Production of Medicinal Maggots
12. Laboratory and Insectary Infrastructure and Equipment
© 2022 Frank Stadler, CC BY-NC 4.0 https://doi.org/10.11647/OBP.0300.12
Medicinal maggot production laboratory infrastructure requirements depend on pre-existing infrastructure, the current research and/or production activities, and on the production objectives—whether medicinal maggots are to be produced for research, therapy, or a combination of both. This chapter provides a typology of production facilities and describes the physical insectary and laboratory infrastructure and equipment necessary to maintain medicinal fly colonies and prepare medicinal maggots for use in human and veterinary medicine. Importantly, reliable production of safe and high-quality medicinal maggots does not necessarily require sophisticated and expensive laboratories and equipment.
Introduction
This chapter is concerned with the physical insectary and laboratory infrastructure and equipment necessary to maintain medicinal fly colonies and prepare medicinal maggots for use in human and veterinary medicine. It focusses specifically on the building, infrastructure, and equipment requirements for medicinal maggot insectaries and laboratories (from now referred to as production laboratories). The establishment of any laboratory can be described as a process that begins with the decision to establish a laboratory, followed by preplanning, planning, design, construction, and post-construction activities. Prospective producers are advised to consult the literature on laboratory planning, construction, and management—particularly the following books:
- Laboratory Design, Construction, and Renovation: Participants, Process, and Production. Committee on Design, Construction, and Renovation of Laboratory Facilities; Board on Chemical Sciences and Technology; Commission on Physical Sciences, Mathematics, and Applications; National Research Council. National Academy Press, Washington D.C. (2000). [1]
- The Sustainable Laboratory Handbook: Design, Equipment, Operation. Dittrich, E. & ProQuest, Ebooks. Wiley-VCH, Weinheim, Germany (2015). [2]
- Guidelines for Laboratory Design: Health, Safety, and Environmental Considerations. DiBerardinis, L. J. & ProQuest, Ebooks. Wiley, Hoboken, New Jersey (2013). [3]
The first is an excellent guide on the planning of laboratories from predesign to postconstruction, and the technical aspects that need to be considered. As a free online resource, it should be essential reading for any prospective producer needing to build or refurbish laboratory facilities—ideally, before planning has commenced! The other two books are much more technical and detailed in nature and are intended to provide professionals involved in the planning, design, construction, and operation of laboratories with essential information to facilitate collaboration.
A detailed discussion of all aspects of laboratory planning, construction and operation are beyond the scope of this book. Instead, this chapter will focus on key considerations as they apply specifically to medicinal maggot production laboratories. First it is important to understand the different types of laboratories that are typically concerned with the production of medicinal maggots and related research, and how these differ depending on their primary objectives and history. This discussion also includes an introduction to the production of medicinal maggots in compromised healthcare settings with mobile and community-based do-it-yourself medicinal maggot laboratories [4]. The remainder of the chapter explains the building and space requirements for research- and commercial-production laboratories, and the equipment needed.
Production Laboratory Typology
Thanks to the robust and adaptable nature of calliphorid fly species suitable for maggot therapy, laboratory and equipment infrastructure can be tailored to suit conditions, needs and resources. What a production facility will look like depends on several factors including a) financial resources, b) pre-existing building infrastructure, c) proximity to the point of care, d) transport infrastructure and distribution logistics, e) the wound burden amenable to maggot therapy, f) access to trained medical or scientific workforce, and more social factors such as g) institutional support for maggot therapy, h) healthcare-worker endorsement of maggot therapy, i) patient acceptance, j) regulatory approval of maggot therapy, and k) insurance cover for maggot therapy either via national health insurance or private health cover.
In general, laboratories that produce medicinal maggots either conduct maggot therapy-related research, forensic research, or they are dedicated medicinal maggot production laboratories. Research laboratories may produce medicinal maggots on a small scale to supply lab experiments or small clinical trials. The market size for medicinal maggots is largely determined by the wound burden, the general acceptance of maggot therapy, regulatory approval, and reimbursement of treatment costs via health insurance schemes. It follows that the scale of operations depends on the actual and/or potential demand for maggot therapy, i.e. from small-scale, not-for-profit production by research laboratories to fully independent and for-profit enterprises. The latter may specialise exclusively in the production of medicinal maggots or have a diversified product range that includes medicinal maggots. Table 12.1 presents a typology of medicinal maggot production organisations and their characteristics.
It appears that commercial producers emerge more often than not from university medical research laboratories that investigate clinical and biochemical aspects of maggot therapy. Large institutions such as universities afford a safety net allowing budding producers to slowly test and build the market through awareness-raising and educational activities, and to initiate the regulatory approval process. Since the regulators require clinical evidence of safety and efficacy, such trials are also best conducted from within a biomedical research organisation with clinical trial support and infrastructure. However, once the regulatory agencies have approved maggot therapy, it is unlikely that the host research organisations have the agility, entrepreneurial mindset, and expertise required for start-up and commercialisation. At this point, the producer ought to create a largely independent spin-off company that still maintains strong research ties with the parent organisation.
Up to recently, there has been little effort to provide high-quality maggot therapy to hard-to-reach patient populations such as in remote locations, at times of disaster or in armed conflict. However, the author’s group has developed a mobile shipping container that is fitted with an insectary and laboratory capable of producing medicinal maggots in remote locations and where logistics infrastructure is failing to guarantee 24- to 48-hour delivery of medicinal maggots. We also developed and tested production and treatment manuals that allow isolated communities to establish and run do-it-yourself (DIY) laboratories with limited resources so they can produce safe medicinal maggots for efficacious maggot therapy (Stadler et al. QVSA). All project material including container lab construction plans, DIY lab instructions, and the treatment manual are available online (www.medmaglabs.com). These resources provide all necessary information for isolated communities to produce safe medicinal maggots.
Table 12.1 Typology of organisations that produce medicinal maggots.
|
Factor |
Scale |
|||
|
Small-scale mostly not-for-profit |
Large-scale or diversified and for-profit |
|||
|
Research laboratory |
Mobile laboratories |
DIY laboratories |
Commercial laboratory |
|
|
Financial resources |
Limited, not core business |
Supported by aid agencies or government |
Very limited |
Investment capital and industry development grants |
|
Pre-existing infrastructure |
Biological, forensic, or biomedical laboratory |
No building infrastructure |
No building infrastructure |
Laboratories, convertible buildings, new buildings |
|
Proximity to point of care |
Close, in-house or local hospitals |
Close, but far from major population centres |
Close, but far from major population centres |
Irrespective of proximity |
|
Distribution logistics |
No need for sophisticated logistics |
Container deployment logistics |
No distribution logistics |
Sophisticated distribution logistics |
|
Wound burden |
Low |
Up to approx. 250 wounds per day |
Low but flexible |
High wound burden/market size |
|
Trained workforce |
Present |
Present |
Untrained laypersons |
Present |
|
Institutional support |
Within the scope of research |
Strong institutional support |
Grass-roots community support |
Independent |
|
Healthcare-worker endorsement |
Limited to collaborating hospitals |
Endorsement restricted to agency and care setting |
Endorsed by local lay- and trained healthcare workers |
Widely endorsed |
|
Patient awareness and acceptance |
Low in greater community but high in treating hospitals |
High |
High |
Generally high |
|
Regulatory approval |
Not approved |
Not necessarily approved |
Not necessarily approved |
Approved |
Mobile and Do-it-yourself Medicinal Maggot Laboratories for Compromised Healthcare Settings
The discussion of production facility establishment has largely focussed on fixed infrastructure such as existing labs, purpose built, or building infrastructure that can be converted into medicinal maggot production facilities. When production is tied to a specific place then this means that the availability of maggot therapy to patients will be dependent on their proximity to the production facility. In advanced economies with highly effective and reliable logistics networks, rapid 24-hour distribution of medicinal maggots over long distances and under cool chain conditions is feasible and standard practice [5]. However, this is not the case where economic disadvantage, poverty, war and conflict, or disasters disrupt logistics infrastructure and supply chains. The answer to this problem may well be mobile and low-resource do-it-yourself medicinal maggot laboratories as mentioned earlier.
The provision of medical services in the field close to the point of care is not a new idea. Portable healthcare facilities provide life-saving emergency care during war and disasters such as earthquakes, tsunamis, infectious disease outbreaks. They may include independently functioning medical units like airborne, floating, or terrestrial truck-mounted infrastructure. Temporary medical infrastructure is transported in parts and assembled and disassembled as required [6]. Tents have been traditionally used for temporary field hospital shelters to house emergency and surgical care, hospital wards, and support services, but their ease of deployment comes with significant disadvantages. For example, they have no rigid flooring and require level ground, they are prone to the elements because they are not insulated, they may leak during rain. In addition, they are difficult to keep clean [7].
In recent times, military field hospitals have been developed and deployed that are composed of a combination of soft and hard portable infrastructure. The new British Army front-line field hospitals use inflatable dome tents in conjunction with pre-configured, fully equipped, containerised units for services such as sterilisation departments [8]. Converted shipping containers have also proven cost-effective and practical solutions for the provision of medical and scientific laboratory services in low-income country healthcare settings [9, 10]. For example, 40-foot modified shipping containers are used by the President’s Malaria Initiative to provide low-cost laboratory infrastructure for mosquito control programmes in Mozambique, Mali, Angola and Liberia [9]. These innovations in mobile healthcare and laboratory services provision suggest strongly that there is a place for mobile medicinal maggot production close to the point of care.
For extremely isolated communities that are cut off from clinical supplies and advanced clinical care by armed conflict, disaster, or simply remoteness, the only way to provide effective limb- and life-saving wound care may be via do-it-yourself medicinal maggot production and maggot therapy—similar to the way maggot therapy had been practiced for millennia in ancient and tribal cultures [11]. The overriding concern, of course, must be patient safety. In today’s age, it would not be acceptable to let wild flies lay eggs on wounds for the maggots to treat the wound of their own accord without quality control—although, this is essentially what had been done in the past and what still happens today when wounds get accidentally colonised by fly maggots. Many cases of such fly colonisation (myiasis) are benign and even beneficial [12, 13], although maggot colonisation can be distressing for the patient, family, and carers because flies and maggots are usually associated with death, decay and filth. Controlled therapy rather than wild infestation is also desirable because of the potential risk of harmful microbes carried in by wild flies above and beyond the microbial burden that is already in the wound [14]. Moreover, a few species of fly such as screw worms can colonise wounds and cause damage to live tissue [15]. Chapter 7 [16] explains in detail the natural history of medicinal flies and related species and Chapters 4 to 6 [17–19] are concerned with the treatment of wounds with maggot therapy.
It follows that if isolated communities are to be encouraged and enabled to produce their own maggots and practice maggot therapy, then any instructions and guidelines need to be based on best practice. Community-based producers will need to be trained in the construction of basic production facilities and equipment, in the identification and culture of safe fly species, in the disinfection of eggs, and the safe treatment of wounds. Such training needs to overcome the material and social constraints of the low-resource setting. Instructions need to be provided in highly visual format to overcome language barriers. Any written material should ideally be provided in multi-lingual format. Minimum requirement would be instructional material in the official national language of the country and easy-to-understand English. The suggested solutions need to be supported by the resources that are locally available, which may vary from place to place. This means the instructions will need to be sufficiently flexible. This will give the end user the freedom to adopt and adapt local resources to achieve production and medicinal maggot quality objectives. The ingenuity and resourcefulness of communities in compromised healthcare settings must not be underestimated. For further information on our own efforts to give isolated communities the wherewithal to treat chronic wounds with safe maggot therapy, please go to www.medmaglabs.com.
Building and Space Requirements
Key activities conducted in the operation of a medicinal maggot production laboratory include the rearing and maintenance of fly colonies, the disinfection of fly eggs and rearing of larvae, the packaging of maggots into primary packaging containers, packaging of consignments for customers, quality control activities, storage of inventory, as well as office-based activities. Each of these has its own space, equipment and consumables requirements (Table 12.2 available at https://hdl.handle.net/20.500.12434/2f7d12xl). This chapter pre-empts by necessity some of the discussion that follows in subsequent chapters explaining fly colony establishment (Chapter 13) [20], medicinal maggot production (Chapter 14) [21], and packaging technology (Chapter 16) [22]. However, the focus is on space and equipment requirements for the establishment and operation of a medicinal maggot production laboratory.
Production spaces are less defined in shared research laboratories that are not exclusively set up for the purpose of medicinal maggot production. Research laboratories must often meet the needs of many users, and maggot therapy-related research and production activities sharing this space need to adapt accordingly. However, when dedicated production facilities are to be established, there is the opportunity to account and plan for the distinct work area requirements, and the workflows between them. It follows that a production facility should have:
Table 12.2 Production facility resources (e.g. equipment, space requirements and consumables) required to undertake production processes and sub-processes. https://hdl.handle.net/20.500.12434/2f7d12xl.
Insectary. An insectary for the rearing and maintenance of medicinal fly colonies. Its specifications arise from the environmental preferences of calliphorid flies, and the need to keep the space clean and pest-free. A constant temperature of 25℃ and 12 hours of light per day will ensure that the flies continue to produce eggs over their useful life span and that their offspring do not enter a diapause or resting phase during pupariation [23]. Humidity should range between approximately 40 and 60% RH. If the air conditioning system is not able to maintain this range, additional humidification or de-humidification systems may need to be installed. If possible, circulated air should be filtered to remove fly-generated dust from the insectary atmosphere. If this is not possible, a stand-alone air purifier can be installed, or lack of air filtration can be compensated for with more frequent cleaning protocols and/or increased personal protection with gloves, dust masks, hair nets, and lab gowns for prolonged insectary activities. Ventilation ports for fresh air intake and air exhaust should be screened with fine insect screens and doors should be fitted with door sweeps to prevent vermin entering the room. All surfaces should be washable and all cracks, gaps, joints, etc. sealed to avoid refugia for vermin and build-up of dirt. The insectary should also have potable water taps and greywater plumbing for a lab sink to be fitted. The insectary is entered via a small room that serves as a change room for protective clothing. The same protective clothing must not be worn in the general and clean lab to maintain hygiene and avoid contamination of disinfected medicinal maggots.
Clean lab. Here, a clean lab is defined as the part of the production laboratory that is dedicated to disinfection of eggs, quality control, and packaging of medicinal maggots into primary packaging. It is ‘clean’ in the sense that no activities such as washing up of dirty equipment, fly diet preparation and regular lab maintenance tasks are performed in this space. The clean lab should not be confused with a clean room, which in medical and pharmaceutical production laboratories provides a sterile work environment. Although possible, clean rooms are not necessary in medicinal maggot production. The sterile environment for work activities is usually provided by laminar flow cabinets (clean benches) that fulfil the same role but cost only a fraction to establish and operate (see equipment requirements).
Overall, the specifications for the clean lab are similar to those for the insectary but lighting is not automatically controlled and there is less stringent requirement to keep temperature and humidity in the optimal range. However, if disinfected eggs and medicinal maggots are to be incubated in the clean lab without an incubator, the temperature must also be kept at around 25℃, unless accelerated growth and development of medicinal maggots at higher temperatures is required. Like the insectary, the clean lab is also entered via a small room used for workers to change into protective clothing.
General lab. A general lab space for food and media preparation, cleaning of laboratory utensils after use, autoclaving of equipment used for clean-bench work, and quality control work other than microbial assays of eggs and maggots. The basic space requirements are identical to those of the clean lab. Again, basic services such as air conditioning, adequate lighting, plumbing and potable water supply should be installed.
Packaging and dispatch room. Product packaging and dispatch room for order processing. Medicinal maggots that have been packed into vials (primary packaging) in various unit sizes and treatment modalities (free-range or bagged) are collated according to customer orders and packed into insulated cool-chain shippers (packaging) and processed for dispatch. Requirements for this space are not as stringent as for the lab spaces but worker comfort should be considered regarding lighting levels, ventilation and air conditioning.
Store room. To hold supplies until they are required. It is best to have separate store rooms for laboratory supplies and office/general operations supplies. This is mainly to locate them in close proximity to the respective work area and facilitate easy access by the main user groups. It may also be necessary for facilities with high production volumes and many courier shipments to store a significant volume of insulated shippers and cool elements. If the packaging and dispatch room is not large enough then an additional store for this inventory may be required. Although hazardous chemical use is minimal in medicinal maggot production laboratories, larger volumes of corrosive or flammable chemicals need to be stored in dedicated safety cabinets according to local regulations.
Restrooms. These may be unisex or separate male/female amenities.
Change and locker rooms. These may also be provided for staff to change into lab uniforms and protective clothing and to store valuables that staff cannot or will not want to take into the labs.
Meeting rooms. Meeting rooms provide staff the opportunity to gather and socialise during breaks and prepare and consume food. A separate board or business meeting room should also be considered. If space is at a premium, the staff room can also serve as a formal meeting room.
Offices. For management and administration of operations including marketing, sales, customer support and customer relations, finance/accounts, quality control, and regulatory compliance. An open-plan office accommodating most office staff may not be advisable due to the different functions performed by staff. For example, it is likely that the sales and marketing team members spend a lot of time on the phone which can be disruptive to colleagues sharing the same space. Therefore, it is necessary to consider ahead of facility establishment which arrangement is most productive.
Actual floor area is not being considered in this discussion because it depends, among other things, on the available overall space, the size of operation/production volume, the number of workers using each workspace at the same time, local health and safety regulations, and building codes. It is, however, a good idea to be generous with floor area allocation when planning a production facility so that future growth in staff and production volume can be accommodated without disruption to operations.
An important consideration that does demand some discussion is the relative position of each workspace in relation to the overall facility layout. The physical infrastructure must facilitate the efficient flow of workers, information, products, and materials. This also includes resources entering and leaving the organisation. Figure 12.1 illustrates this relationship for a medicinal maggot production facility and shows the major flows between the spaces. There should be a clear spatial separation between the production areas and the office and support areas. Access to the labs should be via a single point and the clean lab as well as the insectary should be accessible via the general lab area rather than directly from the office area. There are consequently two main hubs, the office area and the general lab area, for administrative and production facilities, respectively. The medicinal maggot production workflow should be one-directional: 1) eggs are passed from insectary to the general lab for separation and prewashing; 2) from there, they are passed to the clean lab where disinfection, incubation and primary packaging takes place; 3) finally, the packaged maggots are moved on to the dispatch room for order fulfilment and shipping. Other activities such as quality control processes involve workflows that are less directional. It is also desirable that the dispatch room is close to the facility reception and office areas. This ensures good communication with sales who will want to oversee order fulfilment. Shipping-ready consignments also need to be picked up by couriers who may access the premises via the main entrance and reception unless a dedicated service entry exists.
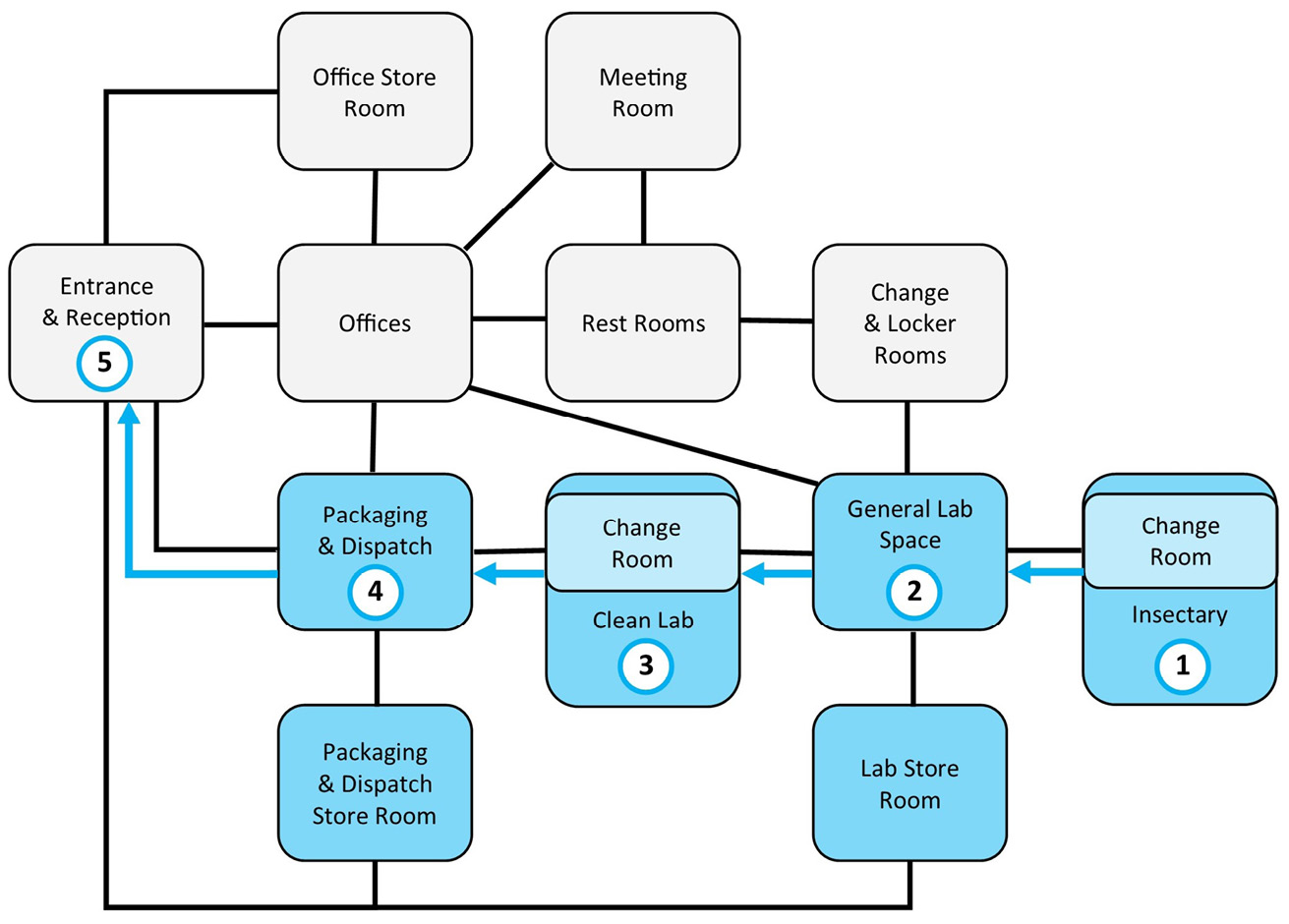
Figure 12.1 Proximity and workflow diagram for production facility. Relative position of bubbles in diagram suggests the spatial layout and proximity of workspaces in the facility. Black lines describe the movement of staff and materials around the facility according to production and administrative workflows. The blue arrows describe the unidirectional production path of medicinal maggots from (1) egg harvest in the insectary to (2) the general lab area for pre-cleaning and perhaps de-agglutination, to (3) the clean lab for disinfection, quality control, and primary packing, to (4) the packing and dispatch area, and (5) the reception for pickup by couriers.
Equipment Requirements
The equipment needs for medicinal maggot production are best discussed in relation to the specific work processes and functions they relate to. This chapter will provide an overview of the equipment needs without being prescriptive regarding specific brands, makes or suppliers. As has been explained earlier, medicinal maggot production affords a great deal of flexibility regarding the scale of operations and the production approaches. Much can be achieved with very little by way of facilities and equipment which makes maggot therapy such an attractive proposition for the low-resource healthcare setting.
The following discussion of equipment needs will guide the prospective producer through the production areas and specific process:
- insectary activities (e.g. adult fly colony maintenance and maggot rearing)
- general lab activities (e.g. diet preparation and quality control)
- clean lab activities (e.g. disinfection, medicinal maggot rearing, and packaging)
- packaging and dispatch activities (packaging of consignments).
The general equipment and furnishing needs for staff support areas (e.g. restrooms, locker rooms, meeting rooms), reception and offices is omitted here because these are not unique to medicinal maggot production facilities. By necessity, the following discussion of equipment and material needs for production facilities foreshadows content discussed in subsequent chapters, particularly those on medicinal maggot production (Chapter 14) [21] and transport packaging (Chapter 16) [22].
Insectary. The insectary is a dedicated space for the maintenance of medicinal flies and the rearing of new fly stock. Disinfected eggs and medicinal maggots should not be incubated in this space. Adult flies can be housed in a variety of cage systems [21]. The main consideration for choosing the right cage model, home-made or commercial, is adequate ventilation, light penetration, volume, and ease of handling and cleaning. These cages are best stored on easy-to-clean shelving that is water resistant and withstands a wide range of cleaning reagents such as bleach and alcohol-based disinfectants.
The maggots are conventionally reared in an inexpensive double-container system. A smaller plastic container holding the diet and maggots is placed inside a larger container holding a pupariation substrate such as sand, sawdust or vermiculite. The larger container is securely covered with fine-mesh muslin or synthetic fabric to provide ventilation but prevent wandering maggots from escaping. Once maggots have pupated, they need to be separated from the substrate by sieving. A variety of expensive metal sieves and inexpensive plastic colanders are readily available so long as they achieve separation of the puparia. This can be a dusty business and workers should wear dust masks or perform the sieving where particulates are quickly removed, either in the open air or under a laboratory exhaust system. Fly larvae shy away from light and therefore maggots are best reared in darkness. In addition, depending on the diet used, maggot rearing can be a smelly business. To make insectary work bearable, maggot containers can be stored in converted cupboards, fridge bodies or similar furniture that is fitted with ventilation that draws room air in and vents it to the outside. Alternatively, such units can be fitted with air filtration systems to limit odour in the room. If air is vented to the outside, it is important to ensure that the exhaust piping is screened with fine mesh to prevent wild flies and fly parasites from accessing the rearing cupboard.
Maintenance of fly colonies will require the removal of cages from the shelves and placement on a work bench. Like the shelving, the work bench or table should be sufficiently large to provide ample room for cages and other equipment. When flies have reached their useful life span and begin to die off, it is time to replace them with young flies. At this point the cage is placed in a freezer for a few hours to euthanise the flies and kill any pests such as mites. For more info on the humane treatment of flies please consult Chapter 19 [24]. The insectary should be fitted with a sink unit and drying racks to allow for easy cleaning of cages and food containers. Where feasible, shelving and other insectary furniture should be fitted with castors so it can be easily rolled about and moved for regular cleaning of the room surfaces.
Diets for maggots or bait to encourage egg laying may be prepared in the insectary or the general lab space. For most producers a household food blender will suffice to allow homogeneous mixing and maceration of various dietary ingredients for either plant- or meat-based diets. Adult flies are fed with a variety of protein-based and carbohydrate-rich food depending on the preference of the producer. These diets and egg-harvesting baits are usually offered with inexpensive and easy-to-clean or disposable plastic containers small enough to fit through the sleeve access in the fly cage. In order not to drown flies accidentally, liquid foods and water are offered with lidded plastic containers that are fitted with a cellulose sponge or rolled cellulose wicks (ideally organic and unstained to avoid poisoning of flies from residues).
Clean lab. The clean lab is where harvested and pre-cleaned fly eggs are disinfected and incubated, where hatched maggots are packaged into primary packaging containers, and where the microbial safety of medicinal maggots is examined via microbial assays. The clean lab should be as uncluttered as possible and may feature some cupboard and benchtop space as required for convenient operations. In practice, however, most activities undertaken in the clean lab take place within the laminar flow cabinet.
The production of medicinal goods such as medicinal maggots requires clean room conditions to maintain sterility during production processes, handling and packaging. There is no need to maintain the entire lab under clean room conditions. The comparatively small production volumes and the small size of the product handled in medicinal maggot production makes it feasible to use laminar flow cabinets, also known as clean benches, for the work that requires a sterile work environment. The number of laminar flow cabinets installed in a medicinal maggot clean lab depends on the volume of production and the time available to undertake required work. For most producers, one such work bench will suffice. When planning the clean lab, enough space should be allocated to be prepared for higher production volumes and to allow for additional clean benches.
There are a number of microbial testing protocols that may be employed by producers to make sure medicinal maggots are adequately disinfected [21]. These tests require a laboratory incubator that can be set and maintained at a constant temperature conducive to the growth of clinically relevant microbes (32℃ to 37℃). Such an incubator is therefore an essential piece of equipment for any sophisticated production facility. It is also convenient to be able to store pre-prepared consumables such as egg incubation media and blood agar plates in a refrigerator within the clean lab to maximise efficient workflow and to maintain hygiene in the clean lab area. A sink and water supply to the clean lab is not essential because clean lab activities do not necessarily require access to running water. Eggs should be already separated and prewashed when they arrive in the clean lab and all water used during the disinfection and packaging processes must be autoclaved prior to use, which is taking place in the general lab space. The small amounts of waste water and disinfection solution are collected and disposed of afterward when the disinfection equipment and utensils are taken to the general lab space for cleaning and autoclaving.
The actual work processes of egg disinfection, quality control, and packaging of medicinal maggots generally require various reagent bottles, measuring cylinders and pipettes, various spatulas or applicators, and a vacuum filtration system (electric or handpump-driven) for the filtration of eggs and perhaps maggots. The exact equipment needs will have to be established through trial as they depend on the volume of work, the disinfection procedure chosen by the lab, and personal preference.
General lab. The general lab area is the central hub of the production laboratory. It provides the support for activities that take place in the insectary and the clean lab. Consequently, the lab should be equipped with cupboards and shelves to hold utensils and consumables and provide ample benchtop workspace for diet preparation and quality control activities.
The general lab has a dedicated wet area with tap, sink and drying racks to clean equipment and utensils. Diets for the incubation of disinfected eggs and for the rearing of fly stock are prepared in the general lab area. A fridge and a freezer should be available to store perishable, meat-based diet ingredients and pre-prepared diets. A kitchen food blender may be used for diet preparation. All sterilisation of equipment, diets and water is conducted with an autoclave, or a medical-grade steam steriliser which is a cost-effective alternative to expensive autoclaves [10].
Quality control work such as monitoring the fly colony performance requires an analytical scale with a sensitivity of at least 0.0001g [25] to be able to determine the weight of individual flies, pupae and older larvae. A stereo dissecting microscope with a magnification range of 10 to 40 times is essential for visual observation of flies and their life stages. Measurement of morphological characters such as wing features may be conducted with digital image analysis software [26] but the length of puparia or maggots can be measured relatively simply with a fine ruler (0.1mm scale) [27], or a geometrical micrometre [28].
Packing and dispatch. The equipment required to support the dispatch of orders will vary depending on the volume of consignments processed per day and the business logistics systems employed. At the very least, the room will have a good amount of bench space to comfortably handle products and packaging, and process orders. For producers shipping their maggots further afield using couriers, equipment is needed to package and handle cool chain consignments (e.g. tape dispensers). Vials holding medicinal maggots are placed into insulated shippers along with phase-change cool elements which have been pre-cooled in a fridge.
Summary
The take-home-message from this chapter is that there is no one typical production laboratory. Production laboratory infrastructure needs will depend on pre-existing building and laboratory infrastructure, on the current research and/or production activities, and on the production objectives—whether only for research or therapy, or a combination of both. There are clearly opportunities to optimise infrastructure and equipment and thereby also work processes when setting up a lab specifically for commercial production. However, it is important to understand that reliable production of safe and high-quality medicinal maggots does not necessarily require sophisticated and expensive laboratories and equipment. Maggot therapy has been performed for millennia in tribal cultures and innovations such as mobile laboratories and community-based laboratories utilising locally-available resources have the potential to produce safe medical-grade maggots where there is little to no access to reliable wound care.
References
1. Anonymous. Laboratory Design, Construction, and Renovation: Participants, Process, and Production. 2000. https://doi.org/10.17226/9799.
2. Dittrich, E., The Sustainable Laboratory Handbook: Design, Equipment, Operation. 2015, Weinheim: Wiley-VCH.
3. DiBerardinis, L.J., Guidelines for Laboratory Design: Health, Safety, and Environmental Considerations. 2013, Hoboken: Wiley.
4. MedMagLabs. MedMagLabs. http://medmaglabs.com.
5. Monarch Labs. Order Form. https://www.monarchlabs.com/Monarch-Labs-Order-Form.pdf.
6. Bitterman, N. and Y. Zimmer, Portable Health Care Facilities in Disaster and Rescue Zones: Characteristics and Future Suggestions. Prehospital and Disaster Medicine, 2018: pp. 1–7, https://doi.org/10.1017/S1049023X18000560.
7. Bricknell, M.C., Organisation and Design of Regular Field Hospitals. Journal of the Royal Army Medical Corps, 2001. 147(2): pp. 161–167, https://doi.org/10.1136/jramc-147-02-09.
8. Apthorp, C. Inside the British Army’s New Front-line Field Hospital. 2016, 30 March. https://www.army-technology.com/features/featureinside-the-british-armys-new-front-line-field-hospital-4809564/.
9. Bridges, D.J., et al., Perspective Piece Modular Laboratories-Cost-Effective and Sustainable Infrastructure for Resource-Limited Settings. American Journal of Tropical Medicine and Hygiene, 2014. 91(6): pp. 1074–1078, https://doi.org/10.4269/ajtmh.14-0054.
10. Boubour, J., et al., A Shipping Container-Based Sterile Processing Unit for Low Resources Settings. PLoS ONE, 2016. 11(3): e0149624, https://doi.org/10.1371/journal.pone.0149624.
11. Kruglikova, A.A. and S.I. Chernysh, Surgical Maggots and the History of Their Medical Use. Entomological Review, 2013. 93(6): pp. 667–674, https://doi.org/10.1134/S0013873813060018.
12. Chan, Q.E., M.A. Hussain, and V. Milovic, Eating out of the Hand, Maggots — Friend or Foe? Journal of Plastic, Reconstructive and Aesthetic Surgery, 2012. 65(8): pp. 1116–1118, https://doi.org/10.1016/j.bjps.2012.01.014.
13. Terterov, S., et al., Posttraumatic Human Cerebral Myiasis. World Neurosurgery, 2010. 73(5): pp. 557–559, https://doi.org/10.1016/j.wneu.2010.01.004.
14. Snyder, S., P. Singh, and J. Goldman, Emerging Pathogens: A Case of Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica bacteremia. IDCases, 2020. 19: e00723, https://doi.org/10.1016/j.idcr.2020.e00723.
15. Zhou, X., et al., Human Chrysomya bezziana Myiasis: A Systematic Review. PLOS Neglected Tropical Diseases, 2019. 13(10): e0007391, https://doi.org/10.1371/journal.pntd.0007391.
16. Harvey, M., The Natural History of Medicinal Flies, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 121–142, https://doi.org/10.11647/OBP.0300.07.
17. Sherman, R., Indications, Contraindications, Interactions, and Side-effects of Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 63–78, https://doi.org/10.11647/OBP.0300.04.
18. Sherman, R., Medicinal Maggot Application and Maggot Therapy Dressing Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 79–96, https://doi.org/10.11647/OBP.0300.05.
19. Sherman, R. and F. Stadler, Wound Aetiologies, Patient Characteristics, and Healthcare Settings Amenable to Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 39–62, https://doi.org/10.11647/OBP.0300.03.
20. Stadler, F., et al., Fly Colony Establishment, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 257–288 (p. 269), https://doi.org/10.11647/OBP.0300.13.
21. Stadler, F. and P. Takáč, Medicinal Maggot Production, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 289–330, https://doi.org/10.11647/OBP.0300.14.
22. Stadler, F., Packaging Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 349–362, https://doi.org/10.11647/OBP.0300.16.
23. Barnes, K.M. and D.E. Gennard, Rearing Bacteria and Maggots Concurrently: A Protocol Using Lucilia sericata (Diptera: Calliphoridae) as a Model Species. Applied Entomology and Zoology, 2013. 48(3): pp. 247–253, https://doi.org/10.1007/s13355-013-0181-7.
24. Stadler, F., The Ethics of Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 405–430, https://doi.org/10.11647/OBP.0300.19.
25. Bala, M. and D. Singh, Development of Two Forensically Important Blowfly Species (Chrysomya megacephala and Chrysomya rufifacies) (Diptera: Calliphoridae) at Four Temperatures in India. Entomological Research, 2015. 45(4): pp. 176–183, https://doi.org/10.1111/1748-5967.12110.
26. Limsopatham, K., et al., A Molecular, Morphological, and Physiological Comparison of English and German Populations of Calliphora vicina (Diptera: Calliphoridae). PLoS ONE, 2018. 13(12), https://doi.org/10.1371/journal.pone.0207188.
27. Tarone, A.M. and D.R. Foran, Generalized Additive Models and Lucilia sericata Growth: Assessing Confidence Intervals and Error Rates in Forensic Entomology. Journal of Forensic Sciences, 2008. 53(4): pp. 942–948, https://doi.org/10.1111/j.1556-4029.2008.00744.x.
28. Villet, M.H., An Inexpensive Geometrical Micrometer for Measuring Small, Live Insects Quickly without Harming Them. Entomologia Experimentalis et Applicata, 2007. 122(3): pp. 279–280, https://doi.org/10.1111/j.1570-7458.2006.00520.x.
