5. Medicinal Maggot Application and Maggot Therapy Dressing Technology
© 2022 Ronald A. Sherman, CC BY-NC 4.0 https://doi.org/10.11647/OBP.0300.05
Maggot therapy dressings are intended to keep maggots on the wound during treatment. Some therapists make their own dressings, others use commercially produced dressings, and many use both, depending on their patients’ wounds. There are two basic designs for maggot dressings. Maggot confinement dressings confine the maggots to the wound bed and allow them complete access to the wound, while maggot containment dressings totally contain the maggots within a net bag that facilitates easy handling but does not allow full access to the wound. This chapter describes the basic principles and goals of the ideal maggot dressing, and provides examples of how that ideal dressing can be achieved.
Introduction
The first question most people ask about maggot therapy is: “How do you get the maggots off?” In fact, removing the maggots is not difficult because the species employed for maggot therapy are “self-extracting”—their instinctive behaviour is to leave the host as soon as they are satiated or as soon as there is no more nutritious food (necrotic tissue or exudates) left in the wound. Since some of the maggots will become satiated earlier than others, the real problem is how to keep the maggots corralled in one spot until the therapist is ready to remove them all. The solution is the maggot therapy dressing.
There is no one single correct or best maggot dressing. Several techniques exist, each with their advantages and disadvantages. Several different commercial dressings are available, but many therapists fashion their own dressings at the bedside. This chapter will describe the basic principles or goals of the ideal maggot dressing, and then provide examples of how that ideal dressing can be achieved.
Maggot Therapy Dressings—Past and Present
The minimal requirements for a maggot dressing are that it be of a porous fabric that allows air to enter and fluid to drain out. This is to prevent the maggots from suffocating or drowning. Also, the dressing should be constructed in such a way that it keeps the maggots from wandering off the wound. Ideally, the dressing should also be comfortable, affordable, and simple to apply, maintain, and remove.
When maggot therapy was commonly used for osteomyelitis and pus-forming wounds in the 1930s, dressings were frequently constructed out of metal screens and/or cloth. Loose-knit gauze does not make an effective barrier because the larvae can easily escape through the large spaces between the woven fibres. The dressing was usually kept in place with an adhesive tape (plaster) and sometimes foam padding was placed between the skin and the maggot dressing [1]. Some dressings were even made to be re-used for repeat applications of maggots to the same wound, with a port to put the young maggots in and take the satiated maggots out.
During the 1990s renaissance of maggot therapy, there was a push to construct maggot dressings from materials readily available on a medical ward [2]. Additionally, many of the patients now receiving therapy are old and frail and have thin sensitive skin prone to tearing. Therefore, efforts were made to identify materials less traumatic to the peri-wound skin, which led to the use of hydrocolloid pads as foundations to which the maggot dressings were then affixed [3]. This and related dressing designs are still commonly used today. Because these dressings confine the maggots to the wound but still provide them with free access to the wound bed and all of its nooks and crannies, they are sometimes called “free-range” or “confined” maggot dressings (Figures 5.1A and 5.1B).
By 2000, Wilhelm Fleischmann developed and patented the concept of containing the maggots within a net bag, which could then be placed over the wound bed without the need for strong tapes or adhesives [4]. Contained or bagged maggots (also sometimes called “tea-bag maggots”) imbibe the liquefied necrotic tissue and wound exudates that enter the net bag, but the maggots cannot leave the bag (Figure 5.1C). Differences between these two dressing designs are described in Tables 5.1 and 5.2.
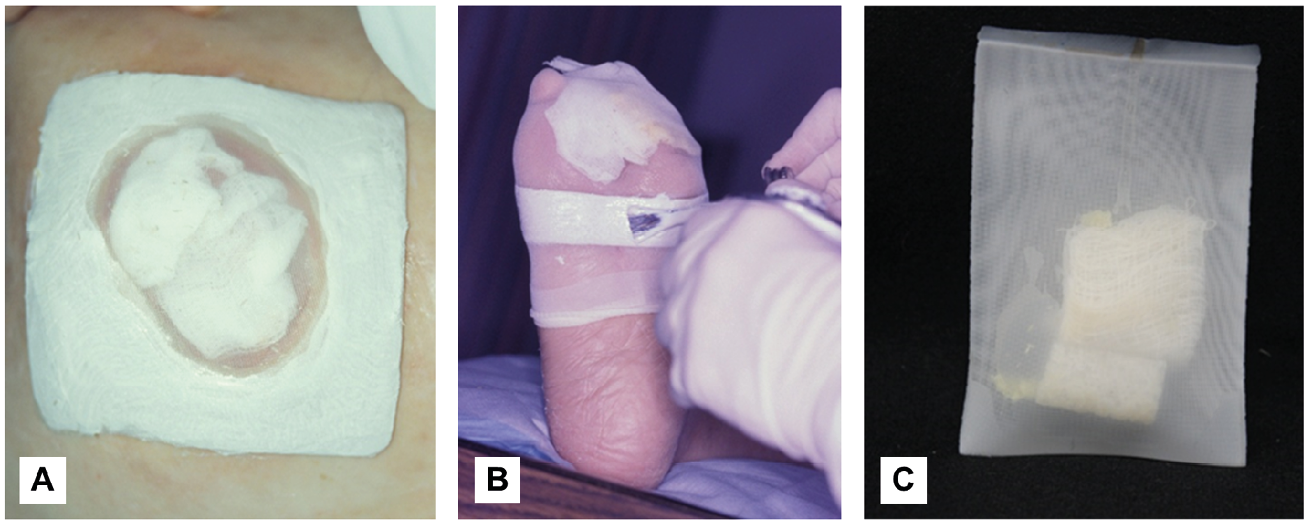
Figure 5.1 Three types of maggot dressings. A) Polyester net fabric was glued to a hydrocolloid pad, the centre of which was cut out to match the wound margins before placing maggot-impregnated gauze over the wound bed. B) Here, a strip of hydrocolloid was placed all around the anterior foot, just proximal to the non-healing toe amputation stump wound. After placing maggot-impregnated gauze over the wound, a nylon stocking (net) was pulled over the anterior foot and glued over the hydrocolloid strip. After covering the adhesive border with water-resistant tape, the excess nylon stocking will be cut off. Many therapists now use only water-resistant tape, not liquid adhesive, to hold the net in place. (Pictures by R.A. Sherman, courtesy of the BioTherapeutics, Education & Research Foundation). C) Maggots are contained within a netted bag. Photos by R. Sherman, Monarch Labs, CC BY.
Table 5.1 Characteristics of confinement and containment dressings.
|
Characteristic |
Confinement Dressing |
Containment Dressing |
|
Efficacy |
Able to access and more efficiently debride undermined areas, sinus tracks, and other crevices |
Valuable for wounds near eyes, mouth, or other sensitive sites where it is imperative to avoid escapes |
|
Debridement Efficiency/Dressing wear-time |
Fast, 48–72 hours |
Slower, 96 hours (4 days) |
|
Wound pain |
Most analyses are in patients with confinement dressings; pain occurring in 5%-30%. In the few comparative reports, there has been no significant difference in the frequency or severity of pain between confinement and containment dressings [5–8]. |
|
|
Escaping maggots |
Inexperienced therapists report more escapes from confinement dressings. Experienced therapists report no more escapes from confinement dressings than from containment dressings [7]. |
|
|
Aesthetics |
Less acceptable |
More acceptable |
|
Cost |
Maggots are less costly to produce, but additional dressing supplies may add cost |
Contained maggots are more costly to produce, but few other dressings are required beyond a gauze wrap |
Table 5.2 Advantages and disadvantages associated with maggot confinement dressings compared to containment dressings.
|
Confined Maggots |
Contained Maggots |
|
|
Advantages |
|
|
|
Disadvantages |
|
|
Applying Maggot Dressings
The method of dressing application depends on several factors, including the type and location of the wound, the type of dressings to be used (i.e., confinement or containment dressings), and the availability of supplies. Here are descriptions of some common dressing methods, followed by increasingly more complex methods, each intended to address a typical problem or complication.
In its simplest iteration, the maggot dressing might be constructed with a simple breathable or net fabric covering a maggot-laden wound (Figures 5.2, 5.3 and 5.4). The net can be affixed to the peri-wound skin with water-resistant tape. When available, a polyester net fabric is very durable, and provides a known pore size, which is optimally 100 um–160 um: small enough that the larvae cannot escape, but large enough to allow the thick, purulent drainage to drain easily. A fixed-weave fabric prevents these pores from expanding. Other fabrics with similar pore sizes are often described as having a mesh size of approximately 80 to 140. If using a stretchable fabric, the pore size may need to be within the smaller range to prevent the maggots from squeezing through and escaping. If such fabrics are not available, one could use appropriate items of clothing such as a T-shirt, blouse, or shirt (Figure 5.4). But beware: if the pores are too small, the thick purulent drainage that accumulates during therapy may not be able to exit through the fabric. This could lead to a fluid build-up and drowning of the maggots, or it could block the pores, which in turn would suffocate the maggots.
The optimal dose of maggots is considered to be 5–10 per cm2 (the upper range is used for wounds with more necrotic or infected tissue). Medicinal larvae are supplied in primary packaging containers, with or without gauze [9]. Larvae in tubes can be rinsed out with sterile saline or clean water and poured onto a piece of gauze or directly onto the polyester net fabric, which is then inverted and placed over the wound. When using maggot-impregnated gauze, it is not necessary to count individual larvae if the gauze is labelled with the concentration of maggots. Simply apply to the wound bed the amount of gauze that contains the approximate number of maggots needed.
Since the maggots will liquefy the necrotic tissue, one should expect a fair amount of wound drainage. To prevent that drainage from settling on healthy tissue as it flows out of the wound and through the net, the net should be covered with an absorptive material such as cotton gauze. The absorptive gauze should not be too thick or else it could obstruct airflow to the maggots. Wet gauze is not permeable to oxygen, so the absorptive dressings should be changed when soiled. This dressing design is sometimes called a “two-layer” maggot dressing: the “cage layer” on the bottom, and the “absorptive layer” on top.
In order to minimise the risk of peri-wound skin becoming macerated by the drainage, the peri-wound skin can be coated with a skin protectant (liquid, fast-drying). Some therapists coat the peri-wound skin with zinc oxide, being careful to avoid applying it in areas where the adhesive will need to stick to the skin.
Patients with skin integrity problems (i.e., elderly or malnourished patients) may develop skin tears when removing strongly adhered tape (less tacky tapes do not hold the maggot cage layer securely enough to prevent maggots from escaping). Therefore, many therapists do not tape the net directly to the skin. Rather, they first place strips of hydrocolloid, hydrogel, or tissue-friendly tape on the skin, and then tape or glue the net to those strips. Alternatively, a hole is cut in a hydrocolloid pad such that the pad now surrounds the wound and completely covers the skin around the wound, such that the larvae are not able to crawl out of the wound and onto the normally innervated skin (Figure 5.1A). This will prevent the itching, tickling, or pain that sometimes occurs when no such barrier blocks the maggots from accessing healthy skin. Though pre-manufactured maggot therapy confinement dressings can be purchased commercially, they are relatively simple and often less costly to construct at the bedside with locally available materials [2] (see also Figures 5.2, 5.3 and 5.4). While this system works well for flat wounds, the flat fabric does not conform well to circumferential leg wounds or stump and foot wounds. For such three-dimensionally challenging dressings, alternative net fabrics such as net bags (performing a sock or glove function) or nylon stockings (Figure 5.1B) may be used [3], or even clothing items (Figure 5.4). In rare instances, non-permeable dressings may be used [10, 11] as long as a source of fresh oxygen can be passively or actively circulated through the dressing.
Since maggots within a containment bag (bagged maggots) do not require additional confinement, they are faster and simpler to apply. The bags of maggots are simply laid over the wound bed and then held in place with gauze wrap or taped gauze pads. The larvae still require plenty of air, and an absorptive dressing layer will need to be changed daily and whenever soiled. In addition, it is recommended that the larvae be provided with water or saline to prevent dehydration, especially during the first day or two. This hydration can be provided by spraying the bags of larvae during the dressing or re-dressing procedure, or by covering them with moist gauze instead of dry gauze [12].
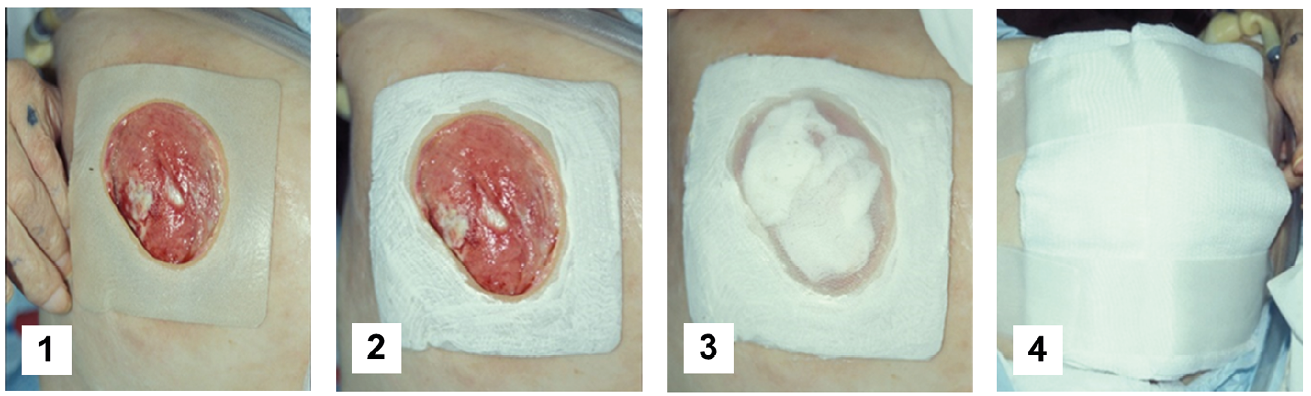
Figure 5.2 How to make a free-range maggot dressing at the bedside using adhesive glue. 1) Place a hydrocolloid pad over the peri-wound skin, with a hole cut out to correspond precisely to the wound perimeter. Alternatively, tape or another simple adhesive could be used to surround the wound. 2) Apply liquid adhesive to the surface of the hydrocolloid pad. 3) As the liquid adhesive sets and becomes tacky, place maggot-impregnated gauze over the wound bed. To prevent escape, apply the net over the wound and hydrocolloid pad immediately thereafter. Apply a second layer of adhesive over the hydrocolloid and net, such that the first and second layers of glue bond through the pores in the net. A layer of water-resistant tape may then be applied over the still sticky hydrocolloid-glue-net-glue “sandwich”, but not on the skin (not shown). 4) Finally, after securing the medicinal maggots within their “cage”, place a layer of absorbent gauze on top to collect the wound drainage (liquefied necrotic tissue and wound exudates). Change the outer gauze dressing whenever it becomes soiled with drainage (about every 8 hours) so that fluid does not leak onto the patient’s skin, and air can continue to reach the maggots through mostly dry gauze. Photos by R. Sherman, BioTherapeutics, Education & Research Foundation, CC BY.
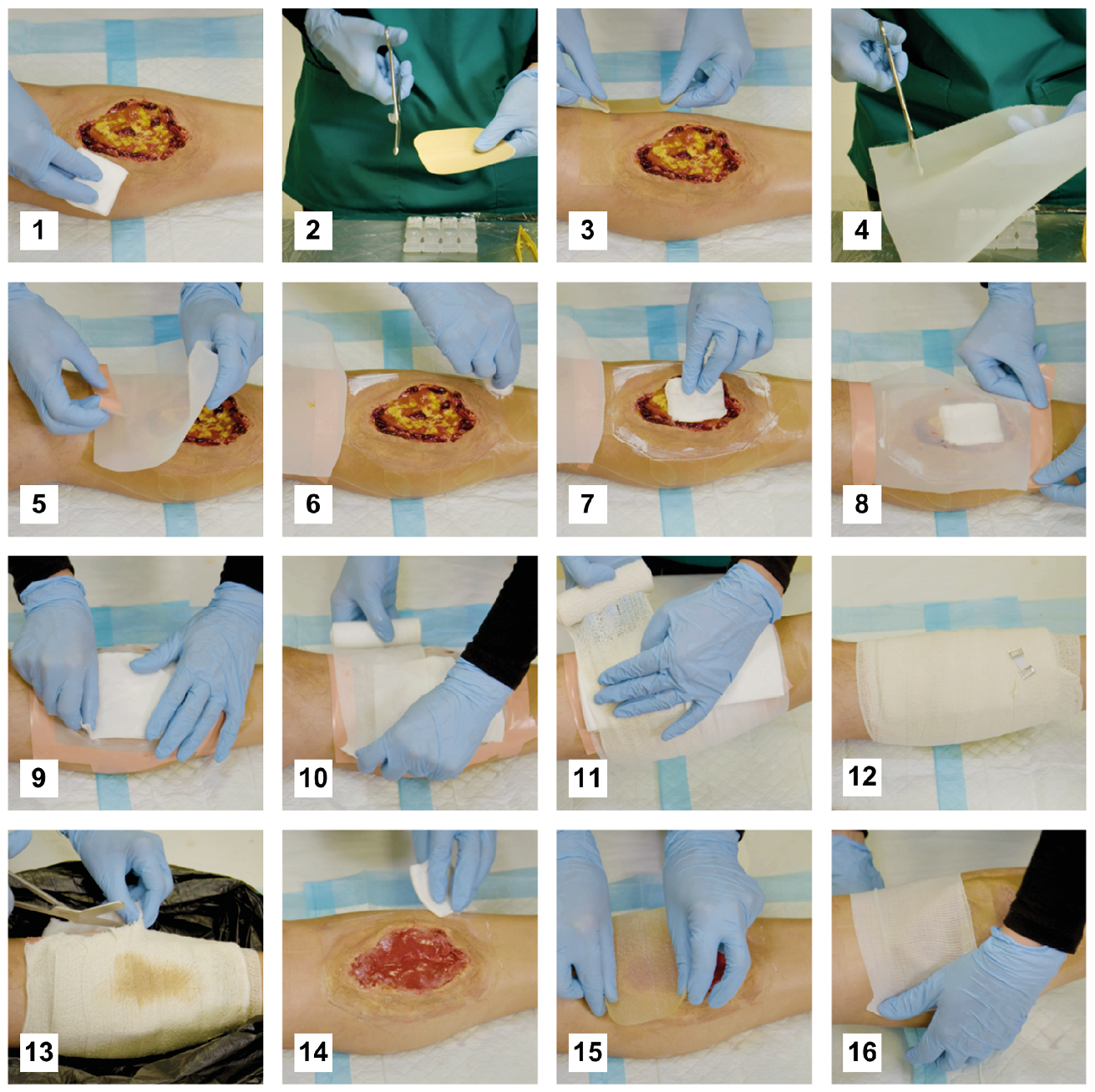
Figure 5.3 Application guidance for a confinement dressing without adhesive glue. 1) Clean the wound and peri-wound area with potable water or saline. 2) Cut hydrocolloid sheets into 2–3 cm-wide strips, perpendicular to the two pieces of plastic film covering the adhesive side of the hydrocolloid. 3) Place hydrocolloid strips around the wound and as close to the wound edge as possible. 4) Cut fine-mesh medical nylon or polyester netting to size. 5) Attach one side of the netting to the hydrocolloid border and flip it out of the way. 6) Apply zinc crème to protect the skin that is not covered by the hydrocolloid. 7) Apply loose medicinal maggots. If the maggots are supplied without a gauze pad, then use some water or saline to wash them out of their primary packaging onto a gauze pad which you apply directly to the wound. 8) Close the netting and secure it on the hydrocolloid strips using water-proof adhesive strips. Alternatively, you can use fast-curing glue to attach the netting to the hydrocolloid. 9) Place a moistened gauze pad on top of the netting. 10) Secure the gauze pad loosely with a bandage. 11) Place dry absorbing gauze pads on the bandage above the wound to absorb any exudate during treatment. Secure them loosely with another bandage. 12) The wound and dressing must be off-loaded during treatment to protect the medicinal maggots. Replace the outer dressings daily or when heavily soiled with exudate. 13) Removal of dressings and maggots is best done over a large, plastic waste bag to easily capture fast-moving maggots, dressing materials and water/saline you may use to rinse the wound. 14) Wash, wipe, suck or pick maggots off the wound. The most convenient method is determined by the wound morphology, the body region and experience of the clinician. Clean the wound and surrounding skin carefully. 15–16) If the wound is free of necrotic tissue, continue regular wound care, or else repeat maggot therapy. Courtesy MedMagLabs and Creating Hope in Conflict: A Humanitarian Grand Challenge, CC BY.
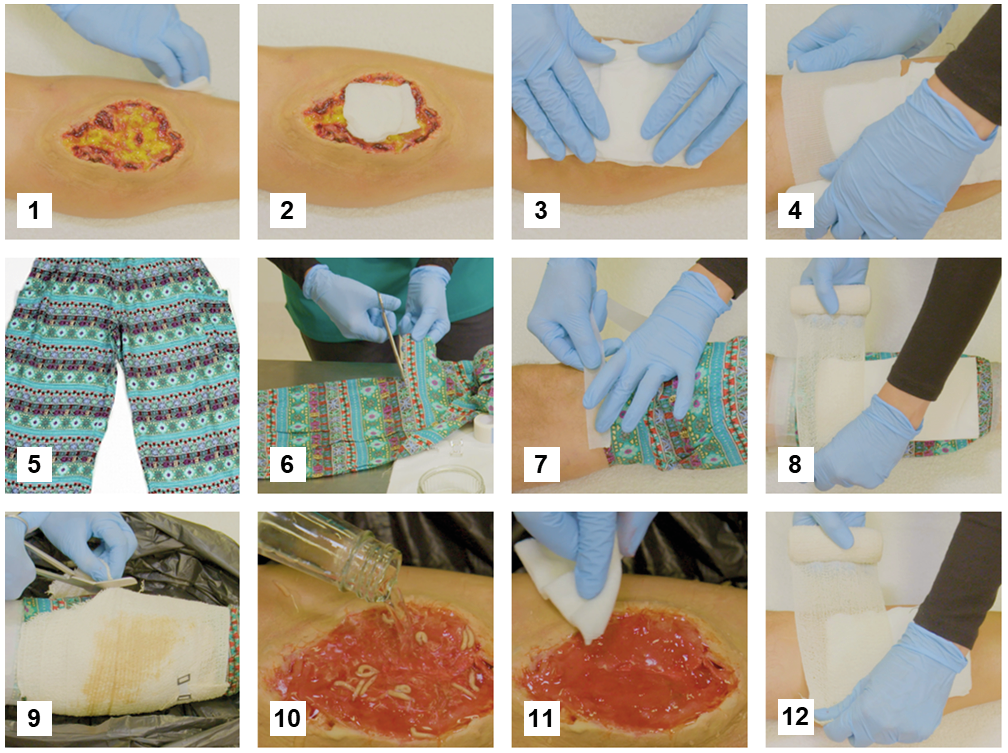
Figure 5.4 Application guidance for a standard confinement dressing in low-resource healthcare settings. 1) Clean the wound and peri-wound area with potable water. You may boil some water for 20 minutes and let it cool before use. 2) Apply loose medicinal maggots. If the maggots are supplied without a gauze pad, you can use some water or saline to wash them out of their primary packaging onto a gauze pad which you apply directly to the wound. Place some larger moistened gauze pads on top of the wound. 4) Secure the gauze pads loosely with a bandage. 5) Use the legs or sleeves of suitable clothing items to confine the maggots on the wound. Make sure the clothing is finely woven to keep maggots in. 6) Cut the leg or sleeve section to size. 7) Tape the fabric tube at the upper and lower end to the leg, making sure there are no gaps for maggots to escape. 8) Place dry absorbing gauze pads on the confinement fabric above the wound to absorb any exudate during treatment and secure them loosely with another bandage. The wound and dressing must be off-loaded during treatment to protect the medicinal maggots. Replace the outer dressing daily or when heavily soiled with exudate. 9) Removal of dressings and maggots is best done over a large, plastic waste bag to easily capture fast-moving maggots, dressing materials, and water/saline that you may use to rinse the wound. 10) Wash, wipe, or pick maggots off the wound. The most convenient method is determined by the wound morphology, the body region and experience of the clinician. 11) Clean the wound and surrounding skin carefully. 12) If the wound is free of necrotic tissue, continue regular wound care, or else repeat maggot therapy. Courtesy MedMagLabs and Creating Hope in Conflict: A Humanitarian Grand Challenge, CC BY.
Dressing Changes, Maintenance & Repair
Guidance for optimal dressing maintenance should always be sought in the package insert. In general, the goal of maintenance is to ensure that the maggots are healthy, active, and contained or confined to the wound. To that end, many authorities recommend dressing inspection at least once daily, though that inspection need not be done by a health professional as long as the observer knows what s/he is looking for.
If there is a lot of drainage or soiling of the outer absorbent gauze dressing, it should be changed in order to optimise aeration of the maggot dressing and minimise the accumulation or spread of fluids and microorganisms. It is not uncommon for wound exudate to increase during maggot therapy and require 2–6 changes or more per day of the outer gauze dressings. If there is a lot of drainage, the fresh gauze dressings may be reapplied dry. If there was not a lot of drainage—say only one or two dressing changes are required per day—the fresh outer gauze should be moist, in order to keep the maggots hydrated. The outer gauze dressing should be changed at least once daily.
When the outer absorbent dressing is changed, also inspect the netted dressings for signs of loosening. Loosened borders can allow the maggots to escape on their own. Reinforce the dressing with extra tape, or extend the border with transparent membrane dressing (such as negative pressure dressing waste). If a hole in the dressing is discovered and maggots are not seen—especially in a dressing over 48 hours old—assume that the maggots have escaped. Open the net dressings to check. If maggots are found, and if they appear not to be satiated (not full size), then the dressing can be repaired with tape or a new net, and left for another day or two longer.
At the time of the dressing change, check on the status of the maggots. If the dressing is only 24 hours old or less, the larvae may not be visible if they are feeding down on the wound bed. If the dressing is 48 hours old, however, then it should be possible to see movement (undulations) of the maggot dressings, if not the maggots themselves. If no movement or live maggots are visible between 48 and 72 hours, the maggots may have escaped or they may be dead. Look for a break in the netting and repair it, as described in detail, above. If the maggots are found to be dead, take down the dressing and dispose of the maggots, as described in the next section. Leaving non-viable maggots in place serves no benefit and may even increase risks. For example, wound infection may worsen under a dressing of dead maggots. If the dressing is opened and the larvae are found to be alive and healthy, then the dressing can be re-mounted, if desired.
Dressing Duration and Removal
Free-range (confined) maggots mature more quickly than bagged (contained) maggots. Over 50% of free-range maggots will likely be satiated and ready to leave the wound by 48 hours. At that point, they will be near the surface and margins of the maggot dressing, looking for a way out. Many therapists remove the dressings at that point, because leaving them in place for longer increases the risk of pain and escapes. Once satiated, the larvae will spend their time attempting to escape from under the dressings until they are finally removed. In patients without wound pain, some therapists chose to leave the maggot dressings in place for up to 72 hours so that the remaining maggots will provide additional debridement. After 72 hours, all of the free-range maggots should have matured, and will be at the surface trying to escape.
When it is time to remove free-range maggots, remember that the larvae are already lined up at the edges of the dressing, ready and eager to crawl far away. Therefore, have all needed materials at the bedside, including wet gauze to wipe up the larvae, and a receptacle for discarding the larvae. Place a barrier (rubbish bag, incontinence pad, etc.) under the work area (wound) in order to catch maggots that may fall off. Remove the outer absorbent gauze and then gently peel back the netted dressing as though it were a banana peel. Meanwhile, wipe the wound with a water- or saline-moistened gauze pad, closely following the peeled net dressing, sandwiching the maggots between the wet pad and the dressing that is being removed. Then drop the dressing and sandwiched maggots into a biohazardous waste bin. If there are any maggots left behind, they can be removed with the aid of another wet gauze pad, a swab or water irrigation. If the remaining maggots are small and appear to be still working to remove more necrotic tissue, then it would be reasonable to replace a gauze pad over the wound and allow the last few maggots to continue working for another day. The gauze dressing can be removed the following day, by which time the last remaining maggots should be satiated and ready to leave the wound. Thoroughly rinse the wound with sterile water or saline once all of the maggots are removed.
Contained maggots grow more slowly, and typically are not satiated until about 96 hours. Since they are contained, the risk of escape is very low; but the risk of pain still increases with the duration of therapy [5]. There is no benefit to leaving the maggots in place longer than the time that they are feeding, since they will stop secreting their digestive enzymes when they are satiated. Contained maggots are easy to remove because they are not running loose. Simply unwrap the outer absorbent gauze wraps and then remove each sachet of bagged maggots, placing them in a biohazard bag. Thoroughly rinse the wound with sterile water or saline.
When the dressings are opened, if the larvae are found to be dead—or not found at all—pay close attention to the wound bed and the condition of the dressings, for they can reveal what went wrong. If few or no maggots are found and there was a breach in the netted dressing layer, then the maggots likely escaped. If there was no breach in the maggot dressing, look for evidence of dead maggots—either dead bodies or remnants of their mouth hooks (little black dots scattered among the wound bed). If either is found, it is highly likely that most or all of the maggots died all at about the same time, by drowning or suffocation. Crushing is also a possibility, but does not commonly kill all the maggots at the same time.
If very few or no maggot bodies are found—and, again, assuming that no maggots escaped—then the maggots may have died slowly, over the course of 24 hours or more. The causes for this may be more difficult to pinpoint. Again, the first place to start the investigation is with a careful examination of the dressing materials to ensure that the maggots did not suffocate or escape. A clean wound suggests that starvation may have played a role, and this is not an uncommon occurrence when maggots are placed on a relatively clean wound for the purpose of maintenance debridement or growth promotion [13]. The live maggots can survive on the decomposing bodies of the dead ones, but soon some of the survivors may, themselves, starve and die. Starvation is not likely to cause maggots to die unless they are very young. Older maggots, when starved, can survive, but they do not grow as quickly or as large. Hostile environmental factors (chemical or physical) are sometimes invoked to explain mass loss of larvae. A few drugs and wound treatments may be harmful to the maggots [13]. Specific bacterial or viral microbes within the wound might be a cause. This has not been demonstrated clinically, but there is laboratory evidence that certain microbes—at least Pseudomonas aeruginosa—can produce maggot-lethal virulence factors [14]. Another possible cause is that the maggots may have been unhealthy to begin with: too old, too starved, exposed to temperature extremes, or contaminated. If poor maggot quality is suspected, look for supporting evidence such as other maggots in the same batch with similar problems.
Often, a definitive cause for widespread maggot death cannot be found. Fortunately, mass maggot death is a rare event when dressings are properly applied, and under these circumstances, a repeat course of therapy will usually proceed without any problem. Some therapists and researchers have speculated that mass maggot death may be due to the host’s immunological response. While allergic reactions are known to exist in animals, such reactions should repeat themselves in a maggot-immune wound. As it happens, the rare instance of massive maggot death even more rarely repeats itself in the same patient, except when one of the causes listed above goes uncorrected.
Reapplication or Follow-up Treatment?
Once the maggots have been removed and the wound rinsed, it is time to determine the next course of action. If necrotic tissue still remains, it is usually desirable to reapply another course of maggot therapy. A subsequent course of maggots may be applied straight away (a useful strategy for out-patients, in order to minimise the number and inconvenience of visits to the clinic), or the maggots may be re-applied according to the schedule that best fits the therapist and/or the maggot lab (for example, every “maggot therapy day” on Monday and Thursday, or once weekly). Keep in mind that the sooner the wound is completely debrided, the better the chances for attaining total debridement and, ultimately, wound-healing. Still, sometimes a patient can benefit from a break for a day or two, especially when anxiety or discomfort causes sleepless nights.
It is difficult to define an “average” number of maggot treatments needed to completely debride a wound because the number of treatments is highly dependent on the wound itself. The thicker or deeper the necrotic tissue and the drier that tissue, the greater number of treatments will be required; the greater the number of healthy maggots that are placed on the wound at one time, the fewer will be the number of treatments required. Most wounds can be completely debrided with one or two applications of maggots; some may require four or more applications. One of the most effective ways to hasten the debridement (decrease the number of treatment cycles required) is to remove as much dry necrotic tissue (eschar) as possible before applying the maggots. This can be done with a scalpel, just prior to application of the maggot dressing, or it can be achieved by softening the existing dry tissue with an autolytic dressing technique overnight (occlusive dressing, hydrocolloid, etc.). This process should be fast and simple, done on the day of or the day before applying the maggots. Spending more effort and time than necessary for a very crude thinning or softening of the necrotic tissue is a waste of time and effort, once the decision has already been made that maggot therapy is the best course of action. The maggots will debride the tissue themselves, dry or not, within a few days.
If the wound bed is now well-debrided, it is time to begin the treatment of choice to effect wound closure. Depending on the therapist, patient and resources, treatment choices can vary widely, and are beyond the scope of this chapter. The important thing to note is that there is no need to delay definitive medical or surgical wound closure after maggot therapy. Maggot therapy does not increase the risk of infection or dehiscence, even if wound closure follows immediately [15]. If a decision about the method of closure cannot be made straight away, it is fine to apply a tissue-supportive dressing (i.e., saline-moistened gauze, non-toxic ointments, honey, hydrogels, etc.) until the definitive decision can be made.
Summary
Maggot therapy dressings are intended to keep the maggots on the wound until most are satiated and have stopped feeding. Some therapists make their own dressings; others use commercially produced dressings. Many therapists use both, depending on their patients’ wounds. All maggot dressings have at least the following characteristics in common: they prevent the maggots from escaping, they allow adequate amounts of oxygenated air to reach the maggots, and they facilitate the drainage of accumulating liquids (liquefied necrotic tissue and exudates). There are two basic designs for maggot dressings: maggot confinement dressings, which confine the maggots to the wound bed but allow them complete access to the wound; and maggot containment dressings, which totally contain the maggots within a net bag that facilitates easy handling but prevents the maggots from direct contact with all the nooks and crannies of the wound. Each has its advantages and disadvantages. Therapists should use the type of dressing that best meets their needs and their patients’ wounds. Once maggot debridement is complete, the appropriate and definitive medical or surgical treatment for closing the wound may begin.
References
1. Fine, A. and H. Alexander, Maggot Therapy: Technique and Clinical Application. The Journal of Bone and Joint Surgery, 1934. 16(3): pp. 572–582.
2. Sherman, R.A., A New Dressing Design for Use with Maggot Therapy. Plastic and Reconstructive Surgery, 1997. 100(2): pp. 451–456, https://doi.org/10.1097/00006534-199708000-00029.
3. Sherman, R.A., J.M. Tran, and R. Sullivan, Maggot Therapy for Venous Stasis Ulcers. Archives of Dermatology, 1996. 132(3): pp. 254–256 https://jamanetwork.com/journals/jamadermatology/fullarticle/vol/132/pg/254.
4. Grassberger, M. and W. Fleischmann, The Biobag — A New Device for the Application of Medicinal Maggots. Dermatology, 2002. 204(4): p. 306, https://doi.org/10.1159/000063369.
5. Dumville, J.C., et al., Larval Therapy for Leg Ulcers (VenUS II): Randomised Controlled Trial. BMJ, 2009. 338(7702): pp. 1047–1050, https://doi.org/10.1136/bmj.b773.
6. Steenvoorde, P., T. Budding, and J. Oskam, Determining Pain Levels in Patients Treated with Maggot Debridement Therapy. Journal of Wound Care, 2005. 14(10): pp. 485–488, https://doi.org/10.12968/jowc.2005.14.10.26846.
7. Steenvoorde, P., C.E. Jacobi, and J. Oskam, Maggot Debridement Therapy: Free-Range or Contained? An in-vivo Study. Advances in Skin & Wound Care, 2005. 18(8): pp. 430–435, https://doi.org/10.1097/00129334-200510000-00010.
8. Steenvoorde, P., et al., Maggot Debridement Therapy of Infected Ulcers: Patient and Wound Factors Influencing Outcome — A Study on 101 Patients with 117 Wounds. Annals of the Royal College of Surgeons of England, 2007. 89(6): pp. 596–602, https://doi.org/10.1308/003588407x205404.
9. Stadler, F., Packaging Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 349–362, https://doi.org/10.11647/OBP.0300.16.
10. DeFazio, M.V., et al., Home Improvement in Maggot Therapy: Designing a Simple, Cost-Effective, Closed-System Habitat to Facilitate Biodébridement of Complex Distal Lower Extremity Wounds. Plastic and Reconstructive Surgery, 2015. 136(5): pp. 722e-723e, https://doi.org/10.1097/prs.0000000000001685.
11. Felder, J.M., 3rd, et al., Increasing the Options for Management of Large and Complex Chronic Wounds with a Scalable, Closed-system Dressing for Maggot Therapy. Journal of Burn Care & Research, 2012. 33(3): pp. e169–175, https://doi.org/10.1097/BCR.0b013e318233570d.
12. All Wales Tissue Viability Nurse Forum. The All Wales Guidance for the Use of Larval Debridement Therapy (LDT). 2013. https://www.wounds-uk.com/download/resource/5850.
13. Sherman, R., Indications, Contraindications, Interactions, and Side-effects of Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 63–78, https://doi.org/10.11647/OBP.0300.04.
14. Andersen, A.S., et al., Quorum-sensing-regulated Virulence Factors in Pseudomonas aeruginosa Are Toxic to Lucilia sericata Maggots. Microbiology (Society for General Microbiology), 2010. 156(2): pp. 400–407, https://doi.org/10.1099/mic.0.032730-0.
15. Sherman, R.A. and K.J. Shimoda, Presurgical Maggot Debridement of Soft Tissue Wounds Is Associated with Decreased Rates of Postoperative Infection. Clinical Infectious Diseases, 2004. 39(7): pp. 1067–1070, https://doi.org/10.1086/423806.
