Press), iii.
Marlowe, Frank W. 2005. ‘Hunter-Gatherers and Human Evolution’, Evolutionary Anthropology: Issues, News, and Reviews, 14.2: pp. 54–67. https://doi.org/10.1002/evan.20046
Massey, Douglas S., Joaquin Arango, Graeme Hugo, Ali Kouaouci, Adela Pellegrino et al. 1994. ‘An Evaluation of International Migration Theory: The North American Case’, Population and Development Review, 20.4, pp. 699–751. https://doi.org/10.2307/2137660
Mathew, Sarah, and Robert Boyd, 2011. ‘Punishment Sustains Large-Scale Cooperation in Prestate Warfare’, Proceedings of the National Academy of Sciences, 108.28: pp. 11375–80. https://doi.org/10.1073/pnas.1105604108
Mattison, Siobhán M. 2011. ‘Evolutionary Contributions to Solving the “Matrilineal Puzzle”: A Test of Holden, Sear, and Mace’s Model’, Human Nature, 22.1–2: pp. 64–88. https://doi.org/10.1007/s12110-011-9107-7
—. 2016. ‘Male-Provisioning Hypothesis’, in Encyclopedia of Evolutionary Psychological Science, ed. by Viviana Weekes-Shackelford, Todd K. Shackelford, and Viviana A. Weekes-Shackelford (Cham: Springer International Publishing), pp. 1–6. https://doi.org/10.1007/978-3-319-16999-6_105-1
—. 2019. ‘Residence’ (Macmillan Encyclopedia of Families, Marriages, and Intimate Relationships).
Mattison, Siobhán M., Bret Beheim, Bridget Chak, and Peter Buston, 2016a. ‘Offspring Sex Preferences among Patrilineal and Matrilineal Mosuo in Southwest China Revealed by Differences in Parity Progression’, Royal Society Open Science, 3.9: pp. 160526 https://doi.org/10.1098/rsos.160526
Mattison, Siobhán M., Melissa J. Brown, Bruce Floyd, and Marcus W. Feldman. 2015. ‘Adoption Does Not Increase the Risk of Mortality among Taiwanese Girls in a Longitudinal Analysis’, PLoS ONE, 10.4: e0122867. https://doi.org/10.1371/journal.pone.0122867
Mattison, S. M., Hare, D., MacLaren, N. G., Reynolds, A. Z., Sum, C.-Y., Liu, R., et al. (2022). Context Specificity of “Market Integration” among the Matrilineal Mosuo of Southwest China. Current Anthropology, 000–00. https://doi.org/10.1086/719266
Mattison, Siobhán M., Christina Moya, Adam Reynolds, and Mary C. Towner. 2018a. ‘Evolutionary Demography of Age at Last Birth: Integrating Approaches from Human Behavioural Ecology and Cultural Evolution’, Phil. Trans. R. Soc. B, 373.1743: pp. 20170060. https://doi.org/10.1098/rstb.2017.0060
Mattison, Siobhán M., and Dawn B. Neill. 2013. ‘The Effects of Residential Ecology on Patterns of Child Work and Mother’s Reproductive Success among Indo-Fijians’, Evolution and Human Behavior, 34.3: pp. 207–15. https://doi.org/10.1016/j.evolhumbehav.2013.01.002
Mattison, Siobhán M., Edmond Seabright, Adam Z. Reynolds, Jingzhe (Bill) Cao, Melissa J. Brown et al. 2018b. ‘Adopted Daughters and Adopted Daughters-in-Law in Taiwan: A Mortality Analysis’, Royal Society Open Science, 5.3: pp. 171745. https://doi.org/10.1098/rsos.171745
Mattison, Siobhán M., and Rebecca Sear, 2016. ‘Modernizing Evolutionary Anthropology’, Human Nature, 27.4: pp. 1–16. https://doi.org/10.1007/s12110-016-9270-y
Mattison, Siobhán M., Eric A. Smith, Mary K. Shenk, and Ethan E. Cochrane. 2016b. ‘The Evolution of Inequality’, Evolutionary Anthropology: Issues, News, and Reviews, 25.4: pp. 184–99. https://doi.org/10.1002/evan.21491
Mayr, Ernst. 1961. ‘Cause and Effect in Biology’, Science, 134.3489: pp. 1501–6. https://doi.org/10.1126/science.134.3489.1501
Mcallister, Lisa, Michael Gurven, Hillard Kaplan, and Jonathan Stieglitz. 2012. ‘Why Do Women Have More Children than They Want? Understanding Differences in Women’s Ideal and Actual Family Size in a Natural Fertility Population’, American Journal of Human Biology, 24.6: pp. 786–99. https://doi.org/10.1002/ajhb.22316
McAllister, Lisa S., Gillian V. Pepper, Sandra Virgo, and David A. Coall. 2016. ‘The Evolved Psychological Mechanisms of Fertility Motivation: Hunting for Causation in a Sea of Correlation’, Phil. Trans. R. Soc. B, 371.1692: p. 20150151. https://doi.org/10.1098/rstb.2015.0151
McAllister, Lisa S., J Scofield, M Zoeller, Adrian V. Jaeggi, and Mary K. Shenk. [n.d.]. ‘Is Human Reproductive Decision Making Sensitive to Priming? A Multi-Level Meta-Analysis of 219 Results from 42 Studies’.
McDade, Thomas W. 2001. ‘Parent-Offspring Conflict and the Cultural Ecology of Breast-Feeding’, Human Nature, 12.1: pp. 9–25. https://doi.org/10.1007/s12110-001-1011-0
McDade, Thomas W., Sharon Williams, and J. Josh Snodgrass. 2007. ‘What a Drop Can Do: Dried Blood Spots as a Minimally Invasive Method for Integrating Biomarkers into Population Research’, Demography, 44.4: pp. 899–925. https://doi.org/10.1353/dem.2007.0038
Mesoudi, Alex, 2017. ‘Migration, Acculturation, and the Maintenance of between-Group Cultural Variation’, BioRxiv: pp. 234807. https://doi.org/10.1101/234807
Migliano, A. B., L. Vinicius, and M. M. Lahr, 2007. ‘Life History Trade-Offs Explain the Evolution of Human Pygmies’, Proceedings of the National Academy of Sciences, 104.51: pp. 20216. https://doi.org/10.1073/pnas.0708024105
Moore, Jim, 1993. ‘Inbreeding and Outbreeding in Primates: What’s Wrong with “the Dispersing Sex”’, The Natural History of Inbreeding and Outbreeding: pp. 392–426.
Mora, Alejandra Núñez-de la, Robert T. Chatterton, Osul A. Choudhury, Dora A. Napolitano, and Gillian R. Bentley, 2007. ‘Childhood Conditions Influence Adult Progesterone Levels’, PLOS Medicine, 4.5: e167. https://doi.org/10.1371/journal.pmed.0040167
Moya, Cristina, Kristin Snopkowski, and Rebecca Sear. 2016. ‘What Do Men Want? Re-Examining Whether Men Benefit from Higher Fertility than Is Optimal for Women’, Phil. Trans. R. Soc. B, 371.1692: pp. 20150149. https://doi.org/10.1098/rstb.2015.0149
Muller, Martin N., and Richard W. Wrangham. 2014. ‘Mortality Rates among Kanyawara Chimpanzees’, Journal of Human Evolution, 66: pp. 107–14. https://doi.org/10.1016/j.jhevol.2013.10.004
Munafò, Marcus R., Brian A. Nosek, Dorothy V. M. Bishop, Katherine S. Button, Christopher D. Chambers et al. 2017. ‘A Manifesto for Reproducible Science’, Nature Human Behaviour, 1.1: p. 0021. https://doi.org/10.1038/s41562-016-0021
Murdock, George P. 1967. Ethnographic Atlas (Pittsburg: University of Pittsburg Press).
Murphy, Lorna, Lynnette Sievert, Khurshida Begum, Taniya Sharmeen, Elaine Puleo et al. 2013. ‘Life Course Effects on Age at Menopause among Bangladeshi Sedentees and Migrants to the UK’, American Journal of Human Biology, 25.1: pp. 83–93. https://doi.org/10.1002/ajhb.22345
Natri, Heini, Angela R. Garcia, Kenneth H. Buetow, Benjamin C. Trumble, and Melissa A. Wilson, 2019. ‘The Pregnancy Pickle: Evolved Immune Compensation Due to Pregnancy Underlies Sex Differences in Human Diseases’, Trends in Genetics, 35.7: pp. 478–88. https://doi.org/10.1016/j.tig.2019.04.008
Navarrete, Ana, Carel P. van Schaik, and Karin Isler. 2011. ‘Energetics and the Evolution of Human Brain Size’, Nature, 480.7375: pp. 91–93. https://doi.org/10.1038/nature10629
Neill, Dawn B. 2007. ‘Exploring the Indo-Fijian Children’s BMI in the Context of Urbanization, Embodied Capital, and Food Choice Trade-Offs’, Human Nature, 18.3: pp. 209–24. https://doi.org/10.1007/s12110-007-9011-3
Nesse, R. M., and Stearns S. C. 2008. ‘The Great Opportunity: Evolutionary Applications to Medicine and Public Health’, Evolutionary Applications, 1.1: pp. 28–48. https://doi.org/10.1111/j.1752-4571.2007.00006.x
Nettle, Daniel. 2010. ‘Dying Young and Living Fast: Variation in Life History across English Neighborhoods’, Behavioural ecology, 21.2: pp. 387–95. https://doi.org/10.1093/beheco/arp202
Nettle, Daniel, Mhairi A. Gibson, David W. Lawson, and Rebecca Sear. 2013. ‘Human Behavioural ecology: Current Research and Future Prospects’, Behavioural ecology, 24.5: pp. 1031–40. https://doi.org/10.1093/beheco/ars222
Newson, L., T. Postmes, S. E. G. Lea, and P. Webley. 2005. ‘Why Are Modern Families Small? Toward an Evolutionary and Cultural Explanation for the Demographic Transition’, Personality and Social Psychology Review, 9.4: pp. 360–75. https://doi.org/10.1207/s15327957pspr0904_5
Núñez-De La Mora, Alejandra, Gillian R. Bentley, Osul A. Choudhury, Dora A. Napolitano, and Robert T. Chatterton, 2008. ‘The Impact of Developmental Conditions on Adult Salivary Estradiol Levels: Why This Differs from Progesterone?’, American Journal of Human Biology, 20.1: pp. 2–14. https://doi.org/10.1002/ajhb.20698
Oeppen, J., and J. W. Vaupel. 2002. ‘Broken Limits to Life Expectancy’, Science, 296.5570: pp. 1029–31. https://doi.org/10.1126/science.1069675
Page, Abigail E., Nikhil Chaudhary, Sylvain Viguier, Mark Dyble, James Thompson et al. 2017. ‘Hunter-Gatherer Social Networks and Reproductive Success’, Scientific Reports, 7.1: pp. 1153. https://doi.org/10.1038/s41598-017-01310-5
Page, Abigail E., Sylvain Viguier, Mark Dyble, Daniel Smith, Nikhil Chaudhary et al. 2016. ‘Reproductive Trade-Offs in Extant Hunter-Gatherers Suggest Adaptive Mechanism for the Neolithic Expansion’, Proceedings of the National Academy of Sciences: pp. 201524031. https://doi.org/10.1073/pnas.1524031113
Panter-Brick, Catherine. 1991. ‘Lactation, Birth Spacing and Maternal Work-Loads among Two Castes in Rural Nepal’, Journal of Biosocial Science, 23.2: pp. 137–54. https://doi.org/10.1017/S0021932000019179
Peccei, Jocelyn Scott. 2001. ‘Menopause: Adaptation or Epiphenomenon?’, Evolutionary Anthropology: Issues, News, and Reviews, 10.2: pp. 43–57. https://doi.org/10.1002/evan.1013
Pennington, Renee, and Henry Harpending. 1993. The Structure of an African Pastoralist Community: Demography, History, and Ecology of the Ngamiland Herero (Oxford: Clarendon Press).
Pepper, Gillian, Lisa McAllister, and Rebecca Sear. 2015. ‘Why Demography Needs Psychologists’, The Psychologist, 29.1: pp. 26–29. https://www.bps.org.uk/psychologist/why-demography-needs-psychologists#
Pepper, Gillian V., and Daniel Nettle. 2013. ‘Death and the Time of Your Life: Experiences of Close Bereavement Are Associated with Steeper Financial Future Discounting and Earlier Reproduction’, Evolution and Human Behavior, 34.6: pp. 433–39. https://doi.org/10.1016/j.evolhumbehav.2013.08.004
Pepper, Gillian V., and Daniel Nettle. 2014. ‘Socioeconomic Disparities in Health Behaviour: An Evolutionary Perspective’, in Applied Evolutionary Anthropology, Advances in the Evolutionary Analysis of Human Behaviour, 1, ed. by Gibson, Mhairi A., and Lawson, David W. (New York: Springer), pp. 225–43. https://doi.org/10.1007/978-1-4939-0280-4_10
Pepper, Gillian V., and Daniel Nettle. 2017. ‘The Behavioural Constellation of Deprivation: Causes and Consequences’, Behavioral and Brain Sciences, 40. https://doi.org/10.1017/s0140525x1600234x
Perry, Gretchen, Martin Daly, and Shane Macfarlan. 2014. ‘Maternal Foster Families Provide More Stable Placements than Paternal Families’, Children and Youth Services Review, 46: pp. 155–59. https://doi.org/10.1016/j.childyouth.2014.08.016
Pollet, Thomas V., Joshua M. Tybur, Willem E. Frankenhuis, and Ian J. Rickard. 2014. ‘What Can Cross-Cultural Correlations Teach Us about Human Nature?’, Human Nature, 25.3: pp. 410–29. https://doi.org/10.1007/s12110-014-9206-3
Prall, Sean P., and Brooke A. Scelza. 2017. ‘Child Fosterage and Sex-Biased Nutritional Outcomes among Namibian Pastoralists’, American Journal of Human Biology, 29.6: pp. 1–10. https://doi.org/10.1002/ajhb.23058
Promislow, D. E. L., and Harvey, P. H., 1990. ‘Living Fast and Dying Young: A Comparative Analysis of Life‐history Variation among Mammals’, Journal of Zoology, 220.3: pp. 417–37. https://doi.org/10.1111/j.1469-7998.1990.tb04316.x
Quinlan, R. J., and E. H. Hagen. 2008. ‘New Genealogy: It’s Not Just for Kinship Anymore’, Field Methods, 20.2: pp. 129–54. https://doi.org/10.1177/1525822x07314034
Quinlan, Robert J. 2006. ‘Human Parental Effort and Environmental Risk’, Proceedings of the Royal Society B: Biological Sciences, 274.1606: pp. 121–25. https://doi.org/10.1098/rspb.2006.3690
Richerson, Peter, and Rob Boyd. 2005. Not by Genes Alone: How Culture Transformed Human Evolution (Chicago: University of Chicago Press).
Richerson, Peter J, and Robert Boyd. 1998. ‘The Evolution of Human Ultra-Sociality’, Indoctrinability, Ideology, and Warfare: Evolutionary Perspectives: pp. 71–95.
Roff, Derek A. 1993. ‘The Evolution of Life Histories: Theory and Analysis’, Reviews in Fish Biology and Fisheries, 3.4, ed. by R. J. Wootton: pp. 384–85. https://doi.org/10.1007/BF00043394
Rogers, Alan R. 1990. ‘Evolutionary Economics of Human Reproduction’, Ethology and Sociobiology, 11.6: pp. 479–95. https://doi.org/10.1016/0162-3095(90)90022-X
Schacht, Ryan, and Karen L. Kramer. 2016. ‘Patterns of Family Formation in Response to Sex Ratio Variation’, PLoS One, 11.8: e0160320. https://doi.org/10.1371/journal.pone.0160320
Schacht, Ryan, Kristin Liv Rauch, and Monique Borgerhoff Mulder. 2014. ‘Too Many Men: The Violence Problem?’, Trends in Ecology & Evolution, 29.4: pp. 214–22. https://doi.org/10.1016/j.tree.2014.02.001
Schoorl, Jeannette, Liesbeth Heering, Ingrid Esveldt, George Groenewold, and Rob Van der Erf. 2000. Push and Pull Factors of International Migration: A Comparative Report (Luxembourg: European Communities, Office for Official Publications).
Sear, Rebecca. 2016a. ‘Beyond the Nuclear Family: An Evolutionary Perspective on Parenting’, Current Opinion in Psychology, 7: pp. 98–103. https://doi.org/10.1016/j.copsyc.2015.08.013
—. 2016b. ‘Evolutionary Demography: A Darwinian Renaissance in Demography’, International Encyclopedia of the Social and Behavioral Sciences, ed. by James D. Wright (Amsterdam: Elsevier), pp. 406–12.
Sear, Rebecca, and David Coall. 2011. ‘How Much Does Family Matter? Cooperative Breeding and the Demographic Transition’, Population and Development Review, 37.S1: pp. 81–112. https://doi.org/10.1111/j.1728-4457.2011.00379.x
Sear, Rebecca, David W. Lawson, Hillard Kaplan, and Mary K. Shenk. 2016. ‘Understanding Variation in Human Fertility: What Can We Learn from Evolutionary Demography?’, Phil. Trans. R. Soc. B, 371.1692: pp. 20150144. https://doi.org/10.1098/rstb.2015.0144
Sear, Rebecca, and Ruth Mace, 2008. ‘Who Keeps Children Alive? A Review of the Effects of Kin on Child Survival’, Evolution and Human Behavior, 29.1: pp. 1–18. https://doi.org/10.1016/j.evolhumbehav.2007.10.001
Sear, Rebecca, Ruth Mace, and Nadine Allal. 2004. ‘Height, Marriage and Reproductive Success in Gambian Women’, in Socioeconomic Aspects of Human Behavioural ecology, Research in Economic Anthropology, 23 vols. (Emerald Group Publishing Limited), xxiii, pp. 203–24. https://doi.org/10.1016/S0190-1281(04)23008-6
Sear, Rebecca, Siobhán M. Mattison, and Mark K. Shenk. 2024. ‘Demography’ in Human Behavioural Ecology, eds Koster, Jeremy, Brooke Scelza and Mary K. Shenk (Cambridge: Cambridge University Press), pp 307-322
Sellen, Daniel W., 2007. ‘Evolution of Infant and Young Child Feeding: Implications for Contemporary Public Health’, Annu. Rev. Nutr., 27.1: pp. 123–48. https://doi.org/10.1146/annurev.nutr.25.050304.092557
Sellen, D. W. 2001. ‘Comparison of Infant Feeding Patterns Reported for Nonindustrial Populations with Current Recommendations’, The Journal of Nutrition, 131.10: pp. 2707–15. https://doi.org/10.1093/jn/131.10.2707
Sheehan, Oliver, Joseph Watts, Russell D. Gray, and Quentin D. Atkinson. 2018. ‘Coevolution of Landesque Capital Intensive Agriculture and Sociopolitical Hierarchy’, Proceedings of the National Academy of Sciences, 115.14: 201714558. https://doi.org/10.1073/pnas.1714558115
Shenk, Mary K. 2007. ‘Dowry and Public Policy in Contemporary India’, Human Nature, 18.3: pp. 242–63. https://doi.org/10.1007/s12110-007-9006-0
Shenk, Mary K., Monique Borgerhoff Mulder, Jan Beise, Gregory Clark, William Irons et al. 2010. ‘Intergenerational Wealth Transmission among Agriculturalists: Foundations of Agrarian Inequality’, Current Anthropology, 51.1: pp. 65–83. https://doi.org/10.1086/648658
Shenk, Mary K., Hillard S. Kaplan, and Paul L. Hooper. 2016. ‘Status Competition, Inequality, and Fertility: Implications for the Demographic Transition’, Phil. Trans. R. Soc. B, 371.1692: pp. 20150150. https://doi.org/10.1098/rstb.2015.0150
Shenk, Mary K., Mary C. Towner, Howard C. Kress, and Nurul Alam. 2013. ‘A Model Comparison Approach Shows Stronger Support for Economic Models of Fertility Decline’, Proceedings of the National Academy of Sciences, 110.20: pp. 8045–50. https://doi.org/10.1073/pnas.1217029110
Shenk, Mary K., Mary C. Towner, Kathrine Starkweather, Curtis J. Atkisson, and Nurul Alam. 2014. ‘The Evolutionary Demography of Sex Ratios in Rural Bangladesh’, in Applied Evolutionary Anthropology, Advances in the Evolutionary Analysis of Human Behaviour, ed. by Mhairi A. Gibson and David W. Lawson (Springer New York), i, pp. 141–73. http://dx.doi.org/10.1007/978-1-4939-0280-4_7
Sieff, Daniela F. 1990. ‘Explaining Biased Sex Ratios in Human Populations: A Critique of Recent Studies’, Current Anthropology, 31.2: pp. 25–48. https://doi.org/10.1086/203823
Silberzahn, Raphael, and Eric L. Uhlmann. 2015. ‘Many Hands Make Tight Work’, Nature, 526.7572: pp. 189–91. https://doi.org/10.1038/526189a
Siler, William. 1979. ‘A Competing‐Risk Model for Animal Mortality’, Ecology, 60.4: pp. 750–57. https://doi.org/10.2307/1936612
Smith, Alexander K., John Z. Ayanian, Kenneth E. Covinsky, Bruce E. Landon, Ellen P. McCarthy et al. 2011. ‘Conducting High-Value Secondary Dataset Analysis: An Introductory Guide and Resources’, Journal of General Internal Medicine, 26.8: pp. 920–29. https://doi.org/10.1007/s11606-010-1621-5
Smith, Eric Alden. 2000. ‘Three Styles in the Evolutionary Study of Human Behavior’, in Human Behavior and Adaptation: An Anthropological Perspective, ed. by Lee Cronk, Napolean Chagnon, and William Irons (Hawthorne, NY: Aldine de Gruyter), pp. 27–46.
Smith, Eric Alden, and Bruce Winterhalder. 1992a. Evolutionary Ecology and Human Behavior (Hawthorne, NY: Aldine de Gruyter).
—. 1992b. ‘Natural Selection and Decision-Making: Some Fundamental Principles’, in Evolutionary Ecology and Human Behavior (Abingdon: Routledge), pp. 25–60.
Stacey, Peter B., and J. David Ligon. 1991. ‘The Benefits-of-Philopatry Hypothesis for the Evolution of Cooperative Breeding: Variation in Territory Quality and Group Size Effects’, The American Naturalist, 137.6: pp. 831–46. https://doi.org/10.1086/285196
Stearns, Stephen C. 1992. The Evolution of Life Histories (Oxford, UK: Oxford University Press).
Stieglitz, Jonathan, Benjamin C. Trumble, Hillard Kaplan, and Michael Gurven. 2018. ‘Marital Violence and Fertility in a Relatively Egalitarian High-Fertility Population’, Nature Human Behaviour, 2.8: pp. 565–72. https://doi.org/10.1038/s41562-018-0391-7
Stone, Linda. 2014. Kinship and Gender, 5th Edition (Boulder, CO: Westview Press).
Strassman, Beverly, and Alice Clarke. 1998. ‘Ecological Constraints on Marriage in Rural Ireland’, Evolution and Human Behavior, 19.1: pp. 35–55. https://doi.org/10.1016/s1090-5138(97)00103-7
Stulp, Gert, Rebecca Sear, and Louise Barrett. 2016. ‘The Reproductive Ecology of Industrial Societies, Part I’, Human Nature, 27.4: pp. 422–44. https://doi.org/10.1007/s12110-016-9269-4
Testa, Maria Rita. 2007. ‘Childbearing Preferences and Family Issues in Europe: Evidence from the Eurobarometer 2006 Survey’, Vienna Yearbook of Population Research, 2007: pp. 357–79. https://doi.org/10.1553/populationyearbook2007s357
Tinbergen, N. 1963. ‘On Aims and Methods of Ethology’, Zeitschrift Für Tierpsychologie, 20: pp. 410–33. https://doi.org/10.1111/j.1439-0310.1963.tb01161.x
Towner, Mary C. 1999. ‘A Dynamic Model of Human Dispersal in a Land-Based Economy’, Behavioural ecology and Sociobiology, 46.2: pp. 82–94. https://doi.org/10.1007/s002650050596
—. 2001. ‘Linking Dispersal and Resources in Humans’, Human Nature, 12.4: pp. 321–49. https://doi.org/10.1007/s12110-001-1002-1
—. 2002. ‘Linking Dispersal and Marriage in Humans: Life History Data from Oakham, Massachusetts, USA (1750–1850)’, Evolution and Human Behavior, 23.5: pp. 337–57. https://doi.org/10.1016/S1090-5138(02)00094-6
Towner, Mary C., Ilona Nenko, and Savannah E. Walton. 2016. ‘Why Do Women Stop Reproducing before Menopause? A Life-History Approach to Age at Last Birth’, Phil. Trans. R. Soc. B, 371.1692: pp. 20150147. https://doi.org/10.1098/rstb.2015.0147
Trinkaus, Erik, 1995. ‘Neanderthal Mortality Patterns’, Journal of Archaeological Science, 22.1: pp. 121–42. https://doi.org/10.1016/S0305-4403(95)80170-7
Trivers, Robert, 1972. ‘Parental Investment and Sexual Selection’, in Sexual Selection and the Descent of Man, ed. by B. Campbell (Chicago, IL: Aldine), pp. 136–79.
Trivers, Robert L. 1974. ‘Parent-Offspring Conflict’, American Zoologist, 14.1: pp. 249–64. https://doi.org/10.1093/icb/14.1.249
Tuljapurkar, Shripad, Nan Li, and Carl Boe. 2000. ‘A Universal Pattern of Mortality Decline in the G7 Countries’, Nature, 405.6788: pp. 789–92. https://doi.org/10.1038/35015561
Turke, Paul W. 1988. ‘Helpers at the Nest: Childcare Networks on Ifaluk’, in Human Reproductive Behavior: A Darwinian Perspective, ed. by Laura L. Betzig, Monique Borgerhoff Mulder, and Paul Turke (New York, NY: Cambridge University Press), pp. 173–88.
Turke, P. W. 1989. ‘Evolution and the Demand for Children’, Population and Development Review, 15.1: pp. 61–90. https://doi.org/10.2307/1973405
Valeggia, Claudia. 2007. ‘Taking the Lab to the Field: Monitoring Reproductive Hormones in Population Research’, Population and Development Review, 33.3: pp. 525–42. https://doi.org/10.1111/j.1728-4457.2007.00183.x
Vallois, Henri V. 1961. ‘The Social Life of Early Man: The Evidence of Skeletons’, Social Life of Early Man (Routledge) pp. 214–35.
Vining, Daniel R. 1986. ‘Social versus Reproductive Success: The Central Theoretical Problem of Human Sociobiology’, Behavioral and Brain Sciences, 9.01: pp. 167–87. https://doi.org/10.1017/S0140525X00021968
Voland, Eckart. 2000. ‘Contributions of Family Reconstitution Studies to Evolutionary Reproductive Ecology’, Evolutionary Anthropology: Issues, News, and Reviews, 9.3: pp. 134–46. https://doi.org/10.1002/1520-6505(2000)9:3<134::AID-EVAN3>3.0.CO;2-M
Voland, Eckart, and Robin I. M. Dunbar. 1995. ‘Resource Competition and Reproduction’, Human Nature, 6.1: pp. 33–49. https://doi.org/10.1007/bf02734134
Voland, Eckhart. 1998. ‘Evolutionary Ecology of Human Reproduction’, Annual Review of Anthropology, 27.1: pp. 347–74. https://doi.org/10.1146/annurev.anthro.27.1.347
Wachter, Kenneth W. 2008. ‘Biodemography Comes of Age’, Demographic Research, 19.40: pp. 1501–12. https://doi.org/10.4054/DemRes.2008.19.40
Walker, R., M. Gurven, K. Hill, A. Migliano, N. Chagnon et al. 2006. ‘Growth Rates and Life Histories in Twenty-two Small-scale Societies’, American Journal of Human Biology, 18.3: pp. 295–311. https://doi.org/10.1002/ajhb.20510
Walker, Robert S., Michael Gurven, Oskar Burger, and Marcus J. Hamilton. 2008. ‘The Trade-off between Number and Size of Offspring in Humans and Other Primates’, Proceedings of the Royal Society B: Biological Sciences, 2751636: pp. 827–34. https://doi.org/10.1098/rspb.2007.1511
Weber, Max, 1978. Economy and Society: An Outline of Interpretive Sociology (Berkeley, CA: University of California Press), i.
Weiss, Kenneth M. 1981. ‘Evolutionary Perspectives on Human Aging’, in Other Ways of Growing Old, pp. 25–58.
Wells, Jonathan C. K. 2014. ‘Nutrition in a Changing World: How Economic Growth Drives Chronic Diseases’, in Applied Evolutionary Anthropology, Advances in the Evolutionary Analysis of Human Behaviour, 1, ed. by Mhairi A. Gibson and David W. Lawson (New York: Springer), pp. 245–70. https://doi.org/10.1007/978-1-4939-0280-4_11
Wells, Jonathan C. K., Randolph M. Nesse, Rebecca Sear, Rufus A. Johnstone, and Stephen C. Stearns. 2017. ‘Evolutionary Public Health: Introducing the Concept’, The Lancet, 390.10093: pp. 500–09. https://doi.org/10.1016/S0140-6736(17)30572-X
Wilson, M., and M. Daly. 1997. ‘Life Expectancy, Economic Inequality, Homicide, and Reproductive Timing in Chicago Neighbourhoods’, BMJ : British Medical Journal, 314.7089: pp. 1271–74. https://doi.org/10.1136/bmj.314.7089.1271
Winterhalder, Bruce, and Eric A. Smith. 2000. ‘Analyzing Adaptive Strategies: Human Behavioural ecology at Twenty-Five’, Evolutionary Anthropology, 9.2: pp. 51–72. https://doi.org/10.1002/(sici)1520-6505(2000)9:2%3C51::aid-evan1%3E3.0.co;2-7
Wood, Brian M., and Frank W. Marlowe. 2011. ‘Dynamics of Postmarital Residence among the Hadza’, Human Nature, 22.1–2: pp. 128–38. https://doi.org/10.1007/s12110-011-9109-5
Wood, Brian M., David P. Watts, John C. Mitani, and Kevin E. Langergraber. 2017. ‘Favorable Ecological Circumstances Promote Life Expectancy in Chimpanzees Similar to That of Human Hunter-Gatherers’, Journal of Human Evolution, 105: pp. 41–56. https://doi.org/10.1016/j.jhevol.2017.01.003
Wood, James W., Darryl J. Holman, Kathleen A. O. Connor, and Rebecca J. Ferrell. 2002. ‘Mortality Models for Paleodemography’, Cambridge Studies in Biological and Evolutionary Anthropology: pp. 129–68.
Woodburn, James. 1968. ‘An Introduction to Hadza Ecology’, Man the Hunter, pp. 49–55. https://doi.org/10.4324/9780203786567-7
Worthman, C. M., and E. J. Costello. 2009. ‘Tracking Biocultural Pathways in Population Health: The Value of Biomarkers’, Annals of Human Biology, 36.3: pp. 281–97. https://doi.org/10.1080/03014460902832934
Wrigley, Edward Anthony. 1997. English Population History from Family Reconstitution 1580–1837 (Cambridge: Cambridge University Press), xxxii.
Zuo, Wenyun, Sha Jiang, Zhen Guo, Marcus W. Feldman, and Shripad Tuljapurkar. 2018. ‘Advancing Front of Old-Age Human Survival’, Proceedings of the National Academy of Sciences, 115.44: pp. 11209–14. https://doi.org/10.1073/pnas.1812337115
1 More recently, and in light of worldwide demographic transitions, ecological demographers have begun to explore proxies — especially status — for LRS as ultimate motivators for demographic behaviour (see e.g. Kaplan, 1996; Mattison and Sear, 2016).
2 By fitness, we do not mean fertility. While fertility is sometimes used as a proxy for fitness, as discussed, pursuing maximum fertility often does not maximize fitness.
4 Methodological individualism “holds that properties of groups […] are a result of the actions of its individual members”. (Smith and Winterhalder, 1992b). We use it here to emphasize that EED is primarily interested in variation in demographic behaviour at the individual, rather than group, level.
5 The ecological fallacy refers to incorrect inferences made by assuming relationships observed at the aggregate level represent individual-level processes (Pollet and others, 2014).
6 “Ecological selectionism” asks “What are the ecological forces that select for behavior X?” (Smith, 2000) and thus anticipates behaviours being shaped differently by different environments or socio-ecological contexts.
7 “Ethnographic analogy” is used to project the behavior of such small-scale communities into the distant past, because such populations are thought to be similar to Pleistocene ancestors given the continuity of selective environments (e.g. Marlowe 2005).
8 This is in contrast to mainstream biodemography, which anticipates even higher rates of fertility, given the apparent physiological capacity to reproduce more (e.g., Bongaarts, 1975). We take this up again in describing new questions around the age at last birth.
9 Note that his chapter has been posted on the Open Science Framework website since 04/02/2020, after it was accepted for publication, so the references will reflect when the chapter was written and not the OBP publication date.
