3. Goliath grouper Epinephelus itajara conservation in Cuba: A protected area, ecotourism and fisheries effort
Fabián Pina Amargós,1 Tamara Figueredo Martín, and
Yunier Olivera Espinosa
©2025 Fabián Pina Amargós et al., CC BY-NC-ND 4.0 https://doi.org/10.11647/OBP.0395.03
The goliath grouper Epinephelus itajara is the largest grouper in the western hemisphere and one of the two largest groupers in the world, growing to 250cm in total length (TL) (Heemstra and Randall, 1993) with a maximum weight of 320 kg (Smith, 1971). The goliath grouper is a long-lived species (at least 37 years) and reaches maturity at 6-7 years (120 to 135cm TL) for females and 4–6 years (110 to 115cm TL) for males (Bullock et al., 1992). The species makes ontogenetic, seasonal, and spawning migrations (Coleman et al., 2000) and forms relatively small (10 to 100 individuals) spatially and temporally predictable spawning aggregations (Sadovy and Eklund, 1999). Adult and juvenile goliath grouper show high site fidelity (Eklund and Schull, 2001). These combined features make goliath grouper particularly susceptible to overexploitation. Once relatively abundant throughout its range, goliath grouper populations began to decline in the 1960s, undoubtedly a consequence of both intensive fishing on spawning aggregations and spearfishing on unwary adults (Sadovy and Eklund, 1999). As a result, the goliath grouper is now overexploited and rarely observed (Sadovy and Eklund, 1999). In the USA, population declines led to a fishery closure and catch moratorium in 1990 in all territorial waters (SAFMC, 1990) until recently when a limited catch had been authorised through a harvest permit and tag system due to the recovery of the species (FFWCC, 2024). Internationally, the goliath grouper was listed as Critically Endangered but now is considered Vulnerable (Bertoncini et al., 2018).
In Cuba, the goliath grouper has been poorly studied and, until recently, inadequately protected. A single management approach through fisheries regulation was for many years the common practice, missing the diverse management approaches, based on the goliath grouper conservation success presented in the previous paragraph. For many years, the protection was an arbitrary 40-cm minimum size regulation which permitted landing almost all goliath groupers caught in Cuban waters (Resolution 561/96 Ministry of Fisheries, Resolution 126/09 Ministry of Food (formerly Ministry of Fisheries)). There is only one peer-reviewed manuscript published specifically about goliath grouper, which focused on the species movement patterns in southeastern Cuba (Pina-Amargós and Gonzalez-Sansón, 2009). Claro and Lindeman (2003) reported 21 spawning aggregation sites for snappers and groupers on the Cuban shelf but none of these are reported spawning aggregation sites for goliath grouper. Previous research has investigated the relationship between predator-prey sizes (Claro et al., 2001). Pina-Amargós and Gonzalez- Sansón (2009) used conventional external tagging within and adjacent to the Jardines de la Reina Marine Reserve (JRMR) to understand goliath grouper movement patterns. This information was applied to improve management for this endangered species. Tagging for this study took place in Jardines de la Reina (JR) in 2001, taking advantage of traditional knowledge of abundant populations of goliath grouper and logistic support from the tourism company. Five individuals were tagged in 2001 and tracked until 2003, with 541 underwater resightings through summer 2002 at the tagging sites. None of the tagged goliath groupers were again sighted after July 2002 at JR diving sites. In February 2002, one individual was caught 36km northeast of the tagging site. In August 2002, a second tagged specimen was caught 77km southeast of the tagging site. In August 2003, two individuals were captured 168km southeast of the tagging site, at a possible spawning aggregation site. All recaptures took place outside JRMR boundaries. The authors highlighted that despite the protection afforded to juveniles and adults by the JRMR, individuals obviously remain susceptible to capture during migrations.
Pina-Amargós and Gonzalez-Sansón (2009) recommended that management approaches to conserve the species included combining fisheries regulations and protected areas. The first approach would entail protecting the spawning aggregation sites, if they occur, by means of catch moratoria and gear restrictions; the second would require the creation of small or medium-sized marine protected areas (MPAs) that contain the spawning aggregation sites and migratory corridors in conjunction with other already established MPAs protecting non-spawning grouper habitat. As such, more research is needed to verify and characterize the status of potential goliath grouper spawning aggregations at Punta Macao and Cabo Cruz and provide recommendations for the spatial planning and designation of MPAs.
Pina-Amargós and Gonzalez-Sansón (2009) implicitly recognized the importance of promoting non-consumptive use of the goliath grouper as an ecotourism attraction for SCUBA divers and snorkelers, as has been happening in JR since the 1990s, but it was Figueredo-Martín et al. (2010a) who quantitatively assessed the importance of large fish species for JR ecotourism. Several of these facts were also included in a peer-reviewed paper about MPAs in Cuba (Perera-Valderrama et al., 2018).
Extensive interviews have been conducted throughout the entire country to gather traditional knowledge (ecology and fisheries) of goliath grouper (authors FPA and TFM, unpublished data). Traditional knowledge has proved to be an invaluable source for the gathering of information, and the protection and management of species of which little scientific information is available, such as goliath grouper in Cuba. Traditional knowledge about goliath grouper has been used to assess changes to abundance and distribution (Bravo-Calderon et al., 2021); understand reproduction, feeding, and behavior (Gerhardinger et al., 2006); generate information on spawning aggregation sites (Gerhardinger et al., 2009); consider the impacts of some fishing gears (Giglio et al., 2017); and discuss the effectiveness of certain conservation strategies (Zapelini et al., 2017).
Recently, a comparison of the monetary benefits contributed by large groupers (including the goliath grouper) between fisheries and ecotourism was published (Figueredo-Martín and Pina-Amargós, 2023). Fisheries of large groupers in the fishing zone surrounding Jardines de la Reina National Park (JRNP) represented US$121,707 per year, while ecotourism with these species inside JRNP reach US$417,328 annually. This result showed that the enjoyment of large grouper by SCUBA divers and snorkelers provides 3.4 times more monetary benefits than their consumption as food.
Here we summarize the scientific knowledge of the species in Cuba, including unpublished results on movements outside the spawning aggregation site, dynamics at the spawning aggregation site and fisheries information, and how a combination of tools (protected areas, ecotourism, and fisheries) and stakeholder involvement, has strengthened the protection of goliath grouper in the largest archipelago of the Caribbean. In this chapter we aim to show that diverse sources of information, stakeholders’ involvement and the combination of management tools yield the best results for conserving endangered species such as goliath grouper.

Fig. 3.1 Map of the study sites. Details showing goliath groupers tagged and released outside the spawning aggregation site (red circles) and goliath groupers tagged and released and sighted at the spawning aggregation site (blue circles).
Information about goliath grouper: quantitative and qualitative
This research took place nationwide, but with an underwater fieldwork focus in southeastern parts of Cuba, on Sancti Spíritus, Ciego de Ávila, and Camagüey provinces, mainly in Cayos de Ana María, islands of the Golfo de Ana María and JR (Figure 3.1), spatially expanding upon the previous study (Pina-Amargós and Gonzalez-Sansón, 2009). In 1996, approximately 950km2 of JR was declared as a Marine Reserve (JRMR) where only lobster fishing and limited catch and release recreational fishing was allowed as part of a tourism operation that included SCUBA diving (Figueredo-Martín et al., 2010a, b) (Resolution 562/1996, former Ministry of Fisheries). In 2010, around 2,170km2 were declared as a National Park (JRNP) (Agreement 6803/2010, Council of the State). JRNP regulations are based on fisheries regulations of the JRMR but more detailed zoning was included and regulations were expanded to all uses allowed in the area such as tourism, navigation, anchoring, scientific research.
Ten expeditions were carried out to study the goliath grouper (2013 (March, April–May, July, August, September); 2014 (April–May, July, August, September); 2015 (February)). Fishing effort of set-lines on mangrove shorelines and mangrove channels (0.5–4m deep) was 21,750 hooks.hours during 91 days at 74 sites (Box 3.1, Figure 3.2A). Fishing effort of hand-lines on coral reef slopes and spur and groove (25–45m deep) spawning aggregation sites was 417 hooks.hours during 32 days at one site (Figure 3.2B). Visual census effort on spawning aggregation sites consisted of 52.7 dives.hours during 22 days at 6 sites (Figure 3.2C and 3.3). Logistical constraints prevented surveys during all moon phases, so we decided to focus on before and during the Last Quarter, which corresponds to peak spawning according to the fishers we interviewed. We collected measurements and biological data from goliath groupers caught by fishers at the spawning aggregation site to assess their size, sex structure, and reproductive status. We classified gonad stages according to García-Cagide et al., (2001).

|

|

|

|
Fig. 3.2 Research methods used to study goliath grouper. Legend: A: set-line, B: hand-line, C: visual census, D: interviews.
We interviewed 36 fishers at sea and within their communities to obtain local ecological knowledge about the species ecology and history of goliath grouper fisheries (Figure 3.2D). We used a semi-structured questionnaire for the interviews (see Supplemental Bibliography). We also reviewed fisheries data from Cuba from 2000 to 2013 to assess temporal changes of commercial landings of goliath grouper.
Movements outside the spawning aggregation site
We caught (tagged and released) a total of 15 specimens and sighted a total of five specimens outside spawning aggregations (mean size 92.37 ± 2.83cm) (Table 3.1, Figure 3.1, Figure 3.3). One specimen (69.2 cm) was caught on a patch reef 3m deep outside JRNP. One specimen (89.7 cm) was caught on a wreck in a seagrass channel 3m deep inside the JRNP. Thirteen (81–120.5 cm) were caught in mangrove channels 2.5–3.5m deep inside JRNP. The five sighted goliath groupers were seen in mangrove channels (sizes around 1m and 1–3m deep), inside JRNP. Regardless of the high fishing effort of the project, abundance was very low even in the well protected JRNP. This is not surprising due to the large size of the species and its spatial and feeding requirements (Sadovy and Eklund, 1999) and also owing to the high artisanal and commercial fishing pressure outside the protected area at the time of the study. The smallest specimen was observed in shallow water close to the mainland of Cuba. Medium sized fish (around 1 m) were located around shallow waters far from the mainland. The largest individuals (>1.5 m) were found on deep coral reefs along the shelf edge. This finding is consistent with ontogenetic habitat shifts and differences between juveniles and adults (Coleman et al., 2000).
Table 3.1 Tagging information of goliath grouper on southeastern Cuba. PR: patch reef, MC: mangrove channel, W: wreck.
|
Tagged |
Recapture |
Growth (cm.y-1) |
|||||
|
Date |
Tagged location |
Habitat |
Size (cm) |
Time at liberty (days) |
Recapture location |
Size (cm) |
|
|
03/10/2013 |
Punta Arena |
PR |
69.2 |
||||
|
05/07/2013 |
Auras |
MC |
120.5 |
||||
|
05/14/2013 |
Cachiboca |
MC |
103.2 |
449 |
Same |
110.0 |
5.53 |
|
06/03/2013 |
Estero Guasa |
MC |
100.0 |
||||
|
08/06/2013 |
Auras |
MC |
92.0 |
5 |
Same |
92.0 |
0 |
|
08/07/2013 |
Auras |
MC |
92.5 |
267 |
Same |
101.5 |
12.3 |
|
08/11/2013 |
Auras |
MC |
92.0 |
264 |
Same |
97.0 |
6.91 |
|
05/01/2014 |
Auras |
MC |
93.5 |
90 |
Same |
97.0 |
14.19 |
|
05/01/2014 |
Nicola |
MC |
93.0 |
||||
|
05/02/2014 |
Auras |
MC |
93.0 |
89 |
Same |
97.0 |
16.4 |
|
05/04/2014 |
Juan Grin |
W |
89.7 |
||||
|
05/19/2014 |
Tronconera |
MC |
81.0 |
87 |
Same |
83.0 |
8.39 |
|
05/20/2014 |
Tronconera |
MC |
88.0 |
87 |
Same |
90.0 |
8.39 |
|
08/15/2014 |
Tronconera |
MC |
88.0 |
||||
|
08/15/2014 |
Tronconera |
MC |
90.0 |
||||
It is premature to discuss movement patterns and site fidelity since we have only recaptured eight goliath groupers, all of which were juveniles or early adults (90 -110 cm). All specimens were recaptured no more than 100m from their release point in mangrove channels, after 87 - 267 days (Figure 3.5A). This result is consistent with movement patterns detected for juveniles and early adults elsewhere (Eklund and Schull, 2001). As more movement data come in, we are expecting longer distance movements of adults, as found in Florida, U.S. (Eklund and Schull, 2001) and Jardines de la Reina, Cuba (Pina-Amargós and Gonzalez- Sansón, 2009). Growth was estimated at 10.30 ± 1.53cm per year, similar to those reported elsewhere for the size range of our study (Sadovy and Eklund, 1999; Artero et al., 2015a).
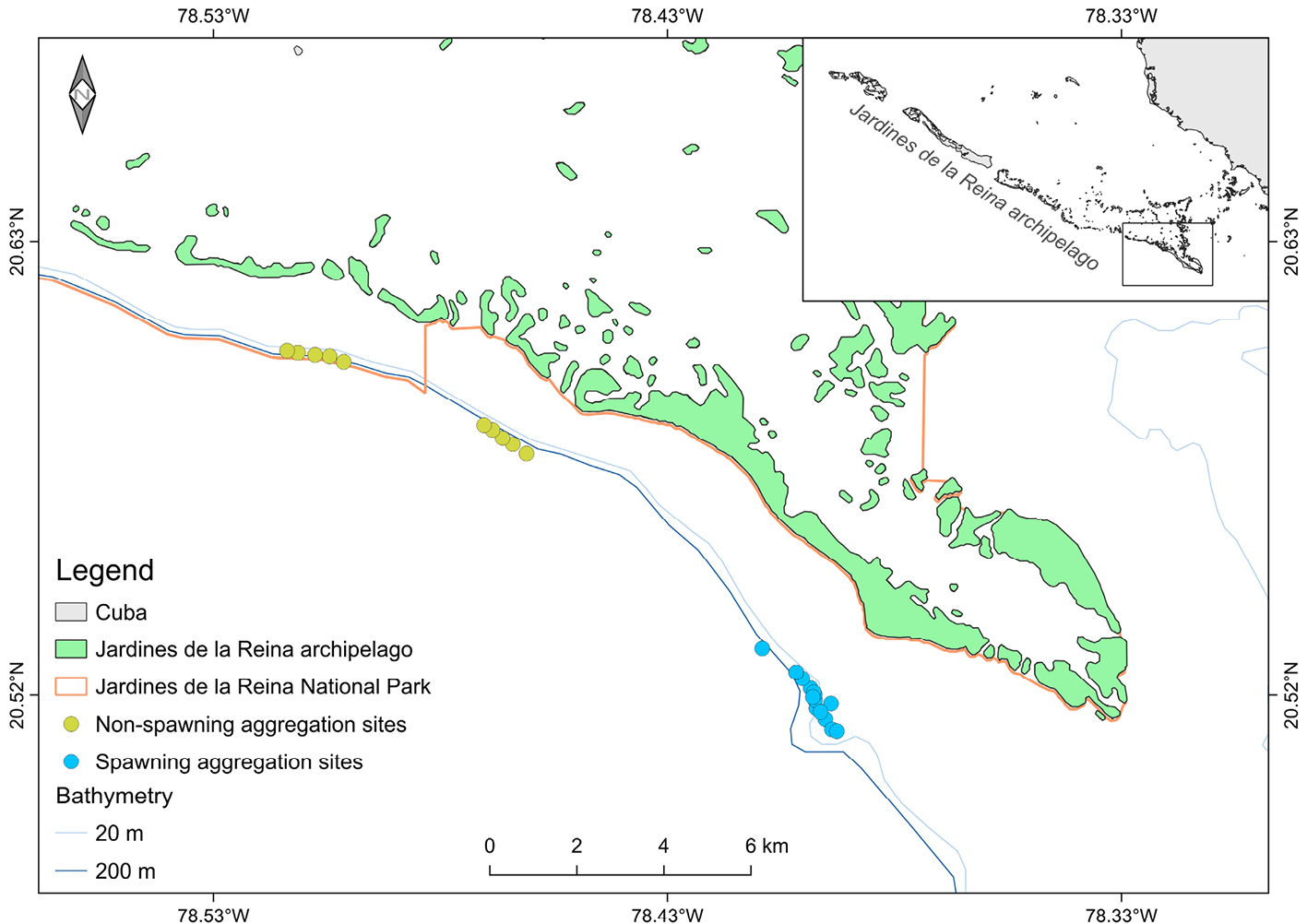
Fig. 3.3 Location of underwater visual census at a goliath grouper spawning aggregation site off Jardines de la Reina, Cuba.
Dynamics at the spawning aggregation site
Goliath groupers were mostly observed during the morning and afternoon during the two moon phases we surveyed (Full Moon and Last Quarter). In a 200m segment of the easternmost site, we counted 21 specimens (average of 5.3 specimens per dive) and caught (tagged and released alive) 11 specimens (Table 3.2, Figure 3.3 and 3.4). Mean size of caught specimens was 160.78 ± 8.57 cm. Our estimate of abundance is among the lowest reported for goliath grouper spawning aggregation sites (GMFMC, 1990; Sadovy and Eklund, 1999) and is presumably an indicator of overfishing. Out at the easternmost site no goliath groupers were sighted.

Fig. 3.4 Size composition of goliath grouper in south eastern Cuba. Legend: Non SpagT: specimens tagged outside the spawning aggregation site, SpagVC: specimens sighted by visual censuses on the spawning aggregation site, SpagT: specimens tagged at the spawning aggregation site, SpagF: specimens caught by fishing boat at the spawning aggregation site.
Table 3.2 Goliath grouper caught at the spawning aggregation in Jardines de la Reina. NI: not identified, M: male, F: female, Gonad stage according to García-Cagide et al. (2001): V: Ovulation and sperm release, VI: spent.
|
Method |
Date |
Size (cm) |
Sex |
Gonad stage |
|
Tagging |
09/02/2013 |
107.4 |
NI |
NI |
|
Fishing |
09/02/2013 |
123.5 |
M |
V |
|
Fishing |
09/02/2013 |
157.4 |
F |
V |
|
Tagging |
09/05/2013 |
178.5 |
NI |
NI |
|
Fishing |
09/05/2013 |
183.7 |
F |
V |
|
Fishing |
09/05/2013 |
142.5 |
M |
V |
|
Tagging |
07/19/2014 |
124.5 |
NI |
NI |
|
Fishing |
07/19/2014 |
171.4 |
F |
VI |
|
Tagging |
07/23/2014 |
168.5 |
NI |
NI |
|
Fishing |
07/23/2014 |
190.0 |
F |
V |
|
Tagging |
08/17/2014 |
136.0 |
NI |
NI |
|
Tagging |
08/18/2014 |
175.5 |
NI |
NI |
|
Tagging |
08/19/2014 |
186.5 |
NI |
NI |
|
Fishing |
08/19/2014 |
201.6 |
F |
VI |
|
Fishing |
08/19/2014 |
134.7 |
M |
V |
|
Fishing |
08/19/2014 |
158.5 |
F |
VI |
|
Tagging |
08/21/2014 |
192.7 |
NI |
NI |
|
Fishing |
08/21/2014 |
180.0 |
F |
V |
|
Fishing |
08/21/2014 |
145.3 |
M |
V |
|
Fishing |
08/21/2014 |
169.5 |
F |
VI |
|
Tagging |
09/16/2014 |
145.5 |
NI |
NI |
|
Fishing |
09/16/2014 |
182.5 |
F |
V |
|
Fishing |
09/16/2014 |
185.7 |
M |
V |
|
Tagging |
09/17/2014 |
164.5 |
NI |
NI |
|
Fishing |
09/17/2014 |
147.5 |
M |
VI |
|
Fishing |
09/17/2014 |
163.2 |
F |
VI |
|
Tagging |
09/18/2014 |
189.0 |
NI |
NI |
We used a generalized additive mixed model (GAMM) (the model) to analyse the data gathered (see details in Supplement section). The model showed an increase in the abundance of goliath groupers in the spawning aggregation site as the Last Quarter moon phase advanced (Table 3.3). Furthermore, the abundance of goliath grouper decreased during the morning and increased toward sunset (Table 3.3, Figure 3.6A).
Table 3.3 Results of the GAMM model applied to goliath grouper abundance and presence/absence by size (TL in meters) at one spawning aggregation site. Significant variables are in bold. St. error: standard error, edf: effective degrees of freedom, sq. Chi: squared Chi.
|
Negative binomial GAMM model (abundance) |
|||||
|
Estimated |
St. error |
Z |
P |
||
|
Intersect |
-0.065 |
0.226 |
-0.288 |
0.773 |
|
|
Day |
0.059 |
0.028 |
2.116 |
0.036 |
|
|
edf |
sq. Chi |
P |
|||
|
Hour |
4.784 |
9.531 |
<0.001 |
||
|
Logistic GAM model (size < 1 m) |
|||||
|
Estimated |
St. error |
Z |
P |
||
|
Intersect |
-0.300 |
0.431 |
-0.695 |
0.487 |
|
|
day_2 |
-27.330 |
78310.0 |
0.000 |
1.000 |
|
|
day_7 |
-0.472 |
0.589 |
-0.801 |
0.423 |
|
|
day_8 |
-0.446 |
0.607 |
-0.735 |
0.463 |
|
|
day_10 |
-0.533 |
0.595 |
-0.897 |
0.370 |
|
|
day_11 |
1.102 |
0.555 |
1.987 |
0.047 |
|
|
day_12 |
0.064 |
1.136 |
0.056 |
0.955 |
|
|
edf |
sq. Chi |
P |
|||
|
Hour |
4.223 |
26.140 |
<0.001 |
||
|
Logistic GAM model (size 1-1.5 m) |
|||||
|
Estimated |
St. error |
Z |
P |
||
|
Intersect |
-1.801 |
0.451 |
-3.994 |
0.000 |
|
|
July.2014 |
0.950 |
0.424 |
2.240 |
0.025 |
|
|
August.2014 |
1.412 |
0.438 |
3.227 |
0.001 |
|
|
Septiembre.2014 |
1.043 |
0.393 |
2.656 |
0.008 |
|
|
day_2 |
-31.630 |
790700.0 |
0.000 |
1.000 |
|
|
day_7 |
0.513 |
0.434 |
1.182 |
0.237 |
|
|
day_8 |
0.640 |
0.441 |
1.453 |
0.146 |
|
|
day_10 |
-0.311 |
0.461 |
-0.675 |
0.500 |
|
|
day_11 |
0.016 |
0.442 |
0.036 |
0.971 |
|
|
day_12 |
-1.856 |
1.063 |
-1.746 |
0.081 |
|
|
Edf |
sq. Chi |
p |
|||
|
Hour |
4.710 |
30.820 |
<0.001 |
||
|
Logistic GAM model (size >1.5 m) |
|||||
|
Estimated |
St. error |
Z |
p |
||
|
Intersect |
-4.258 |
1.707 |
-2.494 |
0.013 |
|
|
July.2014 |
1.386 |
0.582 |
2.383 |
0.017 |
|
|
August.2014 |
1.294 |
0.591 |
2.190 |
0.029 |
|
|
September.2014 |
0.395 |
0.515 |
0.767 |
0.443 |
|
|
day_2 |
-28.500 |
73500.0 |
0.000 |
1.000 |
|
|
day_7 |
-0.016 |
0.793 |
-0.021 |
0.983 |
|
|
day_8 |
0.322 |
0.851 |
0.379 |
0.705 |
|
|
day_10 |
-1.018 |
0.826 |
-1.233 |
0.218 |
|
|
day_11 |
2.058 |
0.799 |
2.577 |
0.010 |
|
|
day_12 |
-2.950 |
1.286 |
-2.294 |
0.022 |
|
|
Depth |
0.136 |
0.052 |
2.585 |
0.010 |
|
|
edf |
sq. Chi |
p |
|||
|
Hour |
3.501 |
28.430 |
<0.001 |
||

|

|

|

|
Fig. 3.5 Field work on goliath grouper in southeastern Cuba. Legend: A: goliath grouper recaptured at mangrove channel, B: goliath grouper caught at the spawning aggregation site landed, C: Testis of goliath grouper full of milt, D: hand line gear.
The model showed significant differences in the days of the lunar phase, with smaller goliath grouper occurring in larger numbers towards the end of the surveys (Table 3.3). Diel patterns were the same as observed when we modelled total abundance, where the presence of small goliath groupers decreased during the morning and increased toward sunset (Table 3.3, Figure 3.6B).
According to the model, medium-sized goliath groupers (1–1.5m TL) sighting frequency showed significant differences between September 2013 and the three months surveyed in 2014, but there were no differences among 2014 months nor among the days of the moon phase (Table 3.3). Diel patterns were consistent with the previous two analyses (Table 3.3, Figure 3.6C).
According to the model, large-sized goliath groupers (>1.5m TL) sighting frequency showed significant differences among September 2013 and July and August 2014 (Table 3.3), with more presence of those large individuals in 2014, but there were no differences among spawning months in 2014. The model showed significant differences in the days of the lunar phase, with large goliath grouper occurring more towards the end of the Last Quarter moon phase (Table 3.3). In the case of depth, large goliath groupers were more abundant in deeper waters. Diel patterns followed the same as the previous three analyses (Table 3.3, Figure 3.6D).

Fig. 3.6 Curves of the GAMM models applied to goliath grouper abundance (A) and to presence/absence by size (TL in meters) (B-D) from a spawning aggregation site at Jardines de la Reina, Cuba. (A) abundance, (B) <1 m, (C) 1-1.5 m, (D) >1.5 m. The shaded area represents the 95 % confidence interval.
Most of our findings related to spawning months are consistent with previous research. Summer spawning has also been confirmed in the south-eastern U.S.A. (Bullock et al., 1992; Eklund and Schull, 2001; Koenig et al., 2011). Previous studies have shown that the New Moon is the peak of the spawning in several places (e.g., Koenig et al., 2011 for U.S.A; and Bueno et al., 2016 for Brazil). In the present study, goliath grouper abundance and catch rates were highest during the Last Quarter moon. As we did not see actual spawning at daylight, we assume spawning of goliath grouper occurs at night as reported by fishers and scientific publications (e.g., Mann et al., 2009). To the best of our knowledge, the two findings of diel abundance changes and large-size specimens being more abundant on deeper reefs have not previously been reported. In all cases, the number of goliath grouper at the spawning aggregation site decreased through the morning, with a minimum at noon, and increased again toward the end of the afternoon. Whether that is a result of a daily movement pattern to deeper/shallower habitats, and what the causes of it are, deserves further research.
The other novel finding is that larger goliath groupers (presumably females according to our data, see below) were consistently observed in deeper waters while medium and small size fish seem to use the whole range of depth surveyed. Sex segregation by depth on spawning aggregations (and out of spawning season as well) has been observed on gag grouper (Mycteroperca microlepis) (Coleman et al., 1996; Koenig et al., 1996; McGovern et al., 1998; Sedberry et al., 2006). Females of this species form pre-spawning aggregations in relatively shallow water (20 m) before moving to shelf-edge reefs (50-100 m) for spawning. Outside of the spawning season, males remain on spawning sites while females move into shallower water. Whether goliath grouper show similar behaviors requires further research. Although statistically significant, diel fluctuations of abundance/presence/absence and size related depth findings were based on small sample sizes, thus these findings should be taken cautiously. An alternative explanation would be that larger specimens are scarcer in shallower water due to fishing. This is supported by anecdotal information showed in the following section but, to the best of our knowledge, this hypothesis has not been tested for this or other species in the Caribbean or elsewhere.
Fisheries information at the spawning aggregation site
We were able to survey one commercial fishing boat fishing at the goliath grouper spawning aggregation site for eight nights in September 2013, and July, August, and September 2014. A total of 16 goliath groupers were caught (123.5–201.6 cm; 32–155 kg gutted; sex ratio 1.66:1 (10 females, 6 males)) (Table 3.2; Figure 3.3, 3.4, 3.5B). This sex ratio is similar to that previously reported (Bullock et al., 1992). The gonads were in spawning condition (stage V, 62%) or spent (stage VI, 38%), as found in previous studies on spawning season (Bueno et al., 2016; Koenig et al., 2016) (Figure 3.5C). The stomachs of all specimens were empty. Previous studies show around half of the stomachs empty (Artero et al., 2015b), likely, due to its larger sample size when compared to our study.
The abundance/presence/absence of goliath grouper detected through underwater visual censuses on the easternmost site, temporal patterns of those variables (day, moon phase and months), the confirmation of the commercial fishing of goliath groupers taking place in the site as well as the active reproductive condition of gonads sampled suggests the existence of a spawning aggregation site, the first detected by science in Cuba.
According to fisheries data and fisher interviews, the goliath grouper is not a highly valued fishery resource in Cuba, despite being considered a high-quality species. Commercial fisheries data from southern Cuba between 1981-2013 show that goliath grouper landings represented an average of 0.02% (average of 8.6 tons, minimum of 0.9 tons and maximum of 23.7 tons) of the total national landings (Figure 3.7). Commercial landings from southern Cuba have decreased steadily since 1981, reflecting overfishing: average landings between 2003 and 2013 (3.2 tons) represented 17% of that between 1981-1991 (18.6 tons). Southern Cuba was the most important goliath grouper fishing ground: average landings represented 76% of the entire country between 1981-2013 (8.6 tons of 11.6 tons) (Figure 3.7). However, this data does not reflect the total fishing mortality since, according to our interviews, goliath grouper is heavily targeted by spear fishers nationwide, which species’ landings likely surpassed those of the commercial fisheries as reported in the Atlantic and Gulf of Mexico coasts of U.S.A. (Sadovy and Eklund, 1999).

Fig. 3.7 Landings of goliath grouper between 1981 and 2013 from the four Cuban fishing zones.
Despite its low commercial fisheries importance, goliath grouper caught are consumed by fishers and their families or marketed informally. Thus, commercial fisheries data do not accurately reflect true catch. The fishery of goliath grouper in Cuba is divided into artisanal and commercial. The artisanal fishery uses spearguns throughout the year, and handlines during the spawning season. Speargun fishers targeted the species at fish aggregating devices, wrecks, piers, deep mangrove channels, patch reefs, and deep coral reef slopes, and spur and groove habitats. During the spawning aggregation season (July to September) artisanal fishers used hand-lines (Figure 3.5D). The gear is made of 2–3mm monofilament and/or 8–10mm rope armed with large hooks baited with large pieces of fish such as barracuda chunks, whole lobsters, or medium-sized live reef fish. 20 or more years ago, hand line fishing for goliath grouper took place around 30m depth, but in 2013-2014, fishers began fishing deeper (e.g., > 50 m). This was likely due to the depletion of spawning populations in shallower waters, though, we saw spawning size specimens around at 30 to 40m deep. Commercial boats fished for goliath grouper as a secondary source of income. All boats had another primary target species, typically deeper water snappers and groupers, but at the end of the fishing day they anchored in a selected spot (known in Cuba as “potala”) and fished for goliath grouper throughout the night until daylight.
Fishers indicated that goliath grouper bite more during the Last Quarter moon between the months of July–September and less in other moon phases during the spawning season. Fishers also reported that during the Last Quarter goliath grouper are caught in larger numbers in shallow waters, but this differs from other phases of the moon where fish are caught in deeper water. Based on our limited catch surveys, and fisher interviews, we estimated that an average of 154 goliath grouper were caught every year from the population at only one site (surveys: 16 specimens caught over 8 nights, commercial fishing effort on the spawning aggregation site averages 77 nights per year). The average weight of those goliath groupers was estimated to be 35.7 kg (154 specimens divided by 5.5 tons (average landing per year of the fishing boat surveyed)). The largest goliath grouper ever caught by the fishing boat surveyed was a 173 kg gutted specimen.
History of goliath grouper conservation in Cuba
There are several tools available for protecting goliath grouper. Fisheries regulation was the only one used in Cuba for long time, with spatial protection and non-consumptive uses such as ecotourism, as the ones Cuba has been implementing for this iconic species in the last few years. Spatial protection of the marine environment is relatively new in Cuba. In the early 1990s, there were no marine protected areas (MPAs) declared under environmental or fisheries legislations, but in 2001, 18 MPAs were designated under environmental legislation (Perera-Valderrama et al., 2018) and 40 marine reserves were declared under fisheries legislation (Kritzer et al., 2014). In 2012, numbers increased to 56 MPAs (Perera-Valderrama et al., 2018) and as of 2021 Cuba legally has approved 64 MPAs (Perera-Valderrama et al., 2021). More than half of these MPAs do not allow fishing inside their boundaries. Even though none of these MPAs have been created specifically to protect goliath grouper, their coverage of Cuban shelf habitats should contribute to its conservation. Currently, a fifth of the entire Cuban shelf, more than a third of Cuban coral reefs, more than a quarter of seagrasses, and more than a third of mangroves are located inside MPAs (Perera-Valderrama et al., 2018). Scientific evidence in Cuba and elsewhere suggest that goliath grouper have a relatively small home range that theoretically should allow even small, protected areas to support its conservation. However, with periodic migrations outside the boundaries of protected areas, they remain highly vulnerable to fishing. In addition to this, enforcement of regulations within many MPAs is still weak and illegal fishing is common practice (Perera-Valderrama et al., 2018).
Among Cuban marine protected areas, JRNP is one of the best examples of strong enforcement. This is mainly due to the ecotourism that takes place there, a successful example of mixing spatial protection and alternative use of the species. SCUBA divers and snorkelers are willing to pay to enjoy large fishes, such as goliath grouper, during their underwater activities (Figueredo-Martín et al., 2010a; Figueredo-Martín and Pina-Amargós, 2023). A portion of the financial benefits are used to support the enforcement of fisheries and environmental regulations within the protected area, to effectively deter illegal fishing and repel poachers. Tourism also supports research by providing the logistical support for long-term continuous monitoring in JRNP. These activities help protect the natural resources upon which tourism depends, and staff conservation ethos is concomitantly high. The high abundance and biomass of large and commercially important fish such as sharks, groupers, and snappers in JRNP, result from proper enforcement and incentives favouring conservation, while allowing humans to make a living from it.
Unfortunately, JRNP is only a small portion of the Cuban shelf, and more is needed to protect goliath grouper nationwide, beyond spatial protection and the promotion of non-consumptive use. Next, we discuss fisheries regulations that promote conservation of goliath grouper in Cuba, their pros and cons, and how a step-wise approach was applied to stakeholder involvement. All of the above, based on the best science available and traditional knowledge, led to success in protecting this endangered and important species.
For many years, the only fishing regulation for goliath grouper in Cuba was a minimum size limit, which was 960 grams (around 25cm TL) (Resolution 561/96 Ministry of Fisheries). That was increased to 40cm more than ten years later (Resolution 126/09 Ministry of Food (former Ministry of Fisheries)). Those regulations allowed almost 100% of goliath groupers caught in Cuban waters to be landed, and obviously contributed nothing to the species’ protection. Increasing the minimum size to 110cm TL, was one of the alternatives we proposed to enhance goliath grouper protection in Cuba. This would allow most goliath groupers to spawn at least once before being caught. It is also a rule relatively easy to enforce among Cuban spear-fishers, since they are capable of selecting specimens by estimating their size. However, it is not very effective for handlines that target spawning aggregations, since hooks, and abrupt pressure change, usually damage internal organs (due to expansion of the swim bladder), causing high mortality rates of released undersized fishes. On the other hand, large minimum sizes such as the one proposed for goliath grouper are hard to enforce, as a consequence of the apparent contradiction that many undersized goliath groupers would be larger than almost all legal-sized fishes of the other species. Furthermore, Cuban fishers are culturally more willing to release small fishes than large ones.
Another fisheries regulation that we assessed to promote conservation of goliath grouper in Cuba, was to prohibit spearfishing of goliath grouper nationwide. Spearfishing is considered the most effective fishing gear for goliath grouper (Sadovy and Eklund, 1999) and is widespread in Cuba. Therefore, implementing this rule would undoubtedly benefit the species. However, there were two main reasons this regulation would be hard to implement. First, it would be considered discriminatory of the spear fishers, with concomitant implication on compliance. Second, its compliance would be even lower because spear fishers target large specimens, and they would not naturally agree to leave goliath groupers alive when spotted.
The fisheries regulation likely to be the most effective for goliath grouper conservation and most accepted by fishers, would be prohibiting fishing around the reported spawning aggregation site of Punta Macao in July, August and September, a combination of spatial and temporal closure, supported by scientific and traditional knowledge. Although fishers usually oppose any regulation limiting their livelihood, the small spatial and temporal scale of the limitation imposed by this regulation, would be expected to produce high compliance, and would protect locally the species on its more vulnerable life cycle phase.
Taking into consideration the pros and cons of the above regulations, we accepted the challenge. At the beginning of 2018, a group of scientists from Avalon Fishing and Diving Center (authors FPA and TFM), and the Center for Coastal Ecosystems Research (author YOE) submitted a petition, supported by the Center for Fisheries Research, to the Consultative Fisheries Commission to advance goliath grouper conservation in Cuba. The petition compiled the state of the knowledge of the species in its natural range, including possible actions to protect the species. It also included the scarce, but important information obtained in Cuba, coming mainly from JRMR: evidence of declining populations nationwide; movement patterns that do not offer full protection even inside a large MPA; higher economic value for ecotourism versus fisheries; the unsustainable nature of the current minimum legal size; the fishing on spawning aggregations; and the existence of a spawning aggregation site without spatial protection. Taking into account the null precedent on bans of fisheries resources in Cuba, the proposal made three recommendations: prohibiting fishing around the reported spawning aggregation site of Punta Macao in July, August and September; establishing a minimum legal size at 110cm of TL; and last, prohibiting spearfishing of goliath grouper nationwide. The proposal was so compelling, that the Consultative Fisheries Commission agreed to propose a complete ban of fishing goliath grouper in Cuba and carry out a nationwide consultation among fishers and other stakeholders.
For several months there were meetings at fishing communities, and the proposal was shared among other stakeholders. Fishers first opposed the proposed regulations for reasons discussed previously, but scientific facts provided them with enough information to support the proposal later. As important as the scientific facts, were the ways we interacted with the fishers, and how we presented the scientific information. There were several formal meetings, but most of the interactions with fishers were informal (taking advantage of our personal relationships with many of them, built during many years of knowing each other), meetings at their homes, harbours and gathering places. We showed them pictures and videos of spawning aggregations of goliath grouper with many specimens, and others where they no longer aggregate, and with divers enjoying the underwater experience with goliath groupers in order to show them the success and failure stories of human interaction with this species. After the involvement with fishers and other stakeholders, consensus was achieved and the Resolution 178/2018 was passed, to fully protect goliath grouper in Cuban waters.
Closing remarks
In this chapter, we aimed to show that diverse sources of information, stakeholders’ involvement, and the combination of management tools, yield the best results for conserving endangered species. The case of goliath grouper conservation in Cuba is an example of the hard, but possible task of protecting traditional fisheries resources. Science, traditional knowledge, political will, stakeholders’ involvement, delivering messages in several ways and venues, international collaboration and multiple management tools acting together, helped to advance conservation for this endangered species. This is a good example how diverse approaches have more profound positive impacts on species conservation, than a single approach. This diverse approach is worthy of application for endangered fish species not only in Cuba, but also in the Caribbean and elsewhere.
Acknowledgements
The authors thank Avalon Fishing and Diving Center for its incredible and long-lasting support for scientific research on Jardines de la Reina, especially G. Omegna (Pepe) and Noel López; and the Pew Fellowship for Marine Conservation and Environmental Defense Fund for contributing to the conservation of goliath grouper in Cuba, through the FPA Fellowship. We are also grateful to the personnel of several institutions who support research and conservation of goliath grouper in Cuba: Center for Coastal Ecosystems Research (Cuba), Center for Environmental Research of Camagüey (Cuba), fishers from Júcaro, Santa Cruz del and Cabo Cruz, Ministry of Food (Cuba), Center for Fisheries Research (Cuba), Center for Marine Research of Havana University (Cuba) and World Wildlife Fund (WWF) Canada.
Supplementary section
Box 3.1 Explanation of hooks.hours and dives.hours.
Hooks.hours and dives.hours are not averages but totals. The fishing effort using set line hooks and the survey effort using dive time are not always the same. Therefore, these methods are the best ways to demonstrate and standardize those efforts. For clarification, please see the following two examples below:
- If we place 32 set line hooks in a mangrove channel for 5 hours, we would have 160 hooks.hours (32 hooks x 5 hours = 160 hooks.hours). Similarly, if we place 50 set line hooks in the same channel for 4.5 hours, we would have 225 hooks.hours. This allows us to calculate the catch per unit of effort by dividing the number of fish caught by either 160 or 225, or the sum of both (385).
- If at a coral reef spawning aggregation site, you have 5 divers and each one stays underwater for 30 minutes (0.5 hour), you would have 2.5 dives.hours (5 dives x 0.5 hours = 2.5 dives.hours). Similarly, if you have 4 divers at the same site and each one stays underwater for 45 minutes (0.75 hour), you would have 3 dives.hours. This allows you to calculate the abundance per unit of effort by dividing the number of fish sighted by either 2.5 or 3, or the sum of both (5.5).
Quantitative data analysis
We used a generalized additive mixed model (GAMM) to characterize the total abundance of goliath grouper at the spawning aggregation site as a function of hour of the day, day of the moon phase, month, and depth, using the mgcv package in R (Wood 2017; R Core Team 2018). The optimum GAMM model incorporated the hour of the day adjusted (cyclic cubic regression splines) and the days of the moon phase. The GAMM model included a spherical correlation structure that adjusted a self-correlation of the days of the moon phase and months. The optimum GAMM model for presence/absence of small size goliath groupers (< 1m TL) incorporated the hour of the day adjusted (cyclic cubic regression splines) and the days of the moon phase. The optimum GAMM model for presence/absence of medium size goliath groupers (1–1.5m TL) incorporated the hour of the day, days of the moon phase, and months. The optimum GAMM model for presence/absence of large size goliath groupers (>1.5m TL) incorporated the hour of the day, days of the moon phase, months, and the depth.
References
Artero, C., D. J. Murie, C. C. Koenig, R. Berzins, C. Bouchon and L. Lampert. (2015a). Age, growth, and mortality of the Atlantic goliath grouper Epinephelus itajara in French Guiana. Endangered Species Research 28: 275-287. https://doi.org/10.3354/esr00691
Artero, C., C. C. Koenig, R. Berzins, C. Bouchon and L. Lampert. (2015b). Ontogenetic dietary and habitat shift in goliath grouper Epinephelus itajara from French Guiana. Endangered Species Research 27: 155-168. https://doi.org/10.3354/esr00661
Bertoncini A. A., A. Aguilar-Perera, J. Barreiros, M. T. Craig, B. P. Ferreira, C. C. Koenig. (2018). Epinephelus itajara (errata version published in 2019). The IUCN Red List of Threatened Species 2018:e.T195409A145206345. https://dx.doi.org/10.2305/IUCN.UK.20182.RLTS.T195409A145206345.en
Bravo-Calderon A., A. Saens-Arroyo, S. Fulton, A. Espinoza-Tenorio, E. Sosa-Cordero. (2021). Goliath grouper Epinephelus itajara oral history, use, and conservation status in the Mexican Caribbean and Campache Bank. Endangered Species Research 45: 283-300. https://doi.org/10.3354/esr01135.
Bueno, L. S., A. A. Bertoncini, C. C. Koenig, F. C. Coleman, M. O. Freitas, J. R. Leite, T. F. De Souza and M. Hostim-Silva. (2016). Evidence for spawning aggregations of the endangered Atlantic goliath grouper Epinephelus itajara in southern Brazil. Journal of Fish Biology 89(4): 2378-2391. https://doi.org/10.1111/jfb.13028
Bullock, L. H., D. Murphy, M. F. Godcharles and M. E. Mitchell. (1992). Age, growth, and reproduction of jewfish Epinephelus itajara in the eastern Gulf of Mexico. Fishery Bulletin 90(1): 243-249.
Claro, R. and K. C. Lindeman. (2003). Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf and Caribbean Research 14(2): 91-106.
Claro, R., K. C. Lindeman and L. Parenti. (eds.). (2001). Ecology of the Marine Fishes of Cuba. Washington, DC: Smithsonian Institution Press.
Coleman, F. C., C. C. Koenig and L. A. Collins. (1996). Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environmental Biology of Fishes 47(2): 129-141.
Coleman, F. C., et al. (2000). Long-lived reef fishes: The grouper-snapper complex. Fisheries 25(3): 14-21.
Eklund, A. M. and J. Schull. (2001). A stepwise approach to investigating the movement patterns and habitat utilization of goliath grouper, Epinephelus itajara, using conventional tagging, acoustic telemetry, and satellite tracking. In J. R. Sibert and J. L. Nielsen (eds.), Electronic tagging and tracking in marine fisheries (pp. 189-216). Philadelphia, USA: Kluwer Academic Publishers.
Figueredo-Martín, T., and F. Pina-Amargós. (2023). Fish Can Be more Valuable Alive than Dead. In Zlatarski, V. N. et al (eds.), Coral Reefs of Cuba (pp. 429-438). Switzerland: https://doi.org/10.1007/978-3-031-36719-9.
Figueredo-Martín, T., F. Pina-Amargós, J. A. Angulo-Valdés and R. Gómez-Fernández. (2010a). Buceo contemplativo en Jardines de la Reina: caracterización y percepción sobre el estado de conservación en el área. Revista de Investigaciones Marinas 31(1): 23-32.
Figueredo-Martín, T., F. Pina-Amargós, J. A. Angulo-Valdés and R. Gómez-Fernández. (2010b). Pesca recreativa en Jardines de la Reina: caracterización y percepción sobre el estado de conservación en el área. Revista de Investigaciones Marinas 31(2): 141-148.
FFWCC (Florida Fish and Wildlife Conservation Commission). (2024). https://myfwc.com/license/recreational/saltwater-fishing/goliath-grouper-harvest-permit/
García-Cagide, A., R. Claro and B. V. Koshelev. (2001). Reproductive patterns of fishes of the Cuban Shelf. In R. Claro, K. C. Lindeman and L. Parenti (eds.), Ecology of the Marine Fishes of Cuba (pp. 73-114). Washington, DC: Smithsonian Institution Press.
GMFMC (Gulf of Mexico Fishery Management Council). (1990). Amendment Number 2 to the Fishery Management Plan for the Reef Fish Fishery of the Gulf of Mexico. GMFMC.
Gerhardinger, L. C., R. C. Marenzi, Á. A. Bertoncini, R. P. Medeiros and M. Hostim-Silva. (2006). Local ecological knowledge on the goliath grouper Epinephelus itajara (Teleostei:Serranidae) in southern Brazil. Neotrop Ichthyol 4: 441-450.
Gerhardinger, L. C., M. Hostim-Silva, R. P. Medeiros, J. Matarezi, Á. A. Bertoncini, M. O. Freitas and B. P. Ferreira. (2009). Fishers’ resource mapping and goliath grouper Epinephelus itajara (Serranidae) conservation in Brazil. Neotrop Ichthyol 7: 93-102.
Giglio, V. J., M. G. Bender, C. Zapelini and C. E. L. Ferreira. (2017). The end of the line? Rapid depletion of a large-sized grouper through spearfishing in a subtropical marginal reef. Perspect Ecol Conserv 15: 115-118.
Heemstra, P. C. and J. E. Randall. (1993). FAO species catalogue. Vol 16. Groupers of the world (Family Serranidae, Subfamily Epinephelinae). An annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper, and lyretail species known to date. FAO Fisheries Synopsis no. 125, FAO.
Koenig, C. C., F. C. Coleman, L. A. Collins, Y. Sadovy and P. L. Colin. (1996). Reproduction of gag (Mycteroperca microlepis) (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. In ICLARM Conference 48. ICLARM Conference Proceeding (pp. 307-323). Manila, Philippines.
Koenig, C. C., F. C. Coleman and K. Kingon. (2011). Pattern of recovery of the goliath grouper (Epinephelus itajara) population in the southeastern US. Bulletin of Marine Science 87(4): 891-911. http://dx.doi.org/10.5343/bms.2010.1056
Koenig, C. C., L. S. Bueno, F. C. Coleman, J. A. Cusick, R. D. Ellis, K. Kingon, J. V. Locascio, C. Malinowski, D. J. Murie and C. D. Stallings. (2016). Diel, lunar, and seasonal spawning patterns of the Atlantic goliath grouper, Epinephelus itajara, off Florida, United States. Bulletin of Marine Science 92(1): 1-16. http://dx.doi.org/10.5343/bms.2016.1013
Kritzer, J. P., C. C. Hicks, B. D. Mapstone, F. Pina-Amargós and P. F. Sale. (2014). Ecosystem-based management of coral reefs and interconnected nearshore tropical habitats. In M. J. Fogarty and J. J. McCarthy (eds.), Marine Ecosystem-Based Management (pp. 369-428). Harvard University Press.
Mann, D. A., J. V. Locascio, F. C. Coleman and C. C. Koenig. (2009). Goliath grouper (Epinephelus itajara) sound production and movement patterns on aggregation sites. Endangered Species Research 7: 229-236.
McGovern, J. C., G. R. Sedberry and P. J. Harris. (1998). Status of stocks of reef fishes in the South Atlantic Bight, 1983-1996. Proceedings of the Gulf and Caribbean Fisheries Institute 50: 871-895.
Perera-Valderrama, S., A. Hernández, J. González, O. Moreno, D. Cobián-Rojas, H. Ferro-Azcona, E. Milián, H. Caballero, P. Alcolado, F. Pina-Amargós, Z. Hernández, L. Espinosa, L. F. Rodríguez. (2018). Marine protected areas in Cuba. Bulletin of Marine Science 94(2): 423-442.
Perera-Valderrama, S., J. González-Méndez, A. Hernández-Ávila, R. Estrada-Estrada, D. Cobián-Rojas, A. Ramón-Puebla, E. de la Guardia-Llansó, H. Ferro-Azcona, J. Hernández-Albernas, Z. Hernández-González, L. Espinosa-Pantoja, A. Lara, F. Pina-Amargós, P. González-Díaz, P. P. Chevalier-Monteagudo, N. Rey-Villiers, J. Antonio Tamayo-Fonseca and H. Caballero-Aragón. (2021). Coral reefs in Cuban marine protected areas. In Z. N. Vassil and B. J. Greenstein (eds.), Coral Reefs of Cuba (Series of Coral Reefs of the World). Springer. In review.
Pina-Amargós, F. and G. González-Sansón. (2009). Movement patterns of goliath grouper Epinephelus itajara around southeast Cuba: implications for conservation. Endangered Species Research 7: 243-247.
Sadovy, Y. and A. Eklund. (1999). Synopsis of biological data on Nassau grouper, Epinephelus striatus (Bloch 1792), and the jewfish, E. itajara (Lichtenstein 1822). NOAA Tech Rep NMFS 146. US Dept Commerce.
safR Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/
SAFMC (South Atlantic Fishery Management Council). (1990). Amendment number 2, regulatory impact review, regulatory flexibility analysis and environmental assessment for fishery management plan for the snapper grouper fishery of the South Atlantic region. SAFMC.
Sedberry, G. R., O. Pashuk, D. M. Wyanski, J. A. Stephen and P. Weinbach. (2006). Spawning locations for Atlantic reef fishes off the southeastern US. Proceedings of the Gulf and Caribbean Fisheries Institute 57: 463-514.
Smith, C. L. (1971). A revision of the American groupers: Epinephelus and allied genera. Bulletin of the American Museum of Natural History 146: 69-241.
Wood, S. N. (2017). Generalized Additive Models: An Introduction with R (2nd edition). Boca Ratón: Chapman and Hall/CRC Press. https://doi.org/10.1201/9781315370279
Zapelini C., V. J. Giglio, R. C. Carvalho, M. G. Bender, L. C. Gerhardinger. (2017). Assessing fishing experts’ knowledge to improve conservation strategies for an endangered grouper in the southwestern Atlantic. J Ethnobiol 37: 478-493.
1 Center for Marine Research of the University of Havana, https://orcid.org/0000-0003-3837-3673
