10. Are mountain and plains zebra hybridising in north-west Namibia?
©2024 ǂKibagu Heinrich Kenneth |Uiseb, CC BY-NC 4.0 https://doi.org/10.11647/OBP.0402.10
Abstract
This chapter focuses on interactions between two animal species critical to the ecosystems of “Etosha-Kunene”, namely mountain zebra (Equus zebra, specifically the subspecies E. z. hartmannae) and plains zebra (E. quagga, specifically the subspecies E. q. burchellii). Large herbivore species are increasingly restricted to fenced protected areas with artificial waterpoints, a situation that limits their opportunities for dispersal and access to natural water sources. This restricted movement may lead to genetic consequences including disruption of gene flow, inflation of “inbreeding”, and the loss of rare alleles supporting local adaptation and genetic fitness. In Namibia’s large protected area of Etosha National Park, mountain zebra are restricted to the dolomite ridges in the far western section of the park, while plains zebra occur throughout the park. Historically, the overlap in range of the two zebra species was limited, as plains zebra confined their movements to the southern and eastern edges of the Etosha Pan during the dry season, and to the open plains west of the Pan during the rainy season. Due to fencing and new waterpoint creation, the current overlap of these two previously geographically separated species creates a potential conservation problem in the form of hybridisation between the two species. This chapter reviews what is known about the hybridisation of these two species, and considers implications for conservation and for future research.
10.1 Introduction
This chapter reports on an ongoing study aiming to assess and understand the mechanisms and extent of hybridisation in naturally occurring populations of mountain zebra (Equus zebra hartmannae) and plains zebra (Equus quagga burchellii). Drawing on integrated genetics and ecological approaches, its focus is Etosha National Park (ENP) and connected landscapes to its west. In this context, hybridisation may arise when these two populations of individuals taxonomically distinguished based on one or more heritable characters may overlap in space and temporarily cross to form viable, and at least partially fertile offspring.1 Concerns may arise in this situation in connection with a wider context of the rapid loss of biodiversity globally in part due to anthropogenic changes to the natural environment.2
The impacts of human activities are observed at all levels of biodiversity, from the modification of ecosystems to the extinction of species and the loss of genetic diversity. Human alteration of the physical landscape and species distribution can additionally affect gene flow and introgression3 by influencing the degree of contact between groups of individuals.4 Large herbivore species are increasingly restricted to fenced protected areas, a situation that limits their opportunities for dispersal and their access to natural water sources.5 This restricted movement may lead to genetic consequences, including disruption of gene flow, inflation of inbreeding, and loss of rare alleles supporting local adaptation and genetic fitness.6
Many protected areas located in Africa use artificial water points to provide water for wildlife in the dry season.7 Availability of vital resources such as water may alter wildlife distribution as some herbivores no longer need to migrate and become localised. This localisation may cause a rapid population increase of water-dependent species such as zebra, increasing competition with more vulnerable low-density species,8 as well as interspecies interaction.9
10.1.1 Study area
Etosha National Park is a wildlife reserve located in northern Namibia between 18’80’ S-19’23 S and 15’70 E-16’5 E, with an average elevation of 1050 m10 (see Figure 10.1). The area that is now ENP was once part of the large connected landscape of about 80,000 km2 named Game Reserve No. 2 at the time of its proclamation under the German colonial regime in 1907 (for details see Chapter 1). In 1947, the north-western part of Game Reserve No. 2 became simultaneously a “native reserve” area home to otjiHerero-speaking peoples (see Chapters 2, 6 and 7), with Khoekhoegowab-speaking peoples also present throughout southern Kunene to north of Puros and towards the coast (see Chapters 1, 12 and 13).11 In 1970 the size and boundaries of ENP as it currently exists were established, its extent now encompassing 22,000 km2 (for details see Chapter 2).12
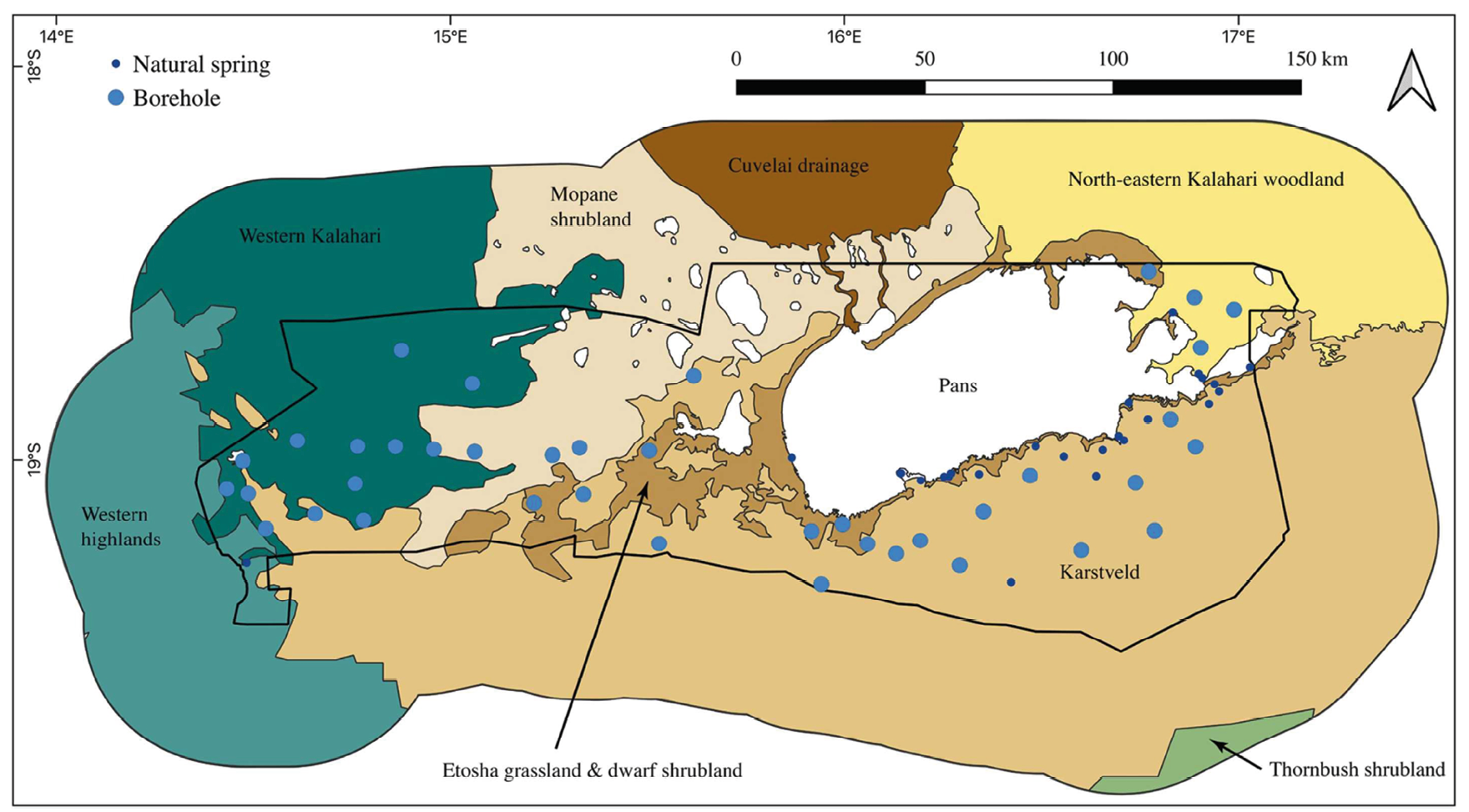
Fig. 10.1 Map showing the major vegetation communities characterising Etosha National Park (signalled by the inner black boundary) in connection with the Greater Etosha Landscape, together with the distribution of boreholes and natural springs. Saline pans are shown in white. Source: © Turner et al. (2022: Figure 2), reproduced with permission, CC BY-NC-ND 4.0.
Almost all ENP may be described as an arid to semi-arid savanna with 250-500 mm average annual rainfall and a highly variable and erratic rainfall pattern.13 The vegetation is classified as arid savanna including open grasslands and groves of woody species.14 Much of the park is covered by mopane (Colophospermum mopane) shrubveld and treeveld, alongside large salt pans with open grasslands along the pans. Seven basic vegetation types are described: bare ground, grassland, steppe, grass savanna, shrub savanna, low tree savanna and high tree savanna.15 These seven vegetation types are further grouped into the following basic habitat types: bare areas (salt pans), open plains (grassland, grass savanna and steppe), shrublands (shrub savanna) and woodlands (high tree and low tree savanna) (Figure 10.1). Common grass species are Cynodon dactylon, Eragrostis micrantha, E. rotifer, Diplachne fusca and Chloris virgata. Mopane is the dominant tree species.16 Etosha National Park has three characteristic seasons: the wet (rainy) season (January-April), the cool-dry season (May-August) and the hot-semi-dry season (September-December). The mean monthly temperatures range from 25°C to 6°C minimum in June and July, to highs of 34-35°C in October-December, and lows of around 18°C in November-February.17 Etosha National Park supports a high density of mammal populations with many herbivores of which zebra and springbok (Antidorcas marsupialis) are the most abundant plains ungulates.18 African wild dog (Lycaon pictus) and Cape buffalo (Syncerus caffer caffer) were known historically in the park area but no longer occur here.19
In ENP, perennial water is found only in fountains and drinking troughs supplied by boreholes. Rivers and water-courses are dependent upon rainfall and as such are not important sources of water for wildlife during the dry season.20 Park boundary fences covering over 850 km were erected in the 1970s,21 blocking wildlife dispersal beyond the park’s boundary, thereby preventing migrations to external water sources in the dry season (see Chapter 2). A consequence of this situation is that several artificial waterholes were established from the 1950s onwards to improve the wildlife viewing experience for tourists and provide water for wildlife within the park.22 Some waterpoints, especially those on the 19th latitude (corresponding roughly with the southern boundary of Etosha Pan), were established to attract elephants (Loxodonta africana) back into the park as a measure to reduce human-elephant conflict in commercial farms close to the protected area (see Chapter 11). There are now over 100 perennial watering points in the Park, including artesian springs, contact seeps and 55 boreholes23 (see Figure 10.1). On the broader implications of changing the hydrology of landscapes in north-west Namibia through the drilling of boreholes also see Chapter 7.
10.1.2 The study species
The large protected area of ENP in north-central Namibia is home to two zebra species, Hartmann’s mountain zebra (E. z. hartmannae) and Burchell’s plains zebra (E. q. burchellii). Mountain zebra are restricted to the dolomite ridges in the far western section of the park while plains zebra occur throughout the park. In this section, I outline the taxonomic relationships between these two zebra species concerning equids in Africa and beyond.
There are seven species of wild equids of which four occur in Africa and three in Asia.24 All equid species are similar in size and body shape, have a polygynous mating strategy, inhabit open grass or shrub-dominated habitats, and are predominantly grazers.25 Equids are highly efficient hind-gut fermenters, adapted to compensate for low-quality food by consuming large quantities.26 African wild ass (Equus africanus), Grevy’s zebra (Equus grevyi), mountain zebra (E. z. hartmannae and E. z. zebra) and plains zebra (Equus quagga) are the four equids occurring in Africa. Mountain zebra and plains zebra are the focal species for this study.
Plains zebra range from southern Sudan and southern Ethiopia, east of the Nile River, to southern Angola, northern Namibia and northern South Africa.27 Six morphologically defined subspecies of plains zebra are recognised based on morphological and genetic cline from north to south across its range.28 The total population of plains zebra across its range is estimated at over 500,000 animals. However, a reduction in numbers of 24% has been observed since the last assessment in 2002, and plains zebra is now listed by the International Union for Conservation of Nature (IUCN) as Near Threatened.29
Historically, mountain zebra occurred from the southern parts of South Africa through Namibia and into south-western Angola. Two subspecies of mountain zebra are recognised: Cape mountain zebra were widely distributed along the mountain ranges forming the southern and western edge of the central plateau of Eastern Cape and Western Cape provinces of South Africa, from the Amatola Mountains in the Cathcart District westward and northward to the Kamiesberg in Northern Cape; Hartmann’s mountain zebra––named after Georg Hartmann, the surveyor for the German colony discussed in Chapters 1 and 12––occurs in the mountainous transition zone between the Namib Desert and the central plateau in Namibia, with a marginal extension into south-western Angola.30 Although the Hartmann’s mountain zebra population has increased overall in recent years, the subspecies remain at threat from droughts that may lead to mortalities across their range. A high proportion (>50%) of mountain zebra occurs on private land where during times of drought they may not be prioritised as they compete with livestock for grazing and water: farmers tend to prefer to protect their cattle by increasing the harvest of zebra, putting the population at risk if dry periods are frequent and prolonged. In Kunene’s communal land areas, a marked decline in the number of mountain zebra has occurred as a result of prolonged drought in combination with high offtake levels into this recent drought period (see Chapter 3, Table 3.2 and Figures 3.4 and 3.5). This subspecies is listed as Vulnerable to extinction by IUCN.31
Both mountain zebra and plains zebra occur in Namibia where their natural distribution range overlap in northern Namibia. Figure 10.2 shows the historical, current and introduced range of mountain zebra and plains zebra.
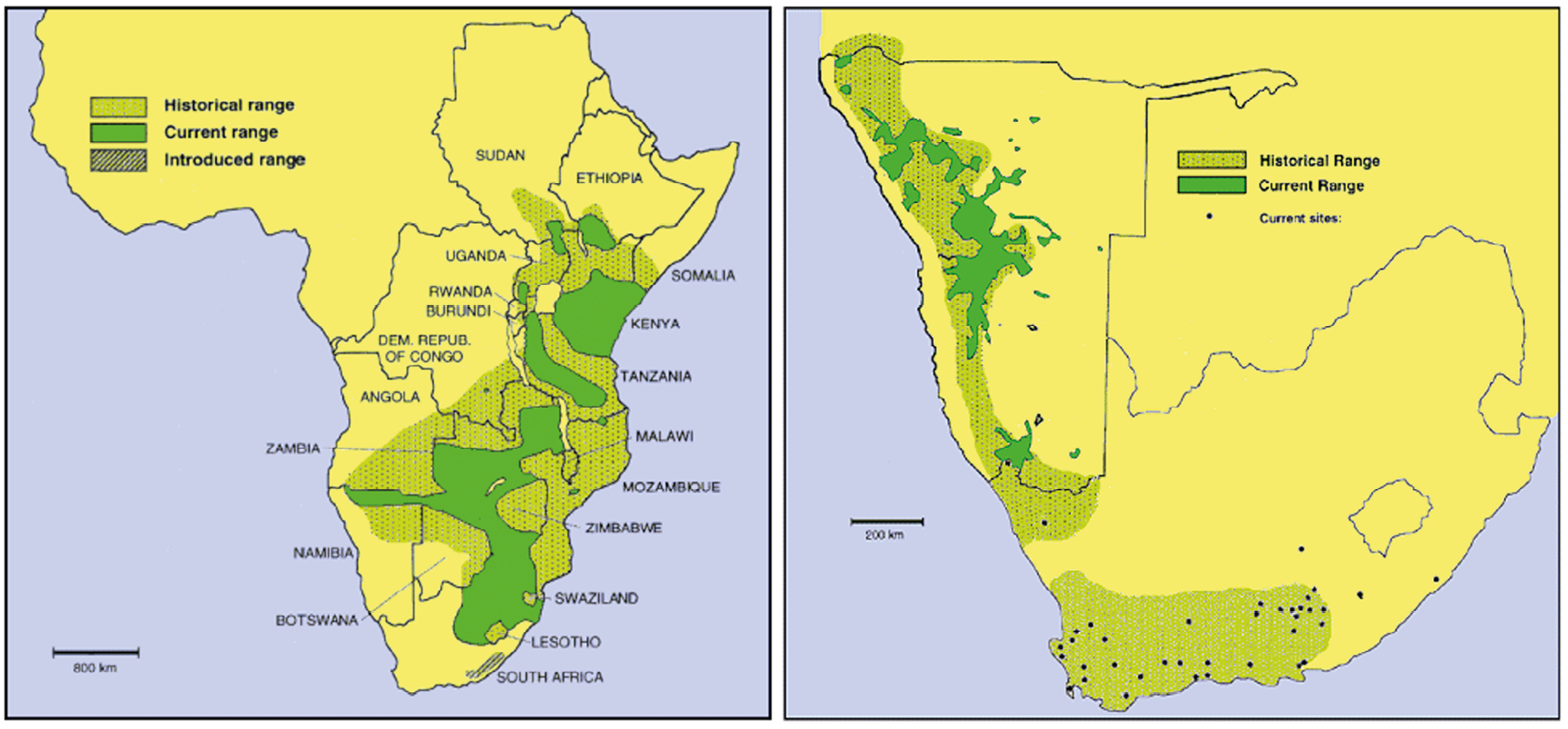
Fig. 10.2 Maps showing the historical, current and introduced range of plains zebra (Equus quagga) (left), and of mountain zebra (Equus zebra) in southern western Africa (right). Source: http://www.equids.org/images/L_PZebra.gif (L) and http://www.equids.org/images/L_MZebra.gif (R) (public domain images), CC BY-NC-ND 4.0.
Historically, the overlap in the range of the two zebra species in the area of ENP was limited. Plains zebra confined their movements to the southern and eastern edges of Etosha Pan during the dry season, and to the open plains west of the Pan during the rainy season.32 Mountain zebra in the park are restricted to the rocky and mountainous western section of the park, and west of the park into the escarpment. With the artificial provision of perennial water sources throughout the park, however, plains zebra expanded their range and now overlap extensively with the mountain zebra range in the west.33 This extended overlap in the range of the two previously geographically separated species in Etosha creates a potential conservation problem in the form of hybridisation between the two species—as discussed in detail in Section 10.2.2. The movement of mountain zebra to the west is restricted by the park boundary fence, while the two species interact at waterholes, and sometimes are observed grazing together.34 Plains zebra occur at a higher density throughout the park compared to mountain zebra.35
Plains zebra mares with foals depicting an intermediate phenotype of plains and mountain zebra were observed in western Etosha in the 1980s during zebra translocation operations,36 as well as along the southern boundary fence near Ombika.37 Hybridisation is thought to be more prevalent in western Etosha where the range of the two species overlap.38 Apart from observations based on the phenotypical evidence of foals with intermediary striping patterns, no in-depth research has been undertaken to understand the circumstances surrounding the phenomenon of zebra hybridisation in ENP. However, a pilot research project was initiated to test for hybridisation between the two zebra species using molecular studies.39 At the same time, there is currently no scientific basis for extrapolating the extent of hybridisation to determine whether or not it is a priority conservation concern for one or both zebra species. There is thus a need to identify and understand the ecological and genetic characteristics and causal mechanisms for hybridisation to inform possible remedial measures to reduce or eliminate associated conservation risks.
10.2 Conceptualising home range, habitats and hybridisation:
A review of literature
As mentioned, provision of artificial water for wildlife, and fencing off of the ENP protected area, is suspected to have facilitated extended overlap between historically separated wildlife species, leading to potential conservation challenges such as hybridisation. In this section, I review literature on an array of ecological and biological factors that may play a part in causing hybridisation between plains and mountain zebra in the area of ENP, to assess hybridisation likelihood and potential conservation consequences.
10.2.1 Home range and habitat use
Understanding wildlife movements and habitat use is critical for species conservation and management.40 Animal space use is a central topic in ecology that has been addressed from two complementary viewpoints, namely geographic and environmental space. Typically, studies rooted in geographic space focus on home range size and spatial distribution, whereas studies focusing on environmental space aim to identify factors determining resource use and selection.41 The most commonly used definition for an animal’s home range is the area traversed by the individual in its normal activities of food gathering, mating and caring for young.42 In this view, occasional ventures outside an area, perhaps exploratory, should not be considered as part of the home range.43 Home ranges differ among animals of different species, among individuals within species, and even in individuals over time.
Home range behaviour is a common pattern of space use although there is also variation in animal home range size. Identifying factors that underlie this variation is fundamental to understanding the distribution and abundance of animals, and ultimately their population regulation, habitat selection and community structure—all relevant for management choices for the conservation of ecosystems.44 Furthermore, home range behaviour is thought to be an expression of an animal’s decision-making process, shaped by natural selection, to access spatially dispersed resources in a manner that increases fitness.45
Biologists track animals to estimate the size and shapes of home ranges, movement patterns within home ranges, home range overlap among individuals, and how home range boundaries vary over time.46 Home range size is influenced by several factors. Generally, home range size has been shown to decrease with decreasing body size, increased forage availability, and intraspecific competition, while interspecies competition leads to increasing home ranges.47 Large mammals have larger home ranges than small mammals because they require more energy and therefore need a greater area in which to find this energy.48 Other factors such as resource heterogeneity, abundance of predators, number of offspring, and anthropogenic disturbance also influence the size of the home range of a species49 (see Chapters 17 and 19 for how these issues manifest concerning lion (Panthera leo)).
Habitat is a theoretical construct used to describe the living space of an organism. It includes the suite of interacting abiotic (e.g. weather, soils, topography, hydrology) and biotic (e.g. vegetation structure and composition, inter- and intra-specific competition, prevalence of diseases) elements influencing whether or not an organism uses a particular location.50 Habitat selection is defined as the disproportionately preferential use of habitat types relative to their availability,51 and is an outcome of individual characteristics, the landscape animals inhabit and relationships among these.52 In their simplest form, habitat studies describe the general distribution of animals, i.e. where they occur in relation to characteristics of their environment.53
Landscape use and the distribution of large mammalian herbivores are primarily driven by the availability of resources and the presence of constraints. Resources are usually related to forage characteristics, while constraints can limit the use of otherwise favourable environments.54 Grass quality and distribution are important characteristics defining the availability of forage resources for herbivores.55
In equids, as with other mammals, resources determine space use and movements. Home ranges of plains zebra, for example, differ across the continent, and across group composition. In East Africa home ranges in Ngorongoro were 80-250 km2, while they were larger in Serengeti where they were influenced by the migratory nature of the zebras; 3-400 km2 in the wet season and 4-600 km2 in the dry season.56 In Kruger National Park (KNP), South Africa, the plains zebra home ranges ranged from 49-566 km2.57 In another study conducted in KNP, the annual home ranges of plains zebra covered 150-250 km2 whereas the seasonal home ranges varied between 30-90 km2.58
Hartmann’s mountain zebra distribution is associated with rainfall patterns, so it has a marked seasonal variation. Their home ranges in Namibia’s winter grazing areas were 6-10 km2 in the fenced area of Daan Viljoen Game Reserve, and 10-20 km2 in the Otjovazandu area of ENP, with much smaller summer grazing areas in both areas.59 The home ranges of Cape Mountain Zebra breeding herds in Cape Mountain Zebra National Park, South Africa, ranged between 3-16 km2.60 The size and shape of the mountain zebra home range are determined by the availability of sufficient grazing, at least one permanent drinking place, mineral licks and sufficient shelter.61 A recent study in Namibia, however, reported much larger home ranges for mountain zebra averaging between 681 and 256 km2 in wet and dry seasons respectively in an unprotected area.62
A suitable habitat is an important factor affecting the distribution and abundance of wild animals.63 Several factors such as variation in structure, abundance and spatial distribution of plant resources,64 local density of herbivores,65 and sex and stage of life resulting in demographic differences,66 may influence habitat selection in herbivores. Preference for a given habitat type is largely determined by the available vegetation within an area which provides herbivores with food, water, minerals, shelter from climatic extremes and cover from predators.67 Of these vegetation features, food is considered the most important factor influencing habitat use among large herbivores.68
Wild and feral equids inhabit diverse grasslands, shrubland and woodland environments around the world and frequently display seasonal changes in home range dimensions or use in response to shifts in water and vegetation availability.69 Plains zebra prefer both open grasslands and woodlands.70 Spatio-temporal variation in habitat selection between open grasslands and woodlands by plains zebra exists as a response to predator avoidance and resource availability.71 A study in ENP established that plains zebra prefer open habitats in wet seasons and wetter years but shifted their selection preferences to woodlands in dry seasons and droughts.72 Mountain zebra are not territorial and could therefore be expected to range freely, selecting those areas that best suit their requirements.73 Mountain zebra were also found to prefer grasslands compared to other habitat types in a study conducted in Mountain Zebra National Park in South Africa.74 Not much more is known about the habitat preferences of Hartmann’s mountain zebra other than their recorded preferences for the mountain escarpment in Namibia.75
10.2.2 Hybridisation and landscape genetics
Hybridisation is a situation in which two populations of individuals distinguishable based on one or more heritable characters overlap in space and temporarily cross to form viable, and at least partially fertile offspring.76 Species boundaries are frequently challenged by lineage divergence and hybridisation. Diverged lineages are maintained by barriers to gene flow that vary in strength over time, space, or the genome.77 For closely related species, the barriers may be permeable, and changes in ecology, behaviour, population dynamics and distribution of species may result in increased levels of spatial and temporal sympatry,78 leading to an increased frequency of hybridisation events.79 Anthropogenic activities such as habitat degradation, domestication and translocation of animal species have recently increased the rate of hybridisation events worldwide as humans have facilitated contact between previously allopatric80 populations.81
Hybridisation between genetically differentiated populations, subspecies or even species often occurs in nature as a consequence of secondary contact: such hybridisation may remain constrained to narrow hybrid zones, or may cause widespread introgression with a variety of novel potentially adaptive genotypes.82 While the evolutionary consequences of natural hybridisation are usually positive, anthropogenic hybridisation can be problematic.83 Hybridisation can occur due to poor habitat, habitat modification, human-mediated introductions, small populations, skewed sex ratios and low mate availability.84 Determining whether hybridisation is “natural” or “anthropogenic” is crucial for conservation, with hybridisation especially problematic for rare species that come into contact with other more abundant species.85
While hybridisation is recognised as an important evolutionary force sometimes leading to the formation of new species, increasing rates of hybridisation in the last 20 years, due to anthropogenically induced habitat decline and the introduction of exotic species, is of concern from a conservation perspective.86 Whether viewed as a threat or opportunity, hybridisation presents challenges for conservation.87 In particular, a high frequency of hybridisation events followed by backcrossing may lead to the formation of a “hybrid swarm”,88 and in the most extreme cases may result in species replacement.89 Hybridisation and introgression may have harmful effects on the fitness of animal populations in the wild, causing loss of genetic diversity due to genetic homogenisation and/or outbreeding depression in local populations.90 It is thus important to strike a balance between these potentially detrimental and beneficial consequences when devising effective conservation strategies.91
Landscape genetics aims to provide information about the interaction between landscape features and micro-evolutionary processes such as gene flow, genetic drift and selection. Viewed as a hybrid between population genetics and landscape ecology, landscape genetics uses spatial genetic patterns as the focus for analysis.92 Landscape genetics treats genetic patterns as multivariate spatial data and seeks to infer ecological understandings by evaluating these patterns either in isolation, or in conjunction with other spatial data.93 This integrated approach allows an assessment of the impacts of landscape composition, configuration and habitat matrix quality on the spatial distribution of neutral and adaptive genetic variation and associated micro-evolutionary processes across natural populations.94 Landscape genetics investigates processes at a fine-spatial scale, generally around the dispersal scale of the organisms—such as the effect of barriers or fine-scale genetic structures with regards to landscape features—and is especially concerned with contemporary and recent processes.95 Issues of landscape effects on population structure, gene flow and identification of barriers, and fragmentation, connectivity and corridors, are some of the questions that can be answered by the study of conservation genetics.96
In the genus Equus, hybridisation has been well documented in captivity, as well as in the wild,97 and has also occurred where equid species have been introduced outside their natural range or where feral equids have interbred with wild equids.98 Cordingley and others99 reported for the first time the evidence of hybridisation between two equid species, plains zebra (E. quagga) and Grevy’s zebra (E. grevyi) in Kenya. Although there are differences in the chromosome numbers of Grevy’s zebra and plains zebra, meaning that fertile hybrid offspring are not expected,100 the hybridisation event in Kenya led to the production of viable hybrid offspring able to raise their young.101 In the Kenyan example, the directionality of gene flow was from Grevy’s zebra to plains zebra, as all known hybrid offspring were sired by male Grevy’s zebra. Dalton and others102 also found evidence of hybridisation between Cape Mountain zebra and plains zebra in South Africa, despite differences in their chromosomal numbers. In the South African example, the direction of gene flow was from plains zebra towards Cape mountain zebra, and the study only detected F1 hybrids103 which may indicate that the hybrids are infertile.104
Studies with a focus on population genetics and hybridisation between equids have clearly been conducted.105 At the same time, these studies lack the aspects of spatial ecology of the studied animals, and how this dimension influences their distribution and gene flow, and therefore the population genetic structuring of the studied populations.
10.2.3 Habitat suitability and landscape connectivity
Habitat suitability is defined as the probability that a species uses a particular habitat. In recent years, predictive modelling of species distribution has become an increasingly important tool to address various issues in ecology, biogeography, evolution, and also in conservation biology and climate change research.106 Habitat suitability models are based on the environmental characteristics of locations used or not used (presence, presence-absence, abundance) by the species in question.107 They can help select reserve networks,108 and evaluate connectivity,109 as these models predict the distribution of suitable habitats or resource patches in a landscape.
Maintaining functional connectivity in ecosystems—i.e. through an area or “corridor” which functions to allow wildlife dispersal without disturbance or hindrance (see Chapters 2, 3, 13 and 19)—
is considered critical for conserving large herbivores; especially those that track dynamic spatiotemporal gradients in resource availability, while minimising predation risk and human interference.110 Landscape connectivity is important for animal dispersal and gene flow in fragmented landscapes, as it allows for the rescuing of declining populations, the (re)colonisation of habitat patches, and prevents inbreeding effects in small populations.111 It is also a critical property in the persistence of spatially structured populations.112 Gene flow is usually restricted by distance, with individuals being genetically more related at shorter than longer geographical distances. Dispersal distance increases greatly when the dispersal route meanders through a fragmented landscape.113 Therefore land use and habitat fragmentation affect landscape connectivity and potentially reduce gene flow.114 Landscape genetic studies have thus incorporated complex landscape measures rather than straight-line distances to give a more realistic estimate of the effective distance between populations.115 Connectivity—the degree to which the landscape facilitates or impedes movement among resource patches—is often species and process-specific, such that a corridor for one species does not necessarily support the movement of other species,116 requiring the use of multi-species connectivity analysis. Such approaches to connectivity analysis can be valuable for prioritising functional conservation strategies that permit herbivore communities to follow changing vegetation productivity through annual cycles.117
Habitat-based and landscape genetic approaches are different but complementary. When combined they can identify important habitats for different life history requirements of a species. Furthermore, the integrated habitat and landscape genetics model also provides valuable information for resource managers to promote connectivity between critical habitats, through designing corridors and conservation areas118 (see Chapter 3). Various studies assessing the habitat suitability and landscape connectivity for equids have been conducted.119 For example, recent work on the population genetics of equids in southern Africa investigated the population genetic structuring of mountain zebra across its range in Namibia,120 and plains zebra across its range in eastern and southern Africa.121 However, all these studies concentrated on habitat suitability, landscape connectivity, and population genetics in isolation, without integrating these dimensions to understand the processes and patterns at the landscape genetics level for the two species. Additionally, most of the studies assessed the habitat suitability and landscape connectivity for single species only. The population genetic studies also focused on single species except in the case of a few studies that investigated hybridisation. Equally, the studies on habitat suitability and connectivity were also focused on single species.
As such, there is an opportunity here to study habitat suitability and landscape connectivity, as well as the population genetics of two co-occurring species of zebra, to understand the spatial and genetic outcomes of their interactions. As highlighted in Chapter 2 historical circumstances have led to the fragmentation and transformation of the wider landscape from Etosha Pan to the Skeleton Coast, giving rise to the permanent overlap in the range of historically separated but closely related species which may then hybridise with conservation consequences.
10.3 To conclude: New research objectives and hypotheses
for assessing zebra genetic integrity for conservation
management in ENP
As a response to the literature review and conceptual dimensions explored in Section 10.2, I now outline the development of a research project exploring the spatial ecology, hybridisation possibilities and conservation implications for mountain and plains zebra in ENP. Data collection is at a preliminary stage, but the research design itself illuminates issues of conservation concern and their management, and further highlights the potentially harmful unintended outcomes that past conservation (and other) policies leading to landscape transformation and fragmentation may have on certain wildlife species in the landscape. This ongoing research is pursuing the following objectives, via a series of hypotheses, as outlined below.
10.3.1 Objective 1: Home ranges and habitat selection
The first objective is to assess home ranges and habitat selection of mountain zebra and plains zebra in Etosha National Park to determine population and species connectivity, isolation or overlap. Here the research is structured by three hypotheses, namely:
- plains zebra have overall larger home range sizes compared to mountain zebra, and these differences in home range sizes remain the same throughout different seasons;
- owing to their similar ecology and physiology, no differentiation in habitat selection is expected for mountain zebra and plains zebra as both zebras will select for the same resources;
- overlap in the home ranges of the two zebra is expected throughout the seasons, and such overlap in home ranges is more profound around wildlife water points.
10.3.2 Objective 2: Hybridisation and genetic connectivity
Based on the literature review shared in Section 10.2, further research will assess hybridisation and genetic connectivity in tandem, by pursuing the following two objectives:
- to assess the extent of hybridisation in mountain zebra and plains zebra populations in the ENP landscape;
- to study genetic connectivity across the landscape to identify potential barriers for gene flow in mountain and plains zebra populations.
It is hypothesised that:
- hybridisation occurs between mountain zebra and plains zebra in the study area, and hybridisation events are restricted to a narrow hybrid zone in the area of overlap between the two species;
- low levels of genetic diversity are expected for mountain zebra in Etosha due to smaller population size and restricted gene flow between mountain zebra populations as a result of movement restrictions by fences;
- plains zebra are expected to have higher levels of genetic diversity owing to their larger and connected population size.
10.3.3 Objective 3: Multi-species habitat suitability and landscape connectivity modelling
The third objective for future research is to conduct multi-species habitat suitability and landscape connectivity modelling to correlate gene flow with landscape connectivity for mountain zebra and plains zebra, and to determine spatial probability for hybridisation. This objective is shaped by the following hypotheses:
- ENP offers limited suitable habitat for mountain zebra and connectivity to available suitable habitat is impaired by anthropogenic factors;
- ENP has suitable habitat for plains zebra whereas connectivity to available suitable habitat outside the park is impaired by anthropogenic factors.
10.3.4 Objective 4: Management recommendations for conserving zebra genetic integrity
The fourth and final objective is to draw on the research outlined above to make management recommendations for the conservation of genetic integrity for mountain zebra and plains zebra, potentially through spatial separation mechanisms. This objective is structured by the following hypotheses:
- it is expected that this study will show that habitat fragmentation restricts the movements of wildlife species and connectivity with suitable habitats elsewhere;
- it is further expected that habitat transformation which facilitates prolonged co-existence between previously allopatric but closely related species has implications for their population and landscape genetics.
To conclude, with this study I hope to shed more light on the home ranges, home range overlap and habitat selection of the two zebra species in the anthropogenically transformed landscape of ENP that has resulted from colonial and post-Independence conservation policies (see Chapters 1, 2 and 3), and how these have impacted on the population genetics of the two zebra species. I further wish to explore and understand the recent and past population genetic structuring of the two species as a result of habitat transformation, while investigating the existence of any gene flow across the landscape. The suitability of areas outside ENP will also be assessed to recommend viable conservation planning for these species that also involves local communities.
Bibliography
Allendorf, F., Leary, R., Spruell, P. and Wenburg, J. 2001. The problems with hybrids: Setting conservation guidelines. Trends in Ecology & Evolution 16: 613–22, https://doi.org/10.1016/S0169-5347(01)02290-X
Bartlam Brooks, H., Beck, P., Bohrer, G. and Harris, S. 2013. In search of greener pastures: Using satellite images to predict the effects of environmental change on zebra migration. Journal of Geophysical Research: Biogeosciences 118(4): 1427–37, https://doi.org/10.1002/jgrg.20096
Bauer, I., Mcmorrow, J. and Yalden, D. 1994. The historic ranges of three equid species in north-east Africa: A quantitative comparison of environmental tolerances. Journal of Biogeography 21(2): 169–82, https://doi.org/10.2307/2845470
Berry, H.H. 1997. Historical review of the Etosha region and its subsequent administration as a National Park. Madoqua 20(1): 3–10, https://hdl.handle.net/10520/AJA10115498_453
Berry, O., Tocher, M., Gleeson, D. and Sarre, S. 2005. Effect of vegetation matrix on animal dispersal: Genetic evidence from a study of endangered skinks. Conservation Biology 19(3): 855-64, https://doi.org/10.1111/j.1523-1739.2005.00161.x
Bevanda, M., Fronhofer, E., Heurich, M., Müller, J. and Reineking, B. 2015. Landscape configuration is a major determinant of home range size variation. Ecosphere 6(10): 195, https://doi.org/10.1890/ES15-00154.1
Binzenhöfer, B., Schröder, B., Strauss, B., Biedermann, R. and Settele, J. 2005. Habitat models and habitat connectivity analysis for butterflies and burnet moths – The example of Zygaena carniolica and Coenonympha arcania. Biological Conservation 126: 247–59, https://doi.org/10.1016/j.biocon.2005.05.009
Brown, C.J. and Jenkins, A.R. 1987. Hybridization between a Hartmann’s Mountain Zebra and a Donkey. Madoqua 15(2): 193–94, https://hdl.handle.net/10520/AJA10115498_361
Burt, W.H. 1943. Territoriality and home range concepts as applied to mammals. Journal of Mammalogy 24: 346–52, https://doi.org/10.2307/1374834
Chabwela, H., Chomba, C., Kaweche, G. and Mwenya, A. 2017. Habitat selection by large mammals in South Luangwa National Park, Zambia. Open Journal of Ecology 7: 179–92, https://doi.org/10.4236/oje.2017.73013
Chetkiewicz, C.-L. and Boyce, M. 2009. Use of resource selection functions to identify conservation corridors. Journal of Applied Ecology 46: 1036–47, https://doi.org/10.1111/j.1365-2664.2009.01686.x
Cordingley, J., Sundaresan, S., Fischhoff, I., Shapiro, B., Ruskey, J. and Rubenstein, D. 2009. Is the endangered Grevy’s zebra threatened by hybridization? Animal Conservation 12: 505–13, https://doi.org/10.1111/j.1469-1795.2009.00294.x
Courbin, N., Loveridge, A., Macdonald, D., Fritz, H., Valeix, M., Makuwe, E. and Chamaillé-Jammes, S. 2016. Reactive responses of zebras to lion encounters shape their predator-prey space game at large scale. Oikos 125: 829–38, https://doi.org/10.1111/oik.02555
Crego, R., Wells, H., Ndung’u, K. et al. 2021. Moving through the mosaic: Identifying critical linkage zones for large herbivores across a multiple-use African landscape. Landscape Ecology 36: 1325–40, https://doi.org/10.1007/s10980-021-01232-8
Crispo, E., Moore, J.-S., Lee-Yaw, J., Gray, S. and Haller, B. 2011. Broken barriers: Human-induced changes to gene flow and introgression in animals: An examination of the ways in which humans increase genetic exchange among populations and species and the consequences for biodiversity. BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology 33: 508–18, https://doi.org/10.1002/bies.201000154
Dalton, D.L., Zimmermann, D., Mnisi, C., Taplin, M., Novellie, P., Hrabar, H. and Kotze, A. 2017. Hiding in plain sight: Evidence of hybridisation between Cape mountain zebra (Equus zebra zebra) and plains zebra (Equus quagga burchelli). African Journal of Wildlife Research 47(1): 59–64, https://doi.org/10.3957/056.047.0059
Dalui, S., Khatri, H., Singh, S., Basu, S., Ghosh, A., Mukherjee, T., Sharma, L., Singh, R., Chandra, K. and Thakur, M. 2020. Fine-scale landscape genetics unveiling contemporary asymmetric movement of red panda (Ailurus fulgens) in Kangchenjunga landscape, India. Scientific Reports 10: 15446, https://doi.org/10.1038/s41598-020-72427-3
du Preez, J.S. and Grobler, I.D. 1977. Drinking times and behaviour at waterholes of some game species in the Etosha National Park. Madoqua 10(1): 61–69, https://journals.co.za/doi/pdf/10.10520/AJA10115498_129
Eckenwalder, J. 1998. Hybridization as evolutionary creation. American Journal of Botany 85(7): 1043–45, https://doi.org/10.2307/2446373
Fischhoff, I., Sundaresan, S., Cordingley, J. and Rubenstein, D. 2007. Habitat use and movements of plains zebra (Equus burchelli) in response to predation danger from lions. Behavioral Ecology 18: 725–29, https://doi.org/10.1093/beheco/arm036
Frank, D., Mcnaughton, S. and Tracy, B. 1998. The ecology of the earth’s grazing ecosystems. Bioscience 48(7): 513–521, https://doi.org/10.2307/1313313
Fynn, R. and Bonyongo, M. 2011. Functional conservation areas and the future of Africa’s wildlife. African Journal of Ecology 49: 175–88, https://doi.org/10.1111/j.1365-2028.2010.01245.x
Galov, A., Fabbri, E., Caniglia, R., Arbanasić, H., Lapalombella, S., Tihomir, F., Škrivanko, M., Galaverni, M. and Randi, E. 2015. First evidence of hybridization between golden jackal (Canis aureus) and domestic dog (Canis familiaris) as revealed by genetic markers. Royal Society Open Science 2(12): 150450, https://doi.org/10.1098/rsos.150450
Geenen, K. 2019. Ecological Impacts of Large Herbivores at Artificial Waterpoints in Majete Wildlife Reserve, Malawi. Unpublished MSc Thesis, Stellenbosch University, https://core.ac.uk/download/pdf/268881676.pdf
Gosling, L. 2014. The Mountain Zebra Project [Progress Report]. Namibia Nature Foundation.
Gosling, L.M., Muntifering, J., Kolberg, H., Uiseb, K.H.K. and King, S.R.B. 2019. Equus zebra ssp. hartmannae. The IUCN Red List of Threatened Species, https://www.iucnredlist.org/species/7958/45171819
Groves, C.P. and Bell, C.H. 2004. New investigations on the taxonomy of the zebras genus Equus, subgenus Hippotigris. Mammalian Biology 69: 182–96, https://doi.org/10.1078/1616-5047-00133
Guisan, A. and Thuiller, W. 2005. Predicting species distribution: Offering more than simple habitat models. Ecology Letters 8: 993–1009, https://doi.org/10.1111/j.1461-0248.2005.00792.x
Hack, M.A., East, R. and Rubenstein, D.I. 2002. Status and Action Plan for the Plains Zebra (Equus burchellii). In Moehlman P.D. (ed.) Equids: Zebras, Asses and Horses. Status Survey and Conservation Action Plan. IUCN/SSC Equid Specialist Group. Gland, Switzerland and Cambridge, UK: IUCN, 43–60.
Hailer, F. and Leonard, J. 2008. Hybridization among three native North American Canis species in a region of natural sympatry. PloS One 3(10): e3333, https://doi.org/10.1371/journal.pone.0003333
Harrington, R., Owen-Smith, N., Viljoen, P., Biggs, H., Mason, D. and Funston, P. 1999. Establishing the causes of roan antelope decline in Kruger National Park, South Africa. Biological Conservation 90: 69–78, https://doi.org/10.1016/S0006-3207(98)00120-7
Harris, G., Thirgood, S., Hopcraft, J.G.C., Cromsigt, J. and Berger, J. 2009. Global decline in aggregated migrations of large terrestrial mammals. Endangered Species Research 7: 55–76, https://doi.org/10.3354/esr00173
Harrison, R. and Larson, E. 2014. Hybridization, introgression, and the nature of species boundaries. The Journal of Heredity 105: 795–809, https://doi.org/10.1093/jhered/esu033
Hobbs, N., Galvin, K., Stokes, C., Lackett, J., Ash, A., Boone, R., Reid, R. and Thornton, P. 2008. Fragmentation of rangelands: Implications for humans, animals, and landscapes. Global Environmental Change 18: 776–85, https://doi.org/10.1016/j.gloenvcha.2008.07.011
Holderegger, R. and Wagner, H. 2006. A brief guide to landscape genetics. Landscape Ecology 21: 793–96, https://doi.org/10.1007/s10980-005-6058-6
Hoffman, L. 1989. An annotated list of amphibians and reptile observations from the Etosha National Park. Madoqua 16(2): 87–92, https://journals.co.za/doi/pdf/10.10520/AJA10115498_320
Huang, Y-H., Joel, H., Küsters, M. et al. 2021. Disease or drought: Environmental fluctuations release zebra from a potential pathogen-triggered ecological trap. Proceedings of the Royal Society B: Biological Sciences 288: 20210582, https://doi.org/10.1098/rspb.2021.0582
Iacolina, L., Corlatti, L., Buzan, E., Safner, T. and Sprem, N. 2018. Hybridisation in European ungulates: An overview of the current status, causes, and consequences. Mammal Review 49(1): 45-59, https://doi.org/10.1111/mam.12140
Janis, C. 1976. The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 30: 757–74, https://doi.org/10.1111/j.1558-5646.1976.tb00957.x
Jarman, P. and Sinclair, A.R.E. 2021. Feeding strategy and the pattern of resource partitioning in ungulates. In Sinclair, A.R.E. and Norton-Griffiths, M. (eds.) Serengeti: Dynamics of an Ecosystem. Chicago: University of Chicago Press, 185–220.
Johnson, D. 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61(1): 65–71, https://doi.org/10.2307/1937156
Joubert, E. 1972. Habitat preferences, distribution and status of the Hartmann zebra Equus zebra hartmannae in South West Africa. Madoqua 7: 5–15, https://hdl.handle.net/10520/AJA10115498_1
Kamath, P. 2011. Characterising Species Boundaries With an Integrated Genetic, Ecological and Behavioural Approach: Implications for Mountain Zebra Conservation in Namibia. Unpublished Research Proposal submitted for funding to the National Science Foundation (USA).
Kebede, F., Bekele, A., Moehlman, P. and Evangelista, P. 2012. Endangered Grevy’s zebra in the Alledeghi Wildlife Reserve, Ethiopia: Species distribution modeling for the determination of optimum habitat. Endangered Species Research 17: 237–44, https://doi.org/10.3354/esr00416
Kie, J., Matthiopoulos, J., Fieberg, J., Powell, R., Cagnacci, F., Mitchell, M., Gaillard, J-M., and Moorcroft, P. 2010. The home-range concept: Are traditional estimators still relevant with modern telemetry technology? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365: 2221–31, https://doi.org/10.1098/rstb.2010.0093
Kigen, C., Kirsteen, O., Konje, M. et al. 2013. Modeling the spatial impact of climate change on Grevy’s Zebra (Equus grevyi) niche in Kenya. Elixir Remote Sensing 62: 17608–11, https://www.elixirpublishers.com/articles/1680170968_201309035.pdf
Kilian, W. 2015. Aerial Survey Report of the Etosha National Park. Windhoek: Ministry of Environment Forestry and Tourism.
King, S. and Moehlman, P. 2016. Equus quagga, Plains Zebra. The IUCN Red List of Threatened Species, https://www.iucnredlist.org/species/41013/45172424
Knüsel, M., Lee, D. and Bond, M. 2019. Correlates of home range sizes of giraffes, Giraffa camelopardalis. Animal Behaviour 149: 143–51, https://doi.org/10.1016/j.anbehav.2019.01.017
Levänen, R., Thulin, C-G., Spong, G. and Pohjoismäki, J. 2018. Widespread introgression of mountain hare genes into Fennoscandian brown hare populations. PLOS ONE 13: e0191790, https://doi.org/10.1371/journal.pone.0191790
Loveridge, A., Valeix, M., Davidson, Z., Murindagomo, F., Fritz, H. and Macdonald, D. 2009. Changes in home range size of African lions in relation to pride size and prey biomass in a semi-arid savanna. Ecography 32: 953–62, https://doi.org/10.1111/j.1600-0587.2009.05745.x
Maier, J., Ver Hoef, J., McGuire, A., Bowyer, R., Saperstein, L. and Maier, H. 2005. Distribution and density of moose in relation to landscape characteristics: Effects of scale. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 35: 2233–43, https://doi.org/10.1139/x05-123
Manel, S., Schwartz, M., Luikart, G. and Taberlet, P. 2003. Landscape genetics: Combining landscape ecology and population genetics. Trends in Ecology and Evolution 18(4): 189–97, https://doi.org/10.1016/S0169-5347(03)00008-9
Mariotti, E., Parrini, F., Louw, J. and Marshal, J. 2020. Habitat use by a large herbivore guild in a fenced South African protected area. African Journal of Wildlife Research 50: 86–101, https://doi.org/10.3957/056.050.0086
Marshal, J., Bleich, V., Krausman, P., Reed, M. and Andrew, N. 2009. Factors affecting habitat use and distribution of desert mule deer in an arid environment. Wildlife Society Bulletin 34: 609–19, https://doi.org/10.2193/0091-7648(2006)34[609:FAHUAD]2.0.CO;2
McKelvey, K., Cushman, S. and Schwartz, M. 2010. Landscape genetics. In Cushman, S.A. and Huettmann, F. (eds.) Spatial Complexity, Informatics, and Wildlife Conservation. Berlin: Springer, 313–27.
McNaughton, S. J. 1987. Adaptation of herbivores to seasonal changes in nutrient supply. In Hacker, J.B. and Ternought, J.H. (eds.) The Nutrition of Herbivores. New York: Academic Press, 391–408.
Metzger, J. and Decamps, H. 1997. The structural connectivity threshold: An hypothesis in conservation biology at the landscape scale. Acta Oecologica 18: 1–12, https://doi.org/10.1016/S1146-609X(97)80075-6
Moehlman, P. 2002. Equids: Zebras, Asses and Horses. Cambridge: IUCN Publication Services Unit.
Montgomery, R. and Roloff, G. 2013. Habitat Selection. In Levin, S.A. (ed) Encyclopedia of Biodiversity. Amsterdam: Elsevier, 59–69, https://doi.org/10.1016/B978-0-12-384719-5.00384-1
Moodley, Y. and Harley, E. 2005. Population structuring in mountain zebras (Equus zebra): The molecular consequences of divergent demographic histories. Conservation Genetics 6: 953–68, https://doi.org/10.1007/s10592-005-9083-8
Montgelard, C., Zenboudji, S., Ferchaud, A.-L., Arnal, V. and Jansen van Vuuren, B. 2014. Landscape genetics in mammals. Mammalia 78(2): 139–57, https://doi.org/10.1515/mammalia-2012-0142
Muntifering, J., Ditmer, M., Stapleton, S., Naidoo, R. and Harris, T. 2019. Characterizing Hartmann’s mountain zebra resource selection and movement behavior within a large unprotected landscape in north-west Namibia. Endangered Species Research 38: 159–70, https://doi.org/10.3354/esr00941
Mwangi, T., Waithaka, E. and Boitt, M. 2018. Ecological niche modeling of zebra species within Laikipia County, Kenya. Journal of Geoscience and Environment Protection 6: 264–76, https://doi.org/10.4236/gep.2018.64016
Nikula, A., Heikkinen, S. and Eero, H. 2004. Habitat selection of adult moose Alces alces at two spatial scales in central Finland. Wildlife Biology 10(2): 121–25, https://doi.org/10.2981/wlb.2004.017
Odhiambo, D.O. 2017. Marker Development and Genetic Structure of Endangered Grevy’s Zebra (Equus Grevyi) in Kenya. Unpublished MSc Thesis, University of Nairobi.
Olivier, A.J. 2019. Ecology and Habitat Suitability of Cape Mountain Zebra (Equus zebra zebra) in the Western Cape, South Africa. Unpublished MSc Thesis, Stellenbosch University.
Ottenburghs, J. 2021. The genic view of hybridization in the Anthropocene. Evolutionary Applications 14(10): 2342–60, https://doi.org/10.1111/eva.13223
Owen-Smith, N. 2004. Functional heterogeneity in resources within landscapes and herbivore population dynamics. Landscape Ecology 19: 761–71, https://doi.org/10.1007/s10980-005-0247-2
Owen-Smith, N., Martin, J. and Yoganand, K. 2015. Spatially nested niche partitioning between syntopic grazers at foraging arena scale within overlapping home ranges. Ecosphere 6(9): 1-17, https://doi.org/10.1890/ES14-00487.1
Pedersen, C-E.T., Albrechtsen, A., Etter, P., Johnson, E., Orlando, L., Chikhi, L., Siegismund, H. and Heller, R. 2018. A southern African origin and cryptic structure in the highly mobile plains zebra. Nature Ecology & Evolution 2: 491–98, https://doi.org/10.1038/s41559-017-0453-7
Penzhorn, B. 1979. Social organisation of the Cape Mountain Zebra Equus Z. Zebra in the Mountain Zebra National Park. Koedoe: African Protected Area Conservation and Science 22(1): a655, https://doi.org/10.4102/koedoe.v22i1.655
Penzhorn, B. 1982a. Home range sizes of Cape Mountain Zebras Equus Zebra Zebra in the Mountain Zebra National Park. Koedoe: African Protected Area Conservation and Science 25(1): a608, https://doi.org/10.4102/koedoe.v25i1.608
Penzhorn, B. 1982b. Habitat selection by Cape mountain zebra in the Mountain Zebra National Park. South African Journal of Wildlife Research 12: 48–54.
Plessis, W.P. 1997. Refinements to the burning strategy in the Etosha National Park, Namibia. Koedoe: African Protected Area Conservation and Science 40(1): a264, https://doi.org/10.4102/koedoe.v40i1.264
Ransom, J. and Kaczensky, P. (eds.) 2016. Wild Equids: Ecology, Management, and Conservation. Baltimore: John Hopkins University Press.
Richard, E., Said, S., Hamann, J-L. and Gaillard, J-M. 2014. Daily, seasonal and annual variations in individual home range overlap of two sympatric species of deer. Canadian Journal of Zoology 92(10), https://doi.org/10.1139/cjz-2014-0045
Rivrud, I.M., Loe, L.E., Vik, J.O., Veiberg, V., Langvatn, R. and Mysterud, A. 2009. Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 90: 699–710, https://doi.org/10.1890/08-0576.1
Roug, A., Muse, E., Clifford, D. et al. 2020. Seasonal movements and habitat use of African buffalo in Ruaha National Park, Tanzania. BMC Ecology 20: a6, https://doi.org/10.1186/s12898-020-0274-4
Roux, C.J.G., Grunow, J.O., Morris, J.W., Bredenkamp, G.J. and Scheepers, J.C. 1988. A classification of the vegetation of the Etosha National Park. South African Journal of Botany 54: 1–10, https://doi.org/10.1016/S0254-6299(16)31355-2
Rubenstein, D. 1989. Life history and social organization in arid adapted ungulates. Journal of Arid Environments 17: 145–56, https://doi.org/10.1016/S0140-1963(18)30901-7
Ryder, O., Epel, N.C. and Benirschke, K. 1978. Chromosome banding studies of Equidae. Cytogenetics and Cell Genetics 20: 332–50, https://doi.org/10.1159/000130862
Shannon, G., Matthews, W., Page, B., Parker, G. and Smith, R. 2009. The effects of artificial water availability on large herbivore ranging patterns in savanna habitats: A new approach based on modelling elephant path distributions. Diversity and Distributions 15: 776–83, https://doi.org/10.1111/j.1472-4642.2009.00581.x
Sharma, B.D., Clevers, J., De Graaf, R. and Chapagain, N.R. 2004. Mapping Equus kiang (Tibetan Wild Ass) habitat in Surkhang, Upper Mustang, Nepal. Mountain Research and Development 24(2): 149–56, https://doi.org/10.1659/0276-4741(2004)024%5B0149:MEKTWA%5D2.0.CO;2
Smuts, G.L. 1975. Home range sizes for Burchell’s zebra Equus burchelli antiquorum from the Kruger National Park. Koedoe: African Protected Area Conservation and Science 18(1): a918, https://doi.org/10.4102/koedoe.v18i1.918
Sommer, S., McDevitt, A. and Balkenhol, N. 2013. Landscape genetic approaches in conservation biology and management. Conservation Genetics 14: 249–51, https://doi.org/10.1007/s10592-013-0473-z
Spalinger, D. and Hobbs, N. 1992. Mechanisms of foraging in mammalian herbivores: New models of functional response. The American Naturalist 140: 325–48, https://doi.org/10.1086/285415
Spencer, W. 2012. Home ranges and the value of spatial information. Journal of Mammalogy 93: 929–47, https://doi.org/10.1644/12-MAMM-S-061.1
Stander, P., Nott, T., Lindeque, P. and Lindeque, M. 1990. Mass marking of zebras in the Etosha National Park, Namibia. Madoqua 17(1): 47–49, https://journals.co.za/doi/pdf/10.10520/AJA10115498_393
Stevens, V., Verkenne, C., Vandewoestijne, S., Wesselingh, R. and Baguette, M. 2006. Gene flow and functional connectivity in the Natterjack toad. Molecular Ecology 15: 2333–44, https://doi.org/10.1111/j.1365-294X.2006.02936.x
Sullivan, S. 1999. Folk and formal, local and national: Damara knowledge and community conservation in southern Kunene, Namibia. Cimbebasia 15: 1–28.
Sullivan, S. and Ganuses, W.S. 2020. Understanding Damara / ǂNūkhoen and ǁUbun indigeneity and marginalisation in Namibia. In Odendaal, W. and Werner, W. (eds.) ‘Neither Here Nor There’: Indigeneity, Marginalisation and Land Rights in Post-independence Namibia. Windhoek: Land, Environment and Development Project, Legal Assistance Centre, 283–324, https://www.lac.org.na/projects/lead/Pdf/neither-13.pdf
Sullivan, S. and Ganuses, W.S. 2021. Densities of meaning in west Namibian landscapes: Genealogies, ancestral agencies, and healing. In Dieckmann, U. (ed.) Mapping the Unmappable? Cartographic Explorations with Indigenous Peoples in Africa. Bielefeld: Transcript, 139–90, https://www.transcript-open.de/doi/10.14361/9783839452417-006
Sullivan, S. and Ganuses, W.S. 2022. !Nara harvesters of the northern Namib: A cultural history through three photographed encounters. Journal of the Namibian Scientific Society 69: 115–39.
Turnbull, P.C.B., Lindeque, P., Roux, J., Bennett, A. and Parks, S. 1998. Airborne movement of anthrax spores from carcass sites in the Etosha National Park, Namibia. Journal of Applied Microbiology 84: 667–76, https://doi.org/10.1046/j.1365-2672.1998.00394.x
Turner, W. and Getz, W. 2010. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha National Park. Journal of Wildlife Diseases 46: 1108–19, https://doi.org/10.7589/0090-3558-46.4.1108
Turner, W.C., Périquet, S., Goelst, C.E. et al. 2022. Africa’s drylands in a changing world: Challenges for wildlife conservation under climate and land-use changes in the Greater Etosha Landscape. Global Ecology and Conservation 36: e02221, https://doi.org/10.1016/j.gecco.2022.e02221
van Moorter, B., Rolandsen, C., Basille, M. and Gaillard, J-M. 2015. Movement is the glue connecting home ranges and habitat selection. Journal of Animal Ecology 85(1): 21–31, https://doi.org/10.1111/1365-2656.12394
Vitousek, P., Mooney, H., Lubchenco, J. and Melilo, J. 1997. Human domination of Earth’s ecosystems. Science 277: 494–99, https://www.science.org/doi/10.1126/science.277.5325.494
Wassermann, M., Aschenborn, O., Aschenborn, J., Mackenstedt, U. and Romig, T. 2015. A sylvatic lifecycle of Echinococcus equinus in the Etosha National Park, Namibia. International Journal for Parasitology: Parasites and Wildlife 4(1): 97–103, https://doi.org/10.1016/j.ijppaw.2014.12.002
Winker, H., Novellie, P., Selier, J., Birss, C. and Hraber, H. 2016. Population Trends and Management Strategy Tools for Cape Mountain Zebra. Pretoria: SANBI (South African National Biodiversity Institute), https://doi.org/10.13140/RG.2.2.11145.60008
Winkler, A. and Owen-Smith, N. 1995. Habitat utilisation by Cape mountain zebras in the Mountain Zebra National Park, South Africa. Koedoe: African Protected Area Conservation and Science 38(1): a308, https://doi.org/10.4102/koedoe.v38i1.308
Wright, S.L. 1943. Isolation by distance. Genetics 28: 114–38.
Wyk, A., Kotz, A., Randi, E. and Dalton, D. 2013. A hybrid dilemma: A molecular investigation of South African bontebok (Damaliscus pygargus pygargus) and blesbok (Damaliscus pygargus phillipsi). Conservation Genetics 14(3): 589–99, https://link.springer.com/article/10.1007/s10592-013-0448-0
Zecherle, L., Bar-David, S., Nichols, H., Templeton, A., Hipperson, H., Horsburgh, G. and Brown, R. 2020. Landscape resistance affects individual habitat selection but not genetic relatedness in a reintroduced desert ungulate. Biological Conservation 252: 108845, https://doi.org/10.1016/j.biocon.2020.108845
Zidon, R., Garti, S., Getz, W. and Saltz, D. 2017. Zebra migration strategies and anthrax in Etosha National Park, Namibia. Ecosphere 8(8): e01925, https://doi.org/10.1002/ecs2.1925
Zielinski, W., Carroll, C. and Dunk, J. 2006. Using landscape suitability models to reconcile conservation planning for two key forest predators. Biological Conservation 133: 409–30, https://doi.org/10.1016/j.biocon.2006.07.003
1 Eckenwalder (1998)
2 Vitousek et al. (1997)
3 The transfer of genetic information from one species to another as a result of hybridisation between them and repeated backcrossing.
4 Crispo et al. (2011)
5 Shannon et al. (2009)
6 Dalui et al. (2020)
7 Geenen (2019)
8 Harrington et al. (1999)
9 Gosling (2014)
10 Zidon et al. (2017)
11 Sullivan (1999), Sullivan & Ganuses (2020, 2021, 2022)
12 Berry (1997)
13 Plessis (1997)
14 Zidon et al. (2017)
15 Huang et al. (2021)
16 Roux et al. (1988)
17 Turner & Getz (2010)
18 Turnbull et al. (1998)
19 Wassermann et al. (2015)
20 du Preez & Grobler (1977)
21 Ibid.
22 Turner & Getz (2010: 3), Wassermann et al. (2015)
23 Hoffman (1989)
24 Moehlman (2002)
25 Rubenstein (1989), Bauer et al. (1994), Moehlman (2002)
26 Janis (1976)
27 Hack et al. (2002), Pedersen et al. (2018)
28 Groves & Bell (2004), King & Moehlman (2016)
29 Ibid.
30 Moodley & Harley (2005), Winker et al. (2016)
31 Gosling et al. (2019)
32 Stander et al. (1990)
33 Gosling (2014)
34 Ibid.
35 Kilian (2015)
36 Louis Geldenhuys, pers. comm., 2015.
37 W. Versfeld, pers. comm., 2015.
38 Gosling (2014)
39 Kamath (2011)
40 Roug et al. (2020)
41 van Moorter et al. (2015)
42 Burt (1943)
43 Kie et al. (2010)
44 Loveridge et al. (2009)
45 Knüsel et al. (2019)
46 Spencer (2012)
47 Bevanda et al. (2015)
48 Penzhorn (1982a)
49 Richard et al. (2014)
50 Montgomery & Roloff (2013)
51 Johnson (1980)
52 Rivrud et al. (2009)
53 Marshal et al. (2009)
54 Mariotti et al. (2020)
55 Ibid.
56 King & Moehlman (2016)
57 Smuts (1975)
58 Owen-Smith et al. (2015)
59 Penzhorn (1982b)
60 Ransom & Kaczensky (2016)
61 Penzhorn (1982a)
62 Muntifering et al. (2019)
63 Chabwela et al. (2017)
64 Spalinger & Hobbs (1992)
65 Maier et al. (2005)
66 Nikula et al. (2004)
67 Jarman & Sinclair (2021)
68 McNaughton (1987)
69 Bartlam Brooks et al. (2013), Muntifering et al. (2019)
70 Courbin et al. (2016), Fischhoff et al. (2007)
71 Ibid., Zidon et al. (2017)
72 Huang et al. (2021)
73 Penzhorn (1979)
74 Winkler & Owen-Smith (1995)
75 Joubert (1972), Muntifering et al. (2019)
76 Eckenwalder (1998)
77 Harrison & Larson (2014)
78 Sympatry is the term used to describe populations, varieties or species that occur in the same place at the same time.
79 Levänen et al. (2018)
80 Allopatry describes a population or species that is physically isolated from other similar groups by an extrinsic barrier to dispersal.
81 Iacolina et al. (2018)
82 Wyk et al. (2013). Genotype refers to the genetic makeup of an organism.
83 Ibid.
84 Dalton et al. (2017)
85 Allendorf et al. (2001)
86 Cordingley et al. (2009), Ottenburghs (2021)
87 Levänen et al. (2018)
88 Defined as a population of hybrids that has survived beyond the initial hybrid generation, with interbreeding between hybrid individuals and backcrossing—i.e. a crossing of a hybrid with one of its parents or an individual genetically similar to its parent, to achieve offspring with a genetic identity closer to that of the parent.
89 Hailer & Leonard (2008)
90 Galov et al. (2015)
91 Ottenburghs (2021)
92 Manel et al. (2003)
93 McKelvey et al. (2010)
94 Sommer et al. (2013)
95 Montgelard et al. (2014)
96 Ibid.
97 Cordingley et al. (2009)
98 Brown & Jenkins (1987)
99 (2009)
100 Ryder et al. (1978)
101 Cordingley et al. (2009)
102 (2017)
103 An F1 hybrid is the first filial generation of offspring of distinctly different parental types.
104 Dalton et al. (2017)
105 Cordingley et al. (2009), Moodley & Harley (2005), Odhiambo (2017), Pedersen et al. (2018)
106 Guisan & Thuiller (2005)
107 Zecherle et al. (2020)
108 Zielinski et al. (2006)
109 Binzenhöfer et al. (2005)
110 Frank et al. (1998), Harris et al. (2009), Hobbs et al. (2008), Owen-Smith (2004)
111 Stevens et al. (2006)
112 Metzger & Decamps (1997)
113 Wright (1943)
114 Berry et al. (2005)
115 Holderegger & Wagner (2006)
116 Crego et al. (2021)
117 Fynn & Bonyongo (2011)
118 Chetkiewicz & Boyce (2009)
119 Sharma et al. (2004), Kebede et al. (2012), Kigen et al. (2013), Mwangi et al. (2018), Olivier (2019)
120 Moodley & Harley (2005)
121 Pedersen et al. (2018)
