15. Establishment of a Medical Maggot Rearing Facility and Maggot Therapy Programme for Human and Veterinary Medicine in Kenya1
© 2022 Chapter Authors, CC BY-NC 4.0 https://doi.org/10.11647/OBP.0300.15
This case study describes the process and experience of establishing a maggot therapy programme in Kenya. Initially, the programme included a technology- and knowledge-transfer initiative which successfully developed production capacity and clinical skills among the surgical and nursing workforce at Kenyatta National Hospital. This work was followed by a pilot study that demonstrated the positive impact mainstreaming of maggot therapy can have on the treatment of patients with chronic and infected wounds. The project highlights the importance of regulatory and supply-chain barriers that need to be addressed from the outset when introducing maggot therapy to new markets.
Introduction
Maggot therapy is the treatment of non-healing or infected wounds with disinfected fly larvae also called maggots. When placed on a wound, medicinal maggots remove dead tissue, fight infection, and promote wound healing [1–3]. Maggot therapy has been used to treat all kinds of chronic wounds including diabetic ulcers, pressure ulcers, burns, gangrene, and osteomyelitis, to name a few [4]. There are two ways medicinal maggots may be applied to a wound—free-range or contained within a mesh bag [5]. During free-range maggot therapy, maggots are placed directly onto the wound and held in place with a cage-like dressing made of a fabric material that allows maggots to breathe and wound exudate to drain off. Alternatively, maggots can be applied within a mesh bag similar to a tea bag. This is possible because maggots do not consume solids but liquefy their food in the wound environment. Digestive secretions and liquefied food easily pass through the mesh material.
Most maggot therapy around the world employs the greenbottle blowfly Lucilia sericata or the closely related L. cuprina, but other blowfly species such as Cochliomyia macellaria [6], Chrysomya putoria [7], and Sarconesiopsis magellanica [8] are also used or explored for potential use. As every medicinal fly should, L. sericata feeds only on dead and devitalised tissue and not on living cells, which does not impede healing. The competitive feeding of L. sericata larvae quickly removes dead tissue and slough and thus the source of nutrition for bacteria. Besides, bacteria are consumed and digested in the process. The removal of dead tissue also allows better diffusion of oxygen into the healthy tissues, which prevents the proliferation of anaerobic bacteria. However, the digestive excretions and secretions of maggots convey therapeutic benefits far beyond debridement. Not only do they contain compounds efficacious against fungal and bacterial pathogens, they also actively promote wound healing through a range of physiological mechanisms and pathways. These multiple therapeutic benefits of maggot therapy are discussed in great detail in Chapters 8 to 10 of this book [1–3].
The biological and life-history characteristics of green bottle maggots make them particularly suitable for biosurgery. They are easily cultured under insectary conditions, have a fast life-cycle and complete metamorphosis. The larvae feed preferentially on decomposing flesh and their performance in the wound is generally unaffected by illicit or therapeutic drugs [9], but topical wound care products must be removed prior to maggot therapy [10]. It is also helpful that medicinal maggots can be stored for a couple of days at cool temperatures as low as 6℃, which arrests their activity and slows down growth. This helps with production scheduling and allows for delivery times of up to 48 hours [11]. All in all, maggot therapy is a highly efficacious wound care modality and well-suited to high-resource and compromised healthcare settings alike.
Maggot Therapy in Kenya
The introduction of a maggot therapy treatment programme in a new jurisdiction requires the establishment of medicinal maggot production facilities and capabilities among the local workforce. This chapter presents the activities and outcomes of a programme that sought to introduce maggot therapy in the Kenyan healthcare system. Transfer of the necessary technology and know-how to the Kenyan setting was achieved via a partnership between Scientica Ltd, the Institute of Zoology at the Slovak Academy of Sciences, and the Kenyan Agriculture & Research Institute (KARI) with financial support from SlovakAid, and diplomatic support from the Ministry of Foreign and European Affairs of the Slovak Republic. Activities included building a laboratory for the production of medicinal maggots and the training of medical and veterinary staff in the clinical aspects and use of maggot therapy. Because maggot therapy had been a new therapy to the Kenyan health system, it was also necessary to demonstrate its benefit via a clinical study. This is why the initial establishment of medicinal maggot production capacity and the training of wound care practitioners was followed by a pilot study at Kenyatta National Hospital which is also described below. The chapter concludes with an update on the current status of maggot therapy in Kenya and some reflections on the Kenyan experience.
Maggot Therapy Technology Transfer and Training
Pre-project assessment of healthcare provider acceptance. In the first instance, it had to be established whether the local healthcare workforce involved in wound care would be accepting of maggot therapy because the buy-in of healthcare providers is critical to the successful introduction of maggot therapy. The Kenyan partners conducted a cross-sectional survey of staff from Kenyatta National Hospital that are involved in wound care. The survey did not find any deep-seated rejection of maggot therapy among the workforce and any concerns raised could be easily addressed with an education and sensitisation programme.
Activities carried out by the Slovak partners during the first half-year of the project, from December 2010 to the end of May 2011, were intended to create conditions for the effective launch of the project. A tender was issued for the supply of instrumentation and other supplies. A draft plan was developed for reconstruction work, which was subsequently discussed in detail with KARI and implementation work begun for breeding and laboratory facilities. Detailed technical specifications of breeding technology were developed, the manufacturer was selected and the first insect-rearing cage built, which was tested at the Institute of Zoology in Bratislava. After some adjustments, the remaining cages were built. In May 2011, the Slovak team completed the first mission to KARI. The trip focussed on the monitoring and assessment of the construction and refurbishment of the laboratory and insectary facilities, evaluation of activities, and the planning of activities for the next period including the required laboratory furniture, appliances, and equipment.
An important aspect of the project was joint awareness raising and stakeholder engagement with KARI Director Dr Ephraim A. Mukusira via publicity, workshops, and project presentations:
- Meeting with the Kenyan State Secretary of the Ministry of Livestock Development, Kennet M. Lusaka EBS.
- Preparation of a training programme for technical staff of KARI.
- Visits to healthcare facilities that were identified as likely early adopters of maggot therapy.
- Project presentation at a business lunch with the Ambassador of the Slovak Republic, Milan Zachar, in Nairobi.
- Interviews with local media representatives concerning the objectives of the project and its potential contribution to society in Kenya.
Project activities during the second six-month period, from June 2011 to December 2011, focussed on finalising the refurbishment works at KARI to create facilities for medicinal maggot production and related research activities. In this period, the Slovak team also finalised the standard operating procedures for the production of L. sericata medicinal maggots and other species that may be utilised for maggot therapy purposes. Finally, the Slovak team coordinated the shipment of purchased insectary and laboratory equipment, furniture, and supplies. Unfortunately, late delivery of the shipping container to Bratislava postponed shipment of equipment by 3 months.
Activities implemented during the six-month period from December 2011 until the end of May 2012 focussed on fine-tuning of the laboratory and insectary refurbishment at KARI (Figure 15.1). This included the shipment of laboratory equipment, furniture, and supplies to Mombasa and then on to KARI. The release of the laboratory equipment was delayed a further six months due to bureaucratic processes regarding tax exemption for imported donated goods. We continuously informed the project manager of Slovak Aid about this unforeseen delay. The installation of equipment and furniture was finally carried out in May 2012.
In addition to the technology transfer, it was necessary to ensure that Kenyan laboratory technicians and wound care providers were trained in the rearing of medicinal flies and the treatment of wounds with maggot therapy. To that end, two technicians (Phoebe Mukiria and Bernard Wanyoyi) were trained in the mass rearing of the greenbottle blowfly L. sericata and medicinal maggot production at Scientica Ltd in Bratislava. The Kenyan clinician Dr Saratian Nyabera Lugia, MD (Chairman of the Diagnostics Division and Head of Accident and Emergency at Moi Teaching and Referral Hospital) also travelled to Slovakia for maggot therapy training at the First Department of Surgery of the Faculty of Medicine in Bratislava, and at the hospital of Čadca and a hospital in the town of Liptovský Mikuláš.
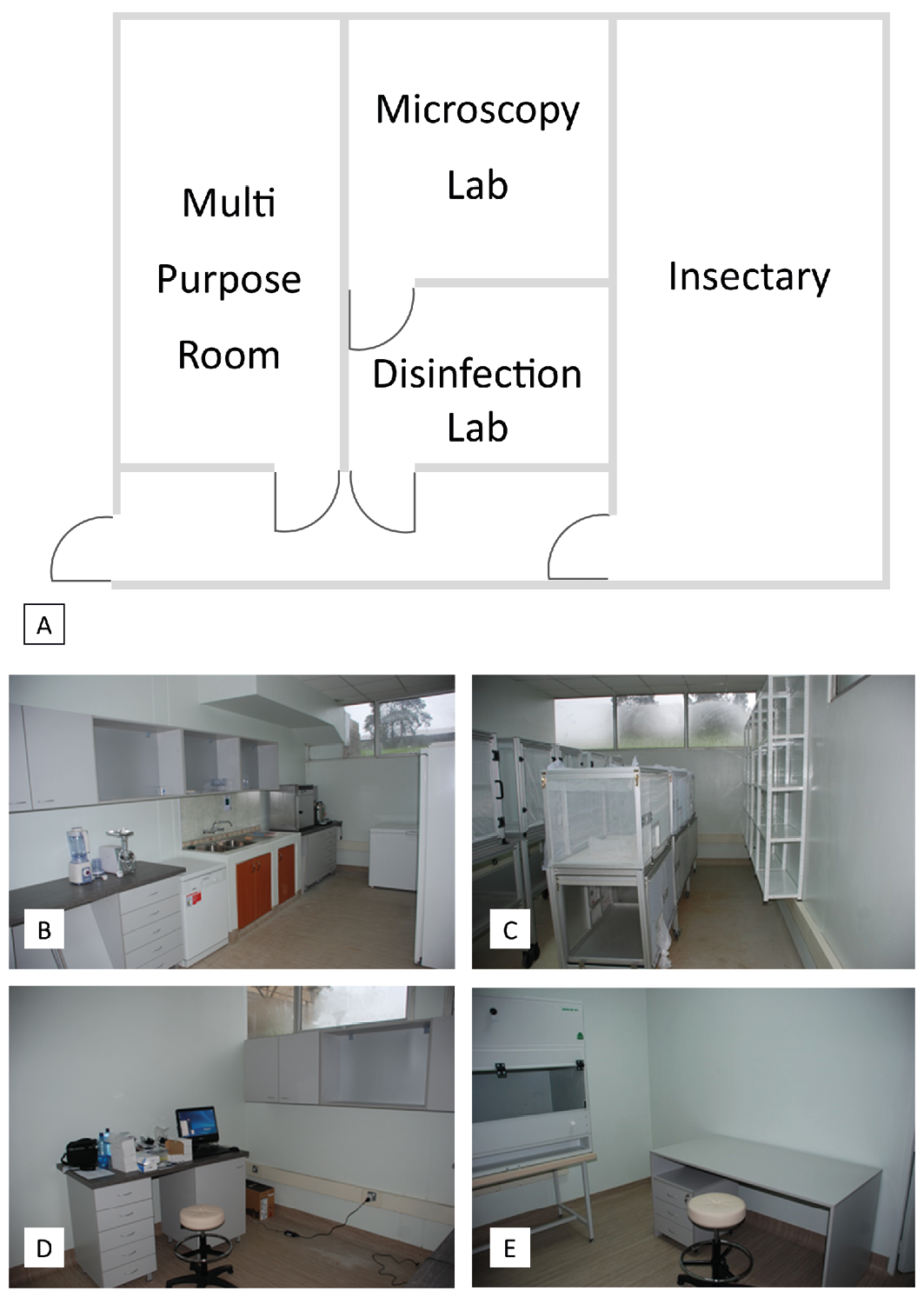
Figure 15.1 Medicinal maggot production and research laboratory facilities established at KARI (now KALRO). A) Plan drawing of the facility layout. An under-utilised building was refurbished to house B) a multipurpose room for washing up, diet preparation, and cool storage of dietary ingredients, C) an insectary, D) a microscopy research laboratory, and E) a dedicated room furnished with a laminar flow cabinet for the disinfection of fly eggs, and the preparation and packaging of medicinal maggots. Photos by P. Takáč, Scientica Ltd and Slovak Academy of Sciences, CC BY-NC.
Activities implemented during the six-month period from June to November 2012 focussed on the successful introduction of maggot therapy to human and veterinary clinical practice in Kenya. Marek Čambal MD, PhD from the First Surgical Clinic of the University Hospital, Comenius University of Bratislava, visited Kenya in July 2012 and trained medical doctors and nursing staff. In addition to these activities, we organised a public lecture at the hospital on the topic of “Maggot debridement therapy—A modality for chronic wound treatment”. There was great interest in the lecture, which was attended by more than 70 doctors and medical staff.
The most important result of this project was the Kenyatta National Hospital (KNH) research and ethics approval for a clinical study entitled “Maggot therapy as a method of treatment of chronic non-healing wounds”. The Slovak and KARI project teams partnered with Dr A Wanjeri and Christopher Kibiwott to pilot maggot therapy at KNH. The ethics and research approval also authorised the KNH group to administer maggot therapy anywhere in Kenya.
Slovak technology transfer activities concluded with the dissemination of project outcomes:
- Presentation and exhibition of project outcomes at the University Library in Bratislava (17 October 2012), attended by 100 participants. The event was organised by the Platform of Development NGOs.
- Presentation of project outcomes at the first Slovak Development Forum in Nairobi (19–21 November 2012). This inaugural forum was organised by the Slovak Embassy in Nairobi with the aim of assessing the almost 17-year history of support provided by Slovak non-governmental and not-for-profit organisations, and the aid contributions to Kenya made by the Slovak government over almost 10 years.
First Clinical Study of Maggot Therapy in Kenya
Study Type and Ethical Clearance
On 26 October 2012, the Kenyatta National Hospital, University of Nairobi (UoN) Ethics and Research Committee (KNH-UoN ERC) approved a pilot study entitled “Maggot Debridement Therapy: The Biotherapeutic Method of Healing Chronic Wounds in Kenya”. The pilot study was conducted between August and December 2013 at KNH. 24 patients were treated with a total of 30 maggot applications. Repeat applications were necessary for some wounds due to the amount of necrotic tissue present. Maggot therapy was carried out by two nurses headed by Dr Wanjeri, a plastic surgeon, and Christopher Kibiwott, a senior nurse.
The Study Site Selection
Two hospitals, Kenyatta National Referral Hospital in Nairobi and Tenwek Mission Hospital in Bomet, were initially selected as study sites due to their high numbers of wound patients. Subsequently, the study was conducted solely at KNH for ease of access and availability of patients. Negotiating the study across two sites with logistical difficulties in the transport of medicinal maggots to Bomet proved too difficult.
Patient Selection and Inclusion Criteria
24 patients were recruited for the pilot study after assessing their wounds and obtaining signed consent. Patients were drawn from the entire in-patient population at KNH. Criteria for selection included the presence of one or more infected wounds that had been debrided more than once without success. Patients and their wounds were excluded from the study for the following reasons:
- Wounds exhibited granulation tissue without necrosis
- Wounds involved a major blood vessel
- Wounds were covered with eschar that required surgical debridement
- Ischemic wounds or presence of arterial insufficiency
- Wounds with significant Pseudomonas aeruginosa infection
- Osteomyelitis
- Patients had severe life-threatening infections
- Patients had an allergy to egg yolk (a component of the maggot-rearing process)
- Patients who had had surgery in the previous 24 to 48 hours
- Patients who refused the therapy
Medicinal Maggot Preparation
Adult L. sericata flies were maintained in the insectary at KARI, Muguga, at 26±2℃, 40–50% relative humidity, and 12 hours of daylight. Newly eclosed flies started producing eggs after 7–10 days. Eggs were collected with an oviposition bait made of minced bovine liver and wheat bran that was offered to flies for two hours. The bait was covered with a plastic container fitted with holes to make a darkened oviposition chamber while providing flies with access. Egg masses were placed into 10 mL Falcon® tubes and disinfected with 1% sodium hypochlorite solution. The number of eggs disinfected depended on the number of patients to be treated. The disinfected eggs were then incubated overnight at 27±2℃ for larvae to emerge and grow sufficiently for medical application. The larvae were washed and packed aseptically into appropriate-size biobags or directly into plastic containers for free-range application. The medicinal maggots were then delivered to KNH.
Maggot Therapy Treatment
A total of 24 patients between 21 and 78 years old with a mean age of 32 years were recruited to the study from the KNH in-patient cohort. Of these, 12 were male and eight females. The aetiologies of the wounds treated included road traffic accidents (31%) including degloving injuries, pressure ulcers, diabetic foot ulcers (42%), fractures, arteriovenous insufficiency, and burns. Biobags were used on only 6 (25%) patients who then needed a repeat treatment with free-range application of maggots. 23 patients completed maggot therapy treatment and one participant chose not to continue after the first application of medicinal maggots. Aseptic technique was strictly observed and the usual wound management protocols followed. The wounds were rinsed with sterile saline and excess moisture was removed with gauze. Where eschar was present, incisions were made to make it easier for maggots to access the necrotic tissue. Maggot therapy was performed using biobags that contained maggots, or free-range (loose) maggots directly into the wound. The mode chosen depended on the patients’ preference and the size and extent of the wound. After placement of maggots the wounds were covered lightly with a gauze bandage and left for 48 hours. Thereafter, the wound was assessed.
Study Outcome
In 16 patients (67%), complete debridement was achieved with only one application of maggots. In seven patients, maggot therapy achieved 70% debridement after the first application and complete debridement after the second application. The wound of the patient who decided to discontinue the therapy after 12 hours was 50% debrided after that short time. The wounds of 18 patients (75%) received regular care after maggot therapy and healed uneventfully without a need for any other intervention. The remaining patients had to undergo procedures such as flap closure and skin grafting. In all patients, maggot therapy successfully controlled infection without additional antibiotic therapy. Case examples are presented in Figure 15.2.
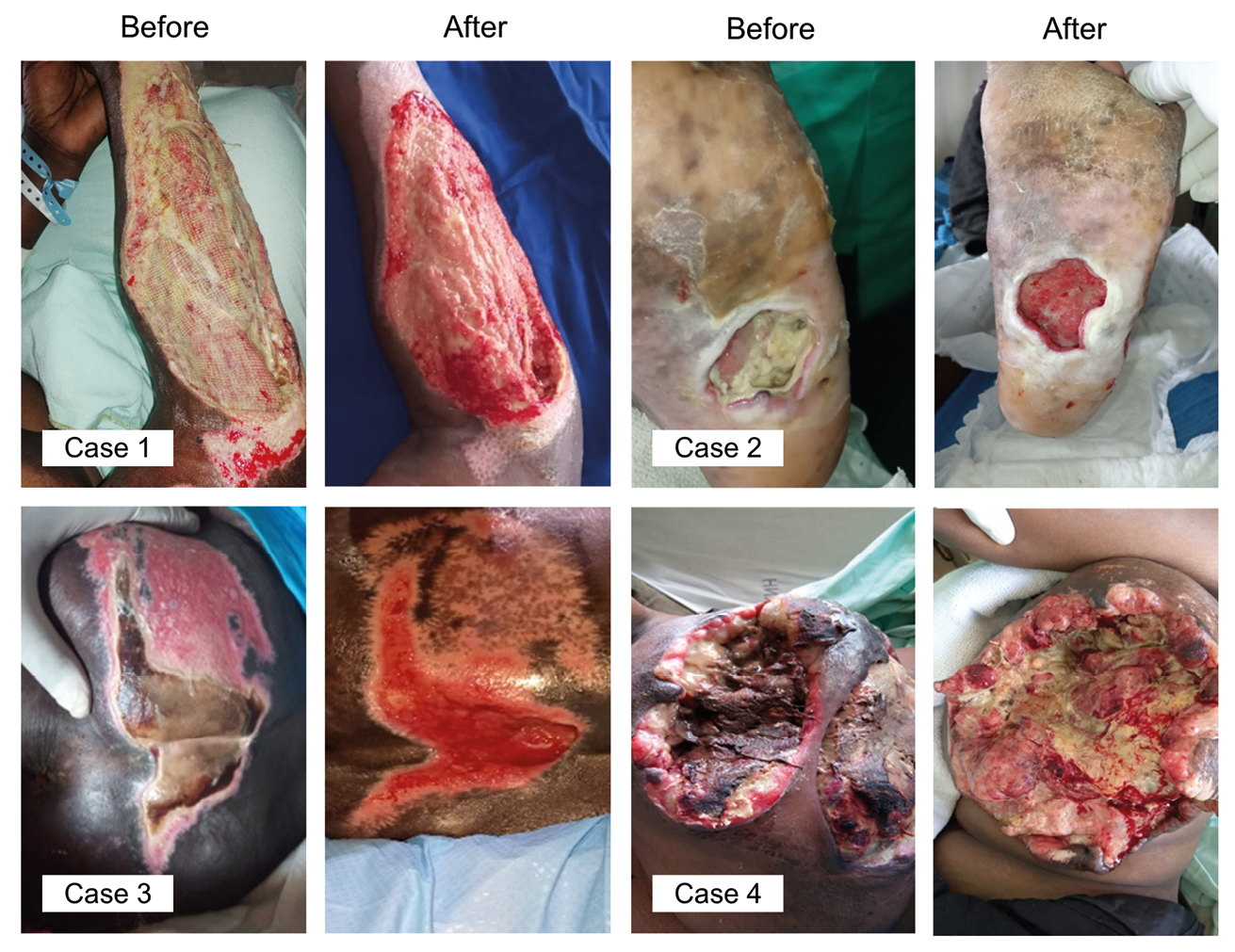
Figure 15.2 Examples of successful maggot therapy treatments during the pilot study. Case 1) Female, age 38, retrovirus disease under treatment, degloving injury of the head and right arm, road traffic accident. History: One day post-admission she was taken to theatre for surgical debridement and closure of the head wound. Due to its size, it was not closed. The degloving injury on the right hand was not debrided. Wound: 15 cm x 8 cm, 80% necrotic tissue with moderate infection. Maggot therapy: 1 treatment, 48 hours. Result: 100% debridement, patient was scheduled for skin grafting the following week, wound fully closed within ten days. Case 2) Female, age 55, diabetic foot ulcer on sole of the left foot. History: Surgical debridement was not successful, patient was scheduled for below knee amputation, antibiotic treatment included Augmentin, Cefuroxime and Meropenem. Wound: 5 cm x 6cm, 4 cm deep, communicating from the small toe to the mid-foot area, edematous to the level of the knee, exudating and filled with slough, odorous, osteomylitis. Maggot therapy: 50% debridement after 1st and 100% after 2nd treatment, no further antibiotic treatment. Discharged one week later with much-improved wound. Case 3) Male, age 72, sacral pressure ulcer. History: Diabetic patient, high blood pressure, prostate cancer. Wound: Stage 3, septic, exudating purulent discharge, slough, odorous. Maggot therapy: 1 treatment, 95% debridement, followed up with negative pressure therapy and conventional dressings. Case 4) Female, age 42, ulcerated tumour on left breast. History: No co-morbidities. Wound: Very large mass, ulcerated, necrotic, filled with thick sloughy tissue, bled easily, odorous. Maggot therapy: 40% debridement after 1st treatment, 80% debridement after 2nd treatment. Antibiotic treatment: Ceftriaxone and metronidazole injection. Photos © Kenyatta National Hospital, Nairobi.
Current Status of Maggot Therapy
Medicinal maggot production capacity. In 2013, KARI was renamed the Kenya Agricultural and Livestock Research Organization. The KALRO insectary houses on average only 3,000 to 4,000 adult flies at any one time. Due to a lack of demand, there is not daily medicinal maggot production, and production occurs only when orders are received. It takes between two to three days to prepare an order depending on the time and date of order placement. However, the facility has the capacity (including equipment, materials, and personnel) to maintain more fly colonies and to produce medicinal maggots on a regular basis.
Nevertheless, strict adherence to established standard operating procedures has led to a shortage of egg yolk powder for medicinal maggot production. However, this does not seriously jeopardise production because egg yolk powder can easily be substituted with fresh poultry egg yolk and albumin. Larger production volumes may lead to stock-outs of net fabric for the construction of maggot confinement and containment dressings, as it has to be sourced from overseas. It, too, is not essential for maggot therapy. Free-range treatment using alternative retention systems could proceed while the netting is sourced. For example, free guidance on maggot therapy in compromised healthcare settings can be accessed via www.medmaglabs.com, which explains the use of ordinary clothing items to construct maggot confinement dressings, including two step-by-step videos describing free-range maggot therapy.
Medicinal maggot supply. KALRO supplies medicinal maggots confined in a biobag or loose maggots in a container for free-range application. The biobag is packaged in a sterile plastic container with a perforated lid for ventilation. The packaged medicinal maggots are placed in a cool box with ice packs and transported immediately to the hospital. The delivery is done through the informal transport system. Delivery to Nairobi-based hospitals or homecare patients is relatively fast but timely and affordable delivery to other parts of Kenya is a challenge.
Demand for medicinal maggots and maggot therapy. Although there is a growing interest in maggot therapy across Kenya, it is still a much under-utilised wound care intervention limited to a few patients. Under current medicinal maggot supply chain arrangements, the reasons for the slow uptake of maggot therapy include i) the outstanding regulatory approval by the Pharmacy and Poisons Board, ii) the high cost of delivery to hospitals outside of Nairobi, and iii) the overall cost of maggot therapy to poor patients. A consignment of medicinal maggots to hospitals or homecare settings around Nairobi costs from USD40 to USD60 for an average treatment. The cost is per application, irrespective of the size of the wound, and is made up of around USD20 for the medicinal maggots and USD20 for delivery [12].
Approval of Maggot Therapy in Kenya. The findings of the pilot study “Maggot Debridement Therapy: The Biotherapeutic Method of Healing Chronic Wounds in Kenya” were presented to the KNH-UoN ERC on 6 August 2014 along with a report. After careful assessment, the KNH-UoN ERC provided a favourable opinion on 20 July 2016, recognising the benefits of maggot therapy for patients with chronic wounds, especially when antibiotics fail. Despite this favourable finding, maggot therapy has not yet been approved by the Kenyan Pharmacy and Poisons Board because there is uncertainty as to whether medicinal maggots are best governed by human therapeutics regulation or livestock production regulation. The original ethics and research approval first granted for the pilot study in 2013 has been renewed on an annual basis to support treatment of patients by the KNH team. This means that as of 2021, seven years after the presentation of their findings, maggot therapy in Kenya is still limited to the KNH team, with a national rollout of the treatment still not possible.
Patients treated and national reach. Between 2013 and 2020, a total of 140 patients were treated by Christopher Kibiwott and Dr Wanjeri at KNH and other hospitals such as Aga Khan Hospital, Texas Cancer Centre, Nyeri Hospital, Oyugis District Hospital in Homa Bay County (about 400 km from Nairobi), Kiambu District Hospital, and patients in home care as far afield as Kisumu, Nyahururu, and Machakos.
Summary
Over ten years later, the introduction of maggot therapy to the Kenyan healthcare system is still an ongoing process. The initial technology and knowledge transfer initiative successfully developed production capacity at KARI/KALRO and clinical skills among the surgical and nursing workforce at KNH. The subsequent pilot study was also a success as it convinced the KNH-UoN ERC of the positive impact that mainstreaming of maggot therapy can have on the treatment of patients with chronic and infected wounds. However, medicinal maggot production and the number of patients treated in 2021 is well below capacity. Without full regulatory approval by the Pharmacy and Poisons Board, this is unlikely to change, which means that hundreds or even thousands of patients each year will miss out on efficacious maggot-assisted wound care. Moreover, what has not yet been addressed is the development of sustainable and affordable distribution logistics and supply chain management solutions for medicinal maggot therapy in Kenya. While good things always take time and the groundwork for a thriving maggot therapy programme has been laid, there is the danger that chronic under-utilisation of the production facility and a lack of funding and sales cashflow will erode institutional commitment to the programme and lead to the closure of the insectary and laboratory.
There are important lessons to be learned. Entrepreneurs and medical professionals wanting to establish a maggot therapy programme in their country or region must invest considerable time and effort to collaborate with and lobby medical regulators to secure approval of maggot therapy. At the same time, it is not enough to establish medicinal maggot production capacity and clinical skills. Medicinal maggots must also reach patients across the country, including in provincial, rural and remote locations, and not only near the production facility. It therefore pays to involve medical supply chain logistics experts and formal as well as informal transport service providers early on. Please refer to Chapter 17 for guidance on distribution logistics [13], and Chapter 18 for drone-assisted distribution of medicinal maggots [14].
References
1. Nigam, Y. and M.R. Wilson, Maggot Debridement, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 143–152, https://doi.org/10.11647/OBP.0300.08.
2. Nigam, Y. and M.R. Wilson, The Antimicrobial Activity of Medicinal Maggots, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 153–174, https://doi.org/10.11647/OBP.0300.09.
3. Nigam, Y. and M.R. Wilson, Maggot-assisted Wound Healing, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 175–194, https://doi.org/10.11647/OBP.0300.10.
4. Sherman, R., Indications, Contraindications, Interactions, and Side-effects of Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 63–78, https://doi.org/10.11647/OBP.0300.04.
5. Sherman, R., Medicinal Maggot Application and Maggot Therapy Dressing Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 79–96, https://doi.org/10.11647/OBP.0300.05.
6. Masiero, F.S., et al., Histological Patterns in Healing Chronic Wounds Using Cochliomyia macellaria (Diptera: Calliphoridae) Larvae and Other Therapeutic Measures. Parasitology Research, 2015. 114(8): pp. 2865–2872, https://doi.org/10.1007/s00436-015-4487-y.
7. Dallavecchia, D.L., R.G. da Silva Filho, and V.M. Aguiar, Sterilization of Chrysomya putoria (Insecta: Diptera: Calliphoridae) Eggs for Use in Biotherapy. Journal of Insect Science (Online), 2014. 14, https://doi.org/10.1093/jisesa/ieu022.
8. Diaz-Roa, A., et al., Evaluating Sarconesiopsis magellanica Blowfly-derived Larval Therapy and Comparing It to Lucilia sericata-derived Therapy in an Animal Model. Acta Tropica, 2016. 154: pp. 34–41, https://doi.org/10.1016/j.actatropica.2015.10.024.
9. Sherman, R.A. and E.A. Pechter, Maggot Therapy: A Review of the Therapeutic Applications of Fly Larvae in Human Medicine, Especially for Treating Osteomyelitis. Medical and Veterinary Entomology, 1988. 2(3): pp. 225–230.
10. Sherman, R.A., Maggot Therapy for Foot and Leg Wounds. International Journal of Lower Extremity Wounds, 2002. 1(2): pp. 135–142, https://doi.org/10.1177/1534734602001002009.
11. Čičková, H., et al., Growth and Survival of Bagged Lucilia sericata Maggots in Wounds of Patients Undergoing Maggot Debridement therapy. Evidence-based Complementary and Alternative Medicine, 2013. 29(4): pp. 416–424, https://doi.org/10.1155/2013/192149.
12. Stadler, F., Supply Chain Management for Maggot Debridement Therapy in Compromised Healthcare Settings. 2018. Unpublished doctoral dissertation, Griffith University, Queensland, https://doi.org/10.25904/1912/3170.
13. Stadler, F., Distribution Logistics, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 363–382, https://doi.org/10.11647/OBP.0300.17.
14. Stadler, F. and P. Tatham, Drone-assisted Medicinal Maggot Distribution in Compromised Healthcare Settings, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 383–402, https://doi.org/10.11647/OBP.0300.18.
1 This work was funded by the Operational Program of Research and Development and co-financed by the European Fund for Regional Development (EFRD). Grant: ITMS 26240220030: Research and development of new biotherapeutic methods and its application in the treatment of some illnesses; and by Slovak Aid, Grant: SAMRS/2010/03/06; The introduction of sterile larval therapy into clinical practice for human and veterinary medicine in the Republic of Kenya.
