17. Integrating remote sensing with CBNRM for desert-adapted lion conservation
©2024 John Heydinger, CC BY-NC 4.0 https://doi.org/10.11647/OBP.0402.17
Abstract
This chapter explains how Global Positioning System (GPS) data on lion movements can contribute to community-oriented conservation. Community-Based Natural Resource Management (CBNRM) of desert-adapted lions presents an array of cultural and scientific challenges to local communities living alongside lions. A significant challenge for lion conservationists is the ability to rigorously monitor lion movements in the unfenced landscapes of north-west Namibia, where monitoring challenges are compounded by low levels of information relevant to lion habitat-use and movement ecology in dryland areas. The chapter documents new uses in this area of data collected via satellite-GPS collars affixed to lions, and via trail cameras placed in designated core wildlife areas within communal conservancies and government concessions. Remote sensing methods of carnivore monitoring are now contributing to lion conservation and the mitigation of “human-lion conflict” on communal lands in Namibia’s Kunene Region. The chapter emphasises how this technology and associated data are being incorporated into the Lion Rangers Programme, a CBNRM initiative in which trained community conservationists take responsibility for monitoring lions and managing human-lion conflict on communal lands.
17.1 Introduction1
The desert-adapted lions (Panthera leo) of north-west Namibia’s Kunene Region are iconic, demonstrating unique grouping patterns2 and movements3 within a “one-of-a-kind” landscape. Inhabiting unfenced lands designated primarily as communally-managed conservancies, the desert-adapted lion population is relatively small, though stable. From 1997–2015 the population recovered from around 20 to around 180 individuals.4 This conservation success story is unique: these are among the only African lion populations to have grown over the last 25 years outside fenced protected areas.5 Since the mid-2010s, however, the population has declined to an estimated 57 to 60 adult (non-cub) individuals,6 and from an estimated density of 0.28-0.35 lions/100 km2,7 to 0.1 lions/100 km2.8 This decline has been driven by retaliatory lion killing following human-lion conflict (HLC) occurring because lions threaten farmers’ safety and livelihoods via livestock destruction, in a context of reduced prey due to drought and high offtake levels (Chapter 3).9 Since 2000, retaliatory killings of lions following HLC incidents have accounted for 89% of recorded lion (non-cub) mortalities in Kunene.10 Human-lion conflict thus poses a serious threat to the viability of the desert-adapted lion population.
Lion presence on conservancy lands is something of a paradox. Conservancy legislation was implemented to enable rural Namibians to benefit from wildlife within communal, multi-use landscapes,11 yet HLC imposes significant costs upon farmers in Kunene conservancies. Recent surveys of communal farmers inhabiting three core lion-range conservancies reveal livestock losses from HLC estimated at USD 2,985 per household, and losses from all predators at USD 10,151 over a three-year period, based on surveys of approximately 90% of livestock-owning households.12 Such losses can be life-altering. Day-to-day household needs may be compromised while funds for emergencies become scarce. HLC and subsequent lion killing is thus both a wildlife conservation and human wellbeing challenge. Furthermore, communal area farmers overwhelmingly (84%) feel they do not benefit from lions inhabiting their conservancy (see Chapters 8 and 14). Even so, 75% of farmers want lions to continue to inhabit their conservancy, the primary reason being that they want future generations to be able to see wild lions.
Strengthening lion monitoring in Kunene Region is important for limiting HLC and supporting farmers’ livelihoods. Kunene’s vast and rugged landscapes, however, create considerable difficulties for monitoring lions. Not only is much of the area difficult to access, but because desert-adapted lions cover such expansive territories, monitoring efforts must be highly-mobile and flexible. Furthermore, because lions in Kunene primarily inhabit communal land, lion monitoring must also engage local communities. Without community participation, lion monitoring and conservation risks alienating conservancy rights over wildlife, with the potential downstream effect of lion management being considered an external imposition. This may engender antagonism towards lion conservation activities, leading to lion-killing as a form of protest.13
The challenge facing Community-Based Natural Resource Management (CBNRM) of lions in Kunene is how to simultaneously limit HLC while building tolerance of lions among farmers. Doing so requires synthesising inclusive, locally-centred efforts with available technologies for monitoring lion movements. This chapter examines how the Lion Rangers Programme14 is actualising community-centred monitoring and conservation of the desert-adapted lions. Building on lion ecologist Philip “Flip” Stander’s work, the Lion Rangers are using cutting-edge remote sensing technologies, including Global Positioning System (GPS)/satellite collars with VHF (Very High Frequency) and early-warning capabilities affixed to lions (as lion collars); and an extensive array of motion-activated cameras taking high-quality photographs (camera traps) deployed across the landscape along key movement corridors and areas where lions concentrate. Using these methods, Lion Rangers Programme researchers are developing an increasingly comprehensive picture of lion movements in Kunene. Yet, the programme is truly centred on the Lion Rangers themselves (see Chapter 18). Composed of 49 conservancy members from 11 lion-range conservancies, the Lion Rangers are employed by their conservancies and capacitated by the Lion Rangers Programme to monitor lions, provide information to other farmers and key conservancy personnel regarding lion movements and behaviour, while supporting farmers’ livelihoods by limiting HLC, thereby increasing local tolerance for living alongside lions. The integration of lion collars and camera traps with the locally-centred work of the Lion Rangers is promoting the active mitigation, management, and prevention of HLC in Kunene.
This chapter examines how the Lion Rangers Programme integrates remote sensing technologies with community-centred monitoring and HLC interventions for desert-adapted lion conservation. I begin by introducing the core desert-adapted lion landscape and providing a brief history of the lion population. This includes an overview of historical and ongoing lion conservation efforts in Kunene, including an examination of the effects of CBNRM on the lion population as well as an introduction to the Lion Rangers Programme. Remote sensing technologies have been an important part of lion monitoring in Kunene for years, but recent advances are enabling such technology to also be an important part of limiting HLC. GPS/satellite collars and camera traps are proving to be invaluable tools for monitoring lions and limiting HLC. I close with a case study of a conflict-causing male lion known as NPL-27, to illustrate how these remote sensing and community-centred methods are being integrated for effective desert-adapted lion conservation.
17.2 Kunene core lion range
The core desert-adapted lion range in Kunene is an area of approximately 40,000 km2. This area encompasses 11 communal area conservancies (Anabeb, Doro !Nawas, Ehi-Rovipuka, ǂKhoadi-ǁHôas, Omatendeka, Orupupa, Puros, Sesfontein, Sorris Sorris, Torra and Tsiseb) as well as the Hobatere, Etendeka, and Palmwag tourism concessions, and part of the Skeleton Coast National Park (SCNP) running approximately from the Hoaruseb riverbed in the north to the Ugab (!Uǂgab) riverbed in the south.15 Dominated by the Northern Namib Desert, primarily composed of sandy dunes with small oases in the west (see Chapter 12), the area also includes rugged mountains and gravel plains bisected by east-to-west ephemeral riverbeds. The area’s basaltic soil is shallow, rocky, and relatively unproductive.16 Other iconic desert-adapted species include black rhinoceros (Diceros bicornis bicornis), elephant (Loxodonta africana), gemsbok (Oryx gazella), and mountain zebra (Equus zebra hartmannae). Rainfall is generally low (50-250 mm per year) and erratic, increasing from west to east. During the wet season (January-May) rains fall in brief, localised downpours. Prey species, including gemsbok, mountain zebra, and giraffe (Giraffa camelopardalis angolensis), follow the rains to fresh grass and often congregate in riverbeds during the dry season (June-December). Springbok (Antidorcas marsupialis) generally stay on the plains, while kudu (Tragelaphus strepsiceros) reside in stands of trees and cliffsides. Surface water is sparse. During the 1970s, however, a government borehole-drilling programme greatly increased year-round water availability (see Chapter 7). Since that time livestock and wildlife are generally grazing- rather than water-limited.17 Boom-and-bust rainfall patterns cause prey numbers to fluctuate widely. Beginning in 2000 the region experienced a relatively wet period, resulting in wildlife and livestock increases. From 2011 to 2021, extensive drought combined with high offtake levels until around 2016, caused the decline of indicator prey species by as much as 60% and livestock by as much as 67%18 (see Chapters 3 and 6). Since 2020, early indications are that a modest increase in rainfall is leading to some recovery of wildlife numbers,19 also complemented by translocations into the area.
This area includes approximately 19,300 rural residents, who primarily identify as Damara/ǂNūkhoen or ovaHerero/ovaHimba. Most are small-scale pastoralists for whom livestock has significant economic and cultural value. Drought and predation are the main threats to these farmers’ livelihoods, with lions accounting for approximately 20% of livestock losses. Although the Namibian government provides limited annual funding to each conservancy to compensate for livestock lost to human-wildlife conflict, 92% of farmers surveyed are dissatisfied with the programme because the compensation money does not equal the monetary value of livestock lost.20 While pastoralism comprises most household incomes, these are often low and insecure:21 40% of Kunene residents earn less than USD 1/day, while 23% earn less than USD 0.73/day.22 Livelihoods have been further hampered by a downturn in tourism receipts stemming from the COVID-19 pandemic.23 Additionally, Kunene has Namibia’s highest primary school drop-out rates; only 55% of residents complete primary school by age 17.24 Such social and economic vulnerability exacerbated by HLC not only worsens livelihood prospects, but may also be straining the conservancy system25 (see Chapters 3 and 5).
17.3 Lions and CBNRM in Kunene
Since the inauguration of Namibia’s communal area conservancy system in 1996, Kunene has become a wellspring of community conservation. During this same period lion numbers rebounded. While lions have long inhabited Kunene, likely in low densities, from the 1980s to 1990s they were nearly eradicated on communal lands.26 Speaking of this period, one Kunene pastoralist remembers that ‘[i]n olden days lions were being killed and they were manageable’.27 The growth of the conservancy system in this century has created a new wildlife conservation paradigm, one in which colonial-era government staff have been replaced by locals as custodians of wildlife; although legal enforcement, e.g. of anti-poaching, remains with the government.
Beginning in the late 1990s, Flip Stander undertook intensive monitoring of the desert-adapted lions, focusing on individuals and groups in the Palmwag Concession and western areas of Puros, Anabeb, Sesfontein and Torra conservancies.28 Already experienced in monitoring lions in Etosha National Park (ENP) and Nyae Nyae Conservancy, Stander’s focus on lions in Kunene coincided with the development of the conservancy system. Simultaneously, the region received relatively good rainfall leading to growing prey populations in the early 2000s. During this period lion numbers rose and lions began re-occupying their former range across Kunene (Figure 17.1). In 1999 and 2000, lion numbers in Kunene grew by 22% and 23% respectively, slowing to about 15% from 2001–2004.29 In 2006, Stander hypothesised a significant linear relationship between the number of lions in Kunene and the size of the range they occupy.30 More lions moving within communal farming areas coincided with increasing HLC incidents (as noted above).
The institutional context of this lion recovery is important for understanding the challenge of HLC. Desert-adapted lions range mostly within communal conservancy lands. As part of a counter-hegemonic conservation movement that gained momentum in the late 1980s to early 1990s, communal area conservancies aim to overcome some of the social, political, and economic injustices stemming from wildlife conservation-oriented interventions during Africa’s colonial era31 (Chapters 1 and 2). Much is written about the history and implementation of communal area conservancies in this volume (see Chapters 3, 5, 6, 11, 13 and 14). Pertinent to lion conservation, under Namibia’s Nature Conservation Amendment Act 5 of 1996, communal area residents may secure limited rights to “huntable game” species via their conservancy. As institutions for securing benefits stemming from wildlife, conservancies may engage in or contract for trophy-hunting based on government-approved quotas, can apply to hunt protected species such as lions, and can trade and sell wildlife products with government approval. Conceptually based upon the CBNRM framework and Nobel Prize winning economist Elinor Ostrom’s Design Principles for Common-Pool Resources,32 communal conservancies use processes of consultation, engagement, and empowerment33 to facilitate collective proprietorship of wildlife for simultaneous conservation and community benefit (although see Chapters 5 and 6 for review of how this institutional structure is playing out in practice).
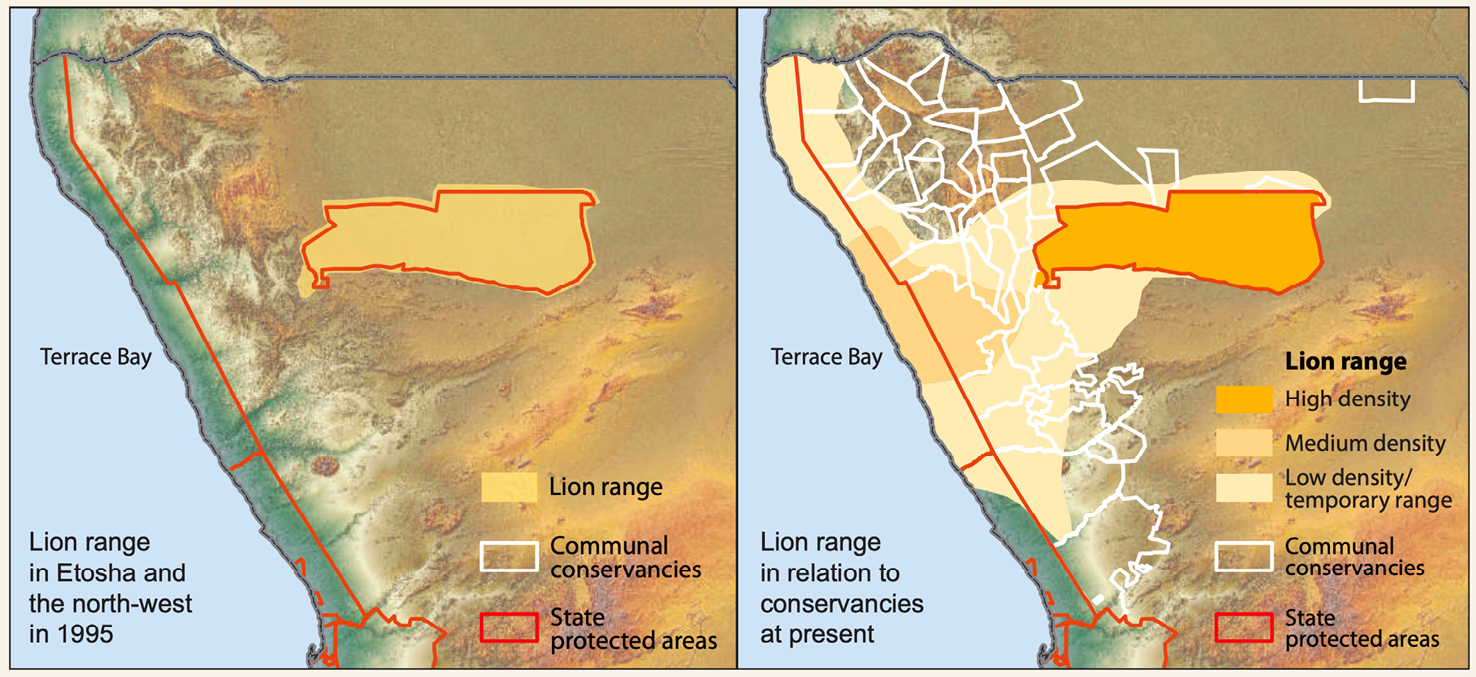
Fig. 17.1 Maps showing lion range expansion in north-west Namibia, 1995–2015. © NACSO (2016: 40), public data,
CC BY-NC-ND 4.0.
Lions, however, prove an awkward fit with the CBNRM paradigm. Lions are a protected species (Nature Conservation Act 4 of 1975), and thus not subject to normal hunting regulations. Heydinger and Muzuma have examined how conservancies constrain residents’ ability to manage and benefit from lions.34 In brief, while conservancy farmers maintain economic and wellbeing risks from living alongside lions, they are unable to directly benefit from lion hunting without government approval. A lack of benefits to match the costs of living with lions is considered a key driver in lion killing. In effect, for many conservancy farmers lion killing is a rational economic response to HLC.
In 2017, Namibia’s Ministry of Environment, Forestry and Tourism (MEFT) released the Human-Lion Conflict Management Plan for North West Namibia (NW Lion Plan).35 By providing a framework for addressing HLC while supporting the rights and development needs of local communities, the NW Lion Plan emphasises the importance of community-centred action and decision-making relevant to lion conservation. Objectives include creating a standardised monitoring system, establishing best practices for HLC mitigation, and creating mechanisms to reduce HLC. Following a regional planning meeting held in September 2017, government, researcher, and conservancy stakeholders agreed to activate and capacitate a group of Lion Rangers.
17.3.1 Lion Rangers
The Lion Rangers are conservancy employees, receiving specialised training and equipment to lead conservancy-level efforts in lion monitoring and limiting HLC. Based on successful CBNRM programmes such the Conservancy Game Guards (CGGs) and Save the Rhino Trust (SRT) trackers in Kunene,36 and the Lion Guardians in Kenya and Tanzania,37 the Lion Rangers are nominated by their conservancies to serve as custodians of lions on communal lands. As a CBNRM programme, tasked with unifying conservation and rural development,38 the Lion Rangers Programme aims at providing lion-centred benefits to conservancies while reducing the costs associated with HLC. This approach is based upon local historical experiences of living with lions39 and contemporary perspectives of HLC.40
From its inception, the Lion Rangers Programme goal has been the long-term sustainable management of HLC in Kunene, centring the work of local conservationists, to ensure the survival of the desert-adapted lions as well as community benefits from their presence. Because Lion Rangers operate within communal conservancies, the programme structure and objectives are founded upon the four conceptual pillars of CBNRM. Adapted from Jones and Murphree, these are:41
- sustainable use as conservation paradigm—As the premier threat to natural habitats and resources, landscape transformation necessitates creating incentives for sustainable resource use, rather than technical interventions or compulsion to limit appropriation. Lion monitoring and conservation are linked to the possibility of conservancy residents potentially benefiting from lion presence;
- economic instrumentalism—Economic considerations are seen to drive resource decisions. Resource provision and appropriation must be economically competitive or else landscape transformation may occur. This includes the creation of supporting structures and access to markets. By providing employment, the Lion Rangers Programme links lion presence to household level benefits and the local economy. Sustainable management of lions may lead to both consumptive (e.g. trophy hunting) and non-consumptive (e.g. tourism and Wildlife Credits) benefits;
- devolution—Responsibility for resources is supported by the authority and entitlement necessary to generate stewardship. Local control enables rights to manage, benefit from, and dispose of resources. Empowered by their conservancy management and trained by programme leadership and other experts, Lion Rangers participate in lion monitoring and HLC interventions and play an active role in decision-making relevant to lion management;
- collective proprietorship—Communities of collective interest are the locus for rights-devolution. Internal legitimacy comes from communities whose membership, boundaries, and constitution are self-defined. External legitimacy comes from legislation. As community members are part of a broader set of stakeholders, Lion Rangers are responsible for representing their conservancy in lion monitoring and management operations.
Operating with government approval, Lion Rangers are the conduit between pastoralists, the conservancy, government, and NGOs concerning HLC. Most Lion Rangers are also pastoralists. They embody the experience of living with lions and are charged to faithfully represent the challenges surrounding HLC. Prior to the activation of the Lion Rangers there was limited monitoring of lions in many lion-range conservancies. The Lion Rangers are the first platform to demonstrate that communities can be trusted to sustainably manage “their” lions. By merging the Lion Rangers’ field deployment with cutting-edge monitoring technologies already in use by area researchers, an emphasis is being placed on devolving responsibility to selected community members, without sacrificing high-quality data collection for evidence-based lion conservation. This empowers local people with the responsibility of lion monitoring and conservation.
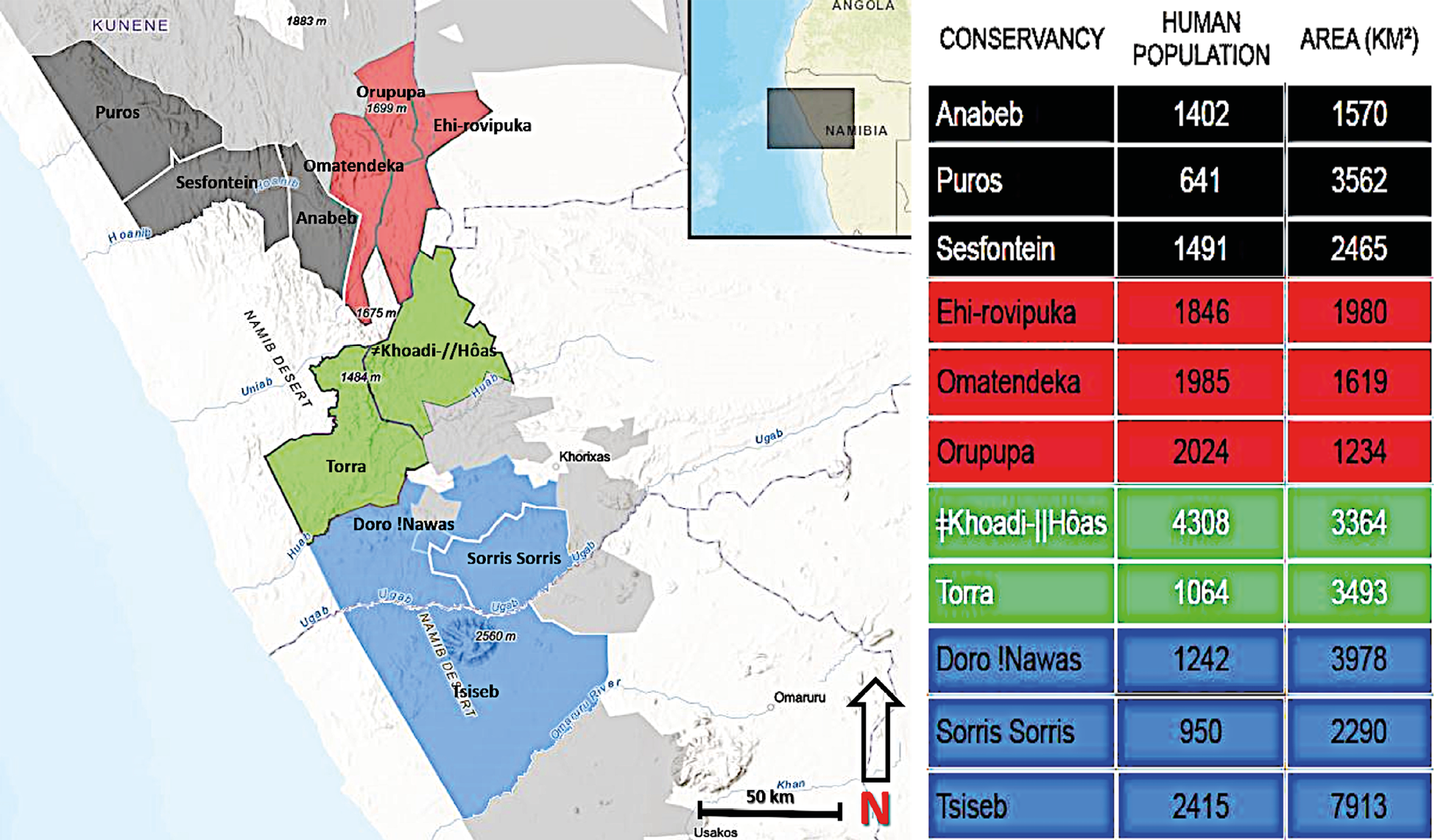
Fig. 17.2 Map showing core lion-range conservancies, separated into Lion Blocks. The Black Block consists of Anabeb, Puros, and Sesfontein; the Red Block of Ehi-Rovipuka, Omatendeka, and Orupupa; the Green Block of ǂKhoadi-ǁHôas and Torra; and the Blue Block of Doro !Nawas, Sorris Sorris, and Tsiseb. © Lion Rangers data, CC BY-NC-ND 4.0.
In Kunene, core lion-range conservancies are grouped into “Lion Blocks” (Figure 17.2). Because lions move freely in this mostly unfenced landscape, it is logical for neighbouring conservancies to partner for monitoring and managing lions. This approach seeks to overcome some of the existing shortfalls related to the conceptual pillars of CBNRM. By linking conservancies together, the justification for and effectiveness of collective proprietorship are strengthened (see Chapter 3); this supports devolution of decision-making to the Lion Block, if not the conservancy, level. Through a forthcoming Wildlife Credits project,42 Lion Block conservancies will be paid for lion presence,43 such that Lion Blocks may forge economic links between lion presence and lion conservation by providing community-level monetary benefits: although clearly there may be some tensions here with government-level decisions for consumptive uses of lions through trophy-hunting.
Within their Lion Blocks, Lion Rangers are deployed on joint-patrols, whereby conservancies pool their resources to get Lion Rangers into the field. Generally, Lion Rangers (Figure 17.3) are deployed to field camps neighbouring HLC hotspots, usually for 10 to 14 days per patrol shifts. Lion Rangers are responsible for performing foot- and vehicle-based patrols, which emphasise monitoring lion and other carnivore movements, as well as livestock movements around nearby farms. In the field, Lion Rangers collect environmental data using the Spatial Monitoring and Reporting Tool (SMART) mobile app.44 This builds on the earlier Event Book System45 to enable environmental data collected by the Lion Rangers to be quickly exported (via either a cellular network or through Wi-Fi) for use by researchers and wildlife managers: as detailed in Chapter 18.

Fig. 17.3 Lion Rangers Rinoveni Tjauira (of Omatendeka Conservancy), Matarakuani Kavetu (of Ehi-Rovipuka Conservancy), and Richard Katira Zaako (of Orupupa Conservancy) on patrol in the ‘Red’ Lion Block, 2022. © John Heydinger, 2022, CC BY-NC-ND 4.0.
17.4 Names, collars, and cameras
Within the text of the NW Lion Plan, the Namibian government affirmed the importance of collecting data on the spatial and temporal patterns of lion movements. These data are an important part of not only responding to and mitigating, but possibly preventing, HLC incidents.
17.4.1 Names
Limiting HLC starts with knowing which lions inhabit the area. Beginning in 1999, VHF collars were deployed by Stander on lions in Kunene, primarily for studying their movements and grouping patterns. Building on his experience in Etosha during the 1980s and 1990s, Stander also began giving lions in Kunene unique identifiers in the form of alpha-numeric names. Whereas the convention in western Etosha had been to name lions WPL-# (‘W’ for western Etosha, ‘PL’ for Panthera leo, plus a unique number to identify the individual), in Kunene individual lions were named according to an XPL-# system (‘X’ for the Xhorixas [Khorixas] district where the study was taking place).46
Naming individuals, in this case nonhumans, is itself a monitoring and governance technology. Individually identifying lions by name, though not unique at the time, was important, both for building a coherent picture of lion movements and grouping patterns, and for creating a discourse in which lions became increasingly known, knowable, and potentially manageable.47 Much as the process of mapping a landscape—including the creation of boundaries and assigning names to certain features—increases humans’ ability to govern and manage that landscape, naming individual lions reinforces researchers, government staff, and the Lion Rangers’ ability to speak with specificity about different lions, in turn enabling us to tailor monitoring and management to lions as individuals. Human-animal historian Etienne Benson has shown that naming research animals is not only useful for differentiating among them, but is also associated with a variety of moral commitments from the researcher towards the subject.48 I have incorporated this understanding into the history of human-lion relationships within Etosha:49 among park staff and tourist visitors to Etosha during the 1950s and 1960s, lions became conceptually transformed from fearsome pests into cosseted individuals who were individually known and in certain cases provisioned with carcasses during drought, and to provide tourist viewing opportunities.
Naming lions in Kunene is indicative of an increased concern for their well-being among researchers, wildlife managers, and the international conservation community. Beginning with XPL-1 and XPL-2 in 1999, to date more than 140 lions in Kunene have received unique alpha-numeric identifiers. To the roster of XPL-# lions have been added OPL-#, for lions inhabiting the greater Ombonde catchment landscape, and NPL-#, for lions monitored by the Namibian Lion Trust,50 a local NGO which is also part of the Lion Rangers Programme. Coinciding with the work of Stander and his NGO Desert Lion Conservation Trust (DLCT),51 the first two decades of the 21st century brought increasing attention to the desert-adapted lions, in the form of research reports and publications, semi-popular articles in conservation magazines, and full-length documentaries focusing on the lives and survival prospects of certain lions.52
17.4.2 Collars
Iterative of Cold War-era technologies developed primarily for military purposes, VHF and later GPS/satellite collaring of elusive, wide-ranging wild animals has been an important part of population biology since the 1960s. Benson provides a thorough and insightful examination of this history.53 From the 1960s, lions were being collared on a small scale in East Africa.54 Beginning in 1984, Craig Packer and his team of researchers began fitting VHF collars to lions in Serengeti. Over the next 30 years they collared more than 300 lions, with 18 to 24 lions collared at any one time.55 This research revealed ground-breaking insights into lion movement patterns and spatial ecology. By the time Stander began working in Kunene, collaring of lions and other similarly elusive and wide-ranging large carnivores was commonplace elsewhere.
Collars enable lions to be monitored remotely. While VHF frequencies are still used, GPS/satellite collars are now available at relatively affordable prices. These have become central to lion research, monitoring, and HLC prevention in Kunene. Once fitted to an individual through a standard process of chemically-induced immobilisation under the supervision of a licensed veterinarian or para-vet,56 GPS/satellite collars begin transmitting location fixes, first to a satellite array, which relays the location fix to a secure online interface.57 Collars can be programmed to transmit location fixes at different intervals. Currently lion collars in Kunene transmit location fixes every four hours during the day, and every two hours at night; these intervals can be reprogrammed, for example when a lion enters a known HLC area. Collars enable researchers and government staff to monitor collar locations online and communicate movements to Lion Rangers and other staff on the ground. These collars are also part of an “early-warning system”. When lions cross a “geofence” boundary, automated messages in the form of SMSs, go out to area Lion Rangers and farmers alerting them to lions’ presence within the area (see Chapter 18). In January 2023, there were 35 active GPS/satellite collars fitted to lions in Kunene. This represents approximately 65% of adult lions which, as a percentage of the total population, is the highest for a free-ranging lion population in Africa.
Collar data provide a dynamic picture of lion movements across Kunene. Not only can current movements be tracked, but as the number of location fixes grows, these locations can be compared to a lion’s historical movements. Over time, a home range for each lion becomes visible. Figure 17.4 is a visualisation of the movements of the lioness OPL-4. Collared on 22 May 2021, over the ensuing 16 months, she maintained a home range of approximately 1,140 km2, centred around the Otjiapa-Okavariona-Otjejekupe waterhole complex in the Omatendeka conservancy. Given that the core of her home-range is far from established farms, and largely contained within a mountainous area, this lioness is normally not considered at high-risk of HLC. During March 2021, however, one can see that OPL-4 did move as far north-west as the mountains south of the Mbakondja/Tsabididi farming area in Anabeb (furthest north-west points)—perhaps in search of prey that had dispersed during the rainy season. This was an aberration compared to her regular movements. When such lions venture beyond the core of their home ranges, potentially into areas they are less familiar with, researchers and the Lion Rangers will pay extra attention to incoming collar data, with the goal of preventing HLC before it occurs.
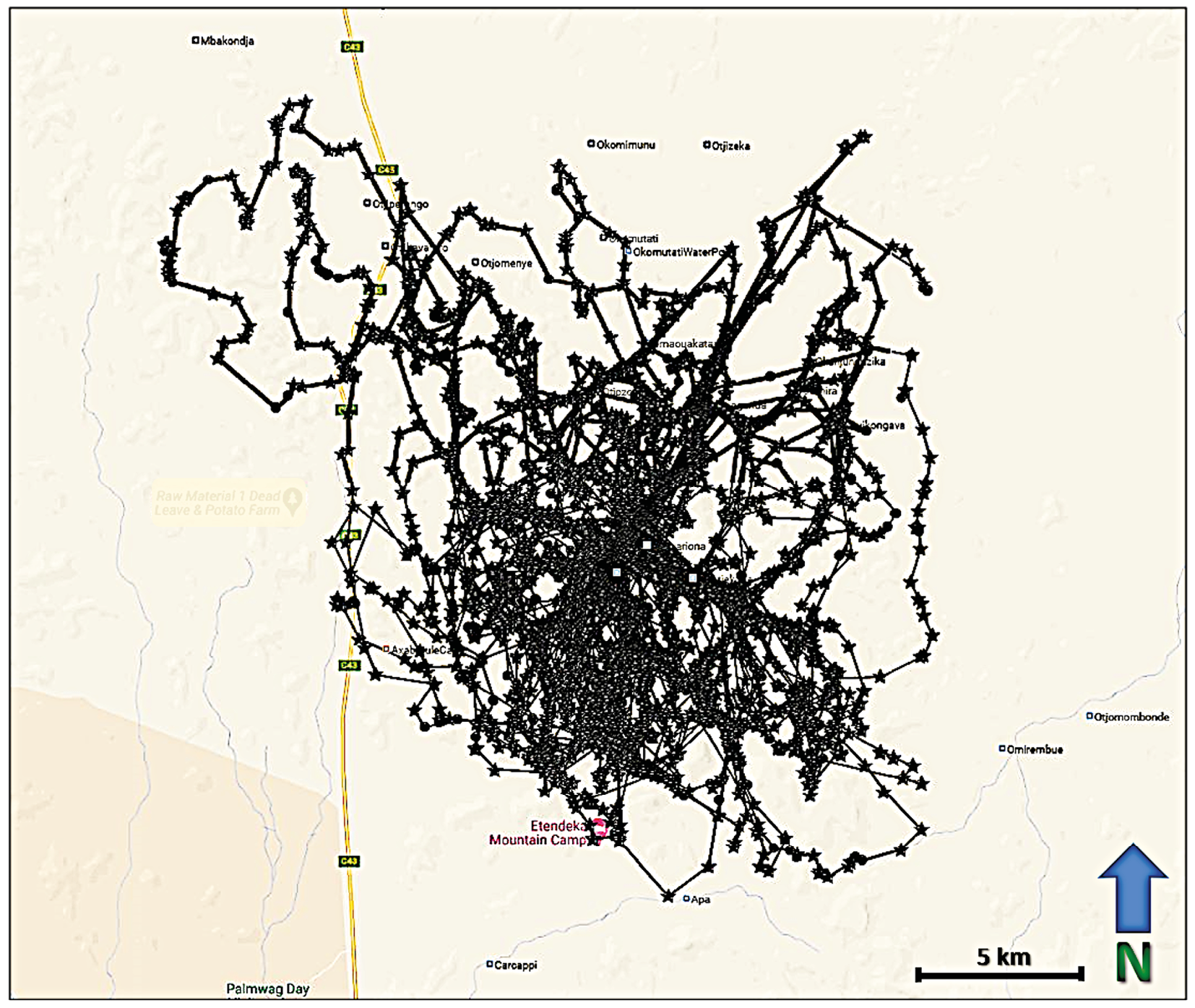
Fig. 17.4 Map showing visualised GPS/satellite collar locations of OPL-4 in the Anabeb and Omatendeka conservancies, from 22.5.2021 to 27.9.2022. © Lion Rangers data, CC BY-NC-ND 4.0.
When collared lion movements are viewed in relation to one another, new insights into lion sociality emerge. It is generally understood that male lions will defend their territory against other males. How these interactions occur at the landscape level can be visualised through available collar data. Figure 17.5 shows the movements of two males, OPL-3 (red fixes) and NPL-28 (blue fixes) over a 25 day period from December 2022 to January 2023 in the Klip River/Tafelberg area of ǂKhoadi-ǁHôas Conservancy. Looking across the 25 days it may seem these two lions are sharing this approximately 800 km2 landscape.
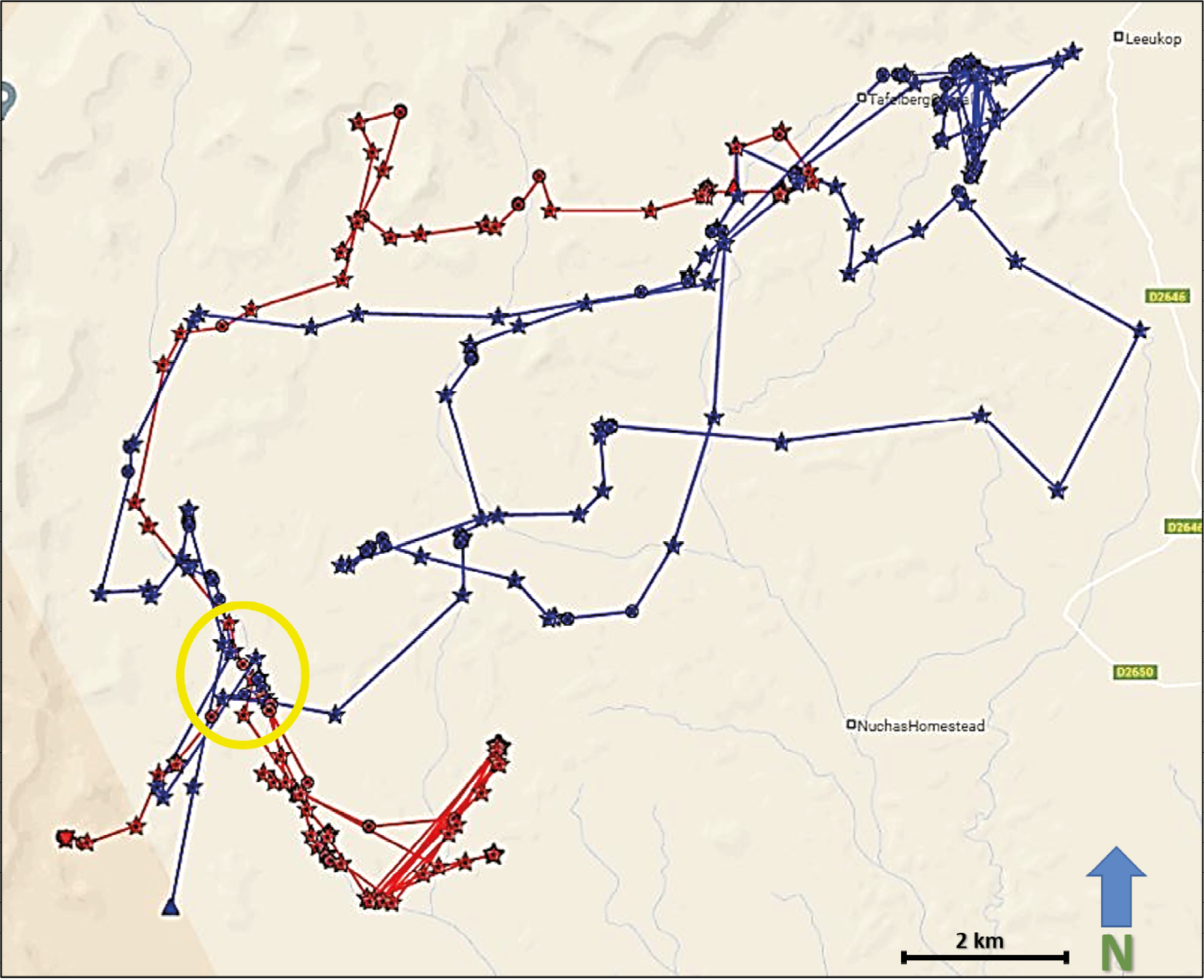
Fig. 17.5 Map showing visualised GPS/satellite collar locations of OPL-3 (red) and NPL-28 (blue) in ǂKhoadi-ǁHôas Conservancy, from 23.12.2022 to 16.1.2023. Yellow circle indicates the area enlarged in Figure 17.6. © Lion Rangers data, CC BY-NC-ND 4.0.
Zooming into specific GPS points, however, reveals interaction dynamics (Figure 17.6). At approximately 0400 on the night of 14 January 2023 (green-circled fixes), OPL-3 and NPL-28 came into close contact about 500 metres west of the Klip riverbed. An apparent altercation resulted in OPL-3 moving further south-west, while NPL-28 briefly returned towards the core of his territory. Less than 24 hours later, NPL-28 appears to have pursued OPL-3, pushing him further south-west while NPL-28 once again returned towards the core of his territory. During the next week, OPL-3 travelled nearly 50 kms north out of the area, while NPL-28 returned to the core of his territory to the north-east. It is noteworthy that while OPL-3 is estimated at between five to six years old and 140 kgs, and has struggled to maintain a consistent home range, NPL-28 is estimated at between seven to eight years old and 180 kgs and has been residing in the area since at least October 2022, when he was collared. From this interaction, combined with demographic and physiological data as well as historical collar data from these two lions, and an absence of other known males in the area, we can infer that while NPL-28 maintains a relatively stable territory in the Klip River/Tafelberg landscape—one that he will defend against potential competitors—OPL-3 does not enjoy similar territorial dominance. Indeed, the recorded home range of each lion since NPL-28 was collared on 10 October 2022 further reveals aspects of each lion’s spatial ecology. While NPL-28 has occupied a range of approximately 900 km2 during this period, OPL-3 has covered an area spanning more than 3,200 km2 during that same time. This further reveals the social and spatial dynamics at work among lions. OPL-3 is considered a nomad in search of a stable territory. His wanderings frequently bring him near to farming areas, increasing the likelihood of HLC. His movements are therefore closely monitored by the Lion Rangers. By comparison, NPL-28 is still considered a relatively low HLC risk because he inhabits an area with no currently established farms or livestock outposts.
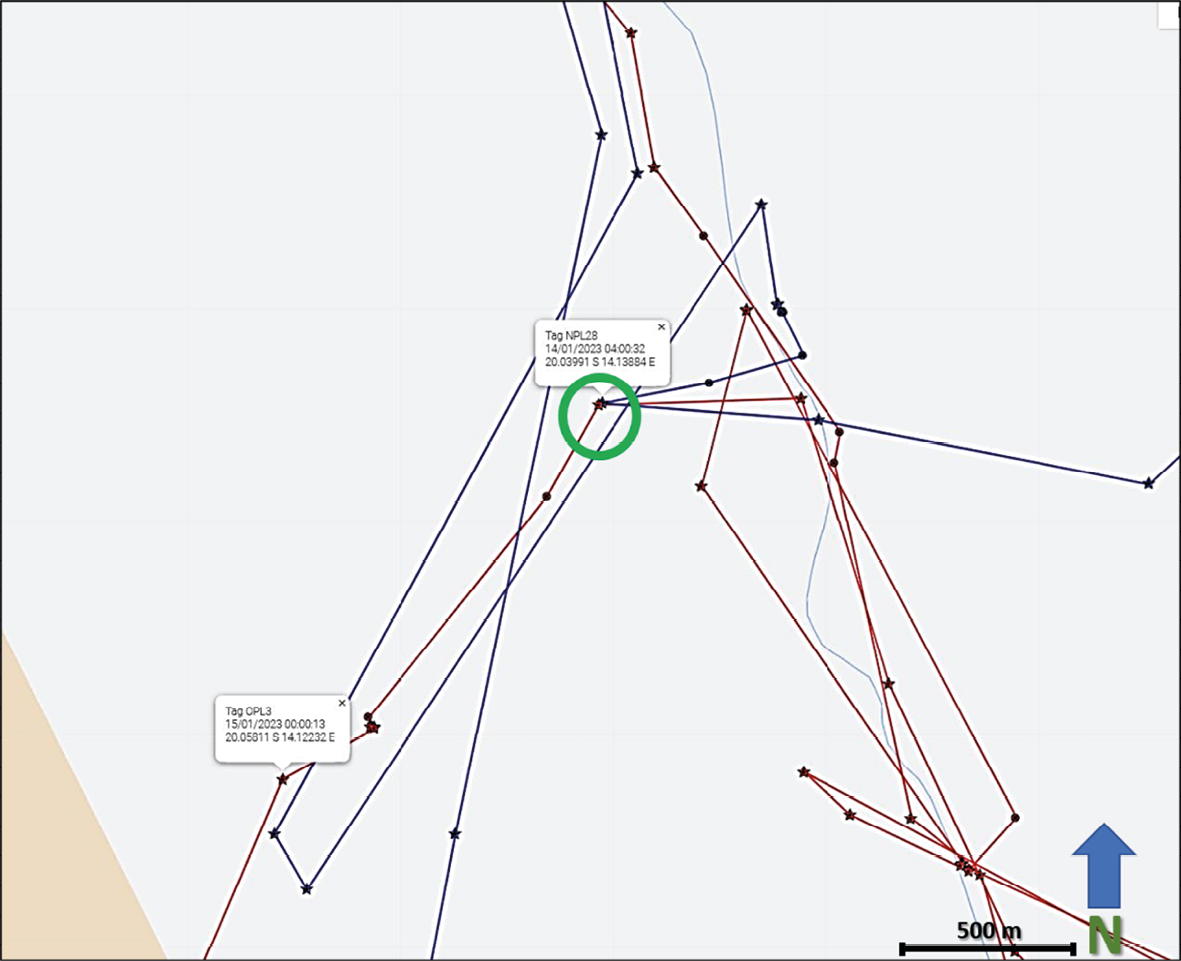
Fig. 17.6 Map showing visualised GPS/satellite collar locations of OPL-3 (red) and NPL-27 (blue) in ǂKhoadi-ǁHôas Conservancy, from 23.12.2022 to 16.1.2023. Area enlarged to emphasise movements from 14-16 January. The green circle indicates a likely conflict event. © Lion Rangers data, CC BY-NC-ND 4.0.
Figure 17.7 shows the movements of nine collared male lions over only a two-week period in January 2023, including areas in which people are living and herding livestock. This image indicates the challenge of monitoring and limiting HLC, even over this approximately 7,600 km2 portion of the landscape. Although these males generally maintain distinct territories, these can overlap, increasing the prospect of HLC at nearby farms. As more lion collar data become available, researchers and Lion Rangers have more information for limiting HLC.

Fig. 17.7 Map showing visualised GPS/satellite collar locations of nine male lions across Kunene, from 10-24.1.2023. Approximate size of areas is 7,600 km2. © Lion Rangers data, CC BY-NC-ND 4.0.
17.4.3 Camera traps
Motion-activated cameras taking high-quality pictures (camera traps) enable researchers and Lion Rangers to intensively monitor key areas where lions are likely present. First developed as a method of estimating tiger population size by Karanth and Nichols,58 camera traps are an increasingly popular tool for monitoring and estimating the abundance of large-bodied species when individuals are identifiable. Large terrestrial carnivores generally demonstrate secretive behaviour and nocturnal habits, existing at low densities while having broad spatial requirements that may cross physical, administrative, and political boundaries.59 This combination of factors presents challenges to intensive monitoring. Camera traps allow for passive collection of presence and abundance data as well as identification of individuals within key areas. When repeated over time, camera traps have been shown to be a useful method for achieving both precise and accurate population estimates for large carnivores.60 Other non-invasive approaches such as track counts have been shown to be less accurate,61 and may even be too imprecise for implementing effective management.62 The predominantly rocky substrates of Kunene also make tracking of lions difficult, and in many areas make it near impossible to use tracks as a measure of abundance.
Beginning in May 2021, we deployed camera trap arrays around key waterpoints and lion movement corridors, centred around the Ombonde lion range landscape in Anabeb, Omatendeka, and Ehi-Rovipuka conservancies and Etendeka and Hobatere Tourism Concessions (Table 17.1). Using a ‘camera blitz’ approach63 we sought to record lion presence where possible with the intended result of collaring key individuals, such as males and pride females. Secondary objectives were to assess the presence of other large carnivores in the landscape, as well as landscape-use overlap among large carnivores. Key individuals were then targeted for collaring, primarily based on HLC considerations, with research data considered a useful by-product.
Table 17.1. Camera trap deployments since May 2021. ‘Capture period’ indicates the dates for which camera arrays were deployed; ‘# cameras’ is the number of individual camera traps deployed and retrieved for each area; ‘effort (trap-days)’ is the sum total number of days cameras were deployed in any area (# of cameras x # of days); ‘target species images’ is the total number of all images of large-bodied mammals photographed during deployment period; ‘lion images’ is the number of captures containing lions.

Fig. 17.8 Selection of camera trap images from Omatendeka 1 deployment Oct.-Dec. 2021, showing the type of quality of lion photos from camera traps. © Lion Rangers data, CC BY-NC-ND 4.0.
Scrutiny of camera trap images allows us to identify lion presence and individuals for collaring operations. While lions have been shown to have near-unique vibrissae (whisker spot) patterns,64 these are rarely visible on camera trap images. However, given the low overall population (56 to 60 individuals) and extremely low density (0.2 lions/100 km2),65 time and location of each photographic capture, along with demographic markers such as sex and age, as well as diagnostic markings such as ear tears, scars, and whether or not the lion is collared, enable us to differentiate among individuals with a high degree of confidence: this may contribute to lion abundance and density estimates going forward. Figure 17.8 shows photos of three collared adult females (OPL-4, OPL-5, and OPL-15) one collared adult male (NPL-27), two uncollared subadult males, and one uncollared adult female.
Camera trap data are enabling researchers and Lion Rangers to make more informed decisions regarding lion monitoring and HLC interventions. Below, I present a brief case study of how collars, camera trap images, and the Lion Rangers’ field monitoring combined to limit HLC, resulting in the translocation of the lion NPL-27 away from a HLC area.
17.5 Case study: Translocation of NPL-27
Combined with the field expertise of the Lion Rangers, lion collar and camera trap data provide an increasingly comprehensive picture of lion movements within Kunene communal areas. The case of NPL-27 (Figure 17.9), an adult male lion estimated at seven to eight years of age, vividly illustrates how these data can be combined to increase the effectiveness of HLC management.

Fig. 17.9 Camera trap photo of NPL-27 taken near Okavariona waterhole, 13.11.2021. © Lion Rangers data, CC BY-NC-ND 4.0.
NPL-27 was first collared by the Namibian Lion Trust on 30 August 2020 in Omatendeka Conservancy. From then until 1 May 2022, he maintained a relatively stable home range of approximately 850 km2, with most of his time spent along a river corridor north-east of the Otjiapa-Okavariona-Otjejekupe waterhole complex (see Figure 17.4). Numerous photos and videos taken by researchers during this period show him enjoying dominance in the area, including fathering at least two litters of cubs.
From 14 October to 15 December 2021, the Lion Rangers research team deployed 80 trail cameras within the waterhole complex area. During this period, equivalent to 4,085 ‘camera trap-days’, 365 images of lions were captured. NPL-27 appears in 90 of these images. Only one other adult male was captured during this period, in three photos from 14 October. Additionally, NPL-27 was frequently captured in photos with area females, who showed signs of pregnancy during this time. These images supported our conclusion that NPL-27 was effectively maintaining a territory in the area, from which he was largely excluding other males (Figure 17.10).
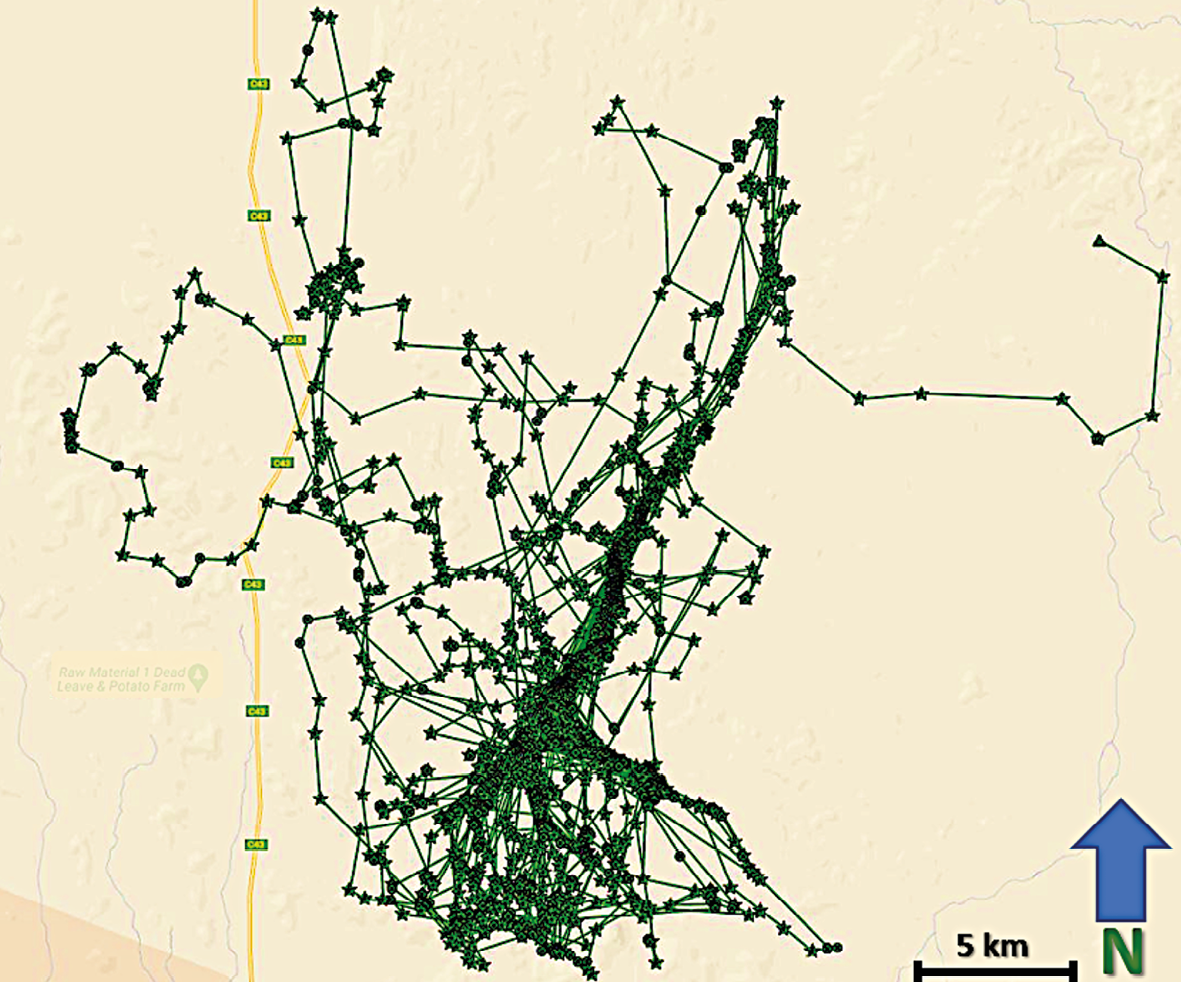
Fig. 17.10 Map showing visualised GPS-satellite collar data illustrating NPL-27 home range in Anabeb and Omatendeka conservancies from 30.8.2019-1.5.2022. © Lion Rangers data, CC BY-NC-ND 4.0.
Abruptly in May 2022, NPL-27’s movements changed dramatically. Between 2 May and 13 June 2022, he covered an area encompassing more than 1,000 km2, in an area distinctly different from his previous range (Figure 17.11). During this period, NPL-27 was responsible for three separate HLC incidents, during which he killed three donkeys in Omatendeka and two cattle in ǂKhoadi-ǁHôas conservancies (Figure 17.12). His movements also brought him into the ǂKhoadi-ǁHôas farming area, where HLC incidents have previously resulted in numerous retaliatory killings of lions by farmers. Following these incidents, the decision was taken by MEFT and the conservancies to have NPL-27 translocated from the ǂKhoadi-ǁHôas farming area and hopefully away from further HLC trouble.
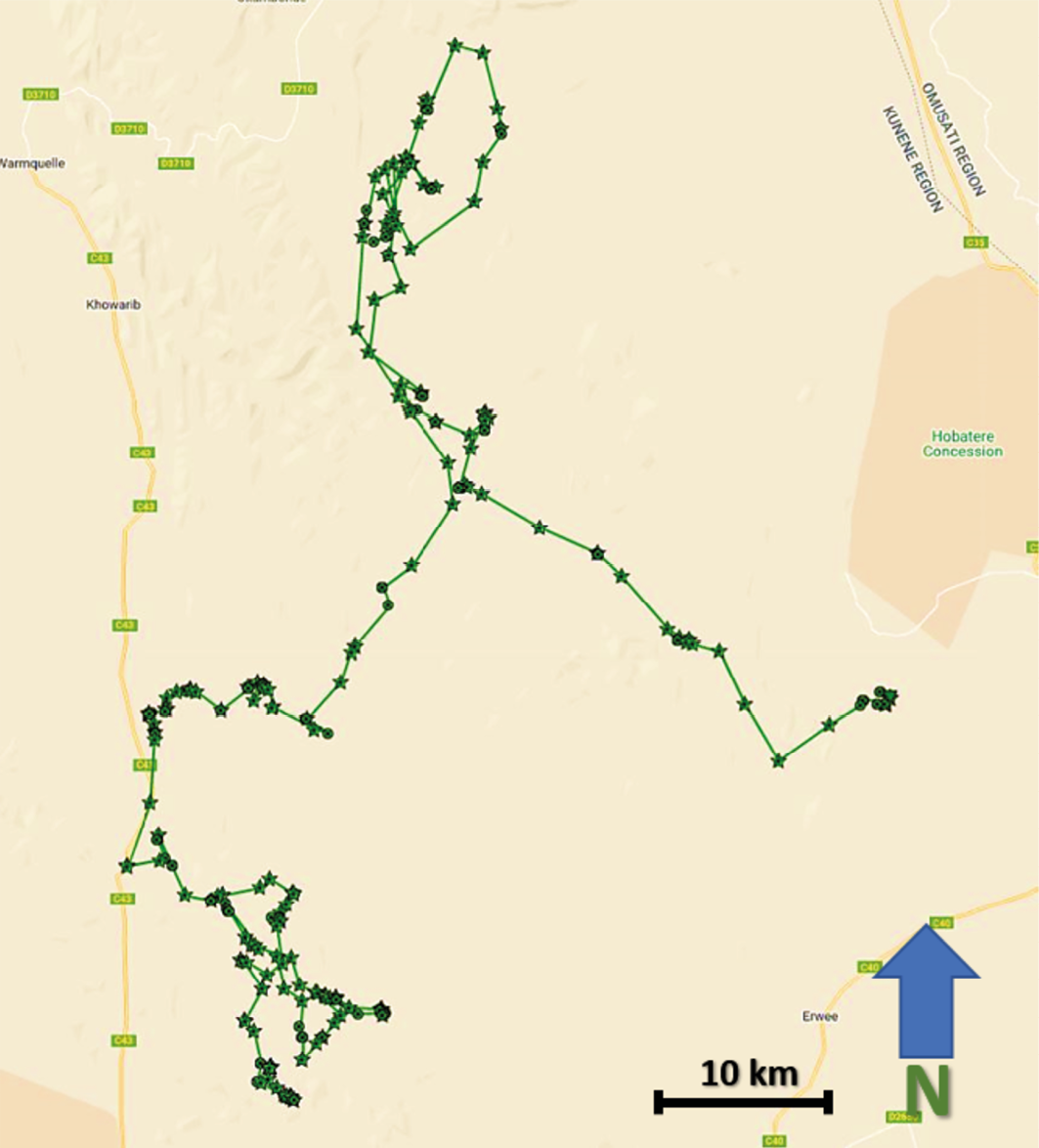
Fig. 17.11 Map showing visualised GPS-satellite collar data illustrating NPL-27 movements in Anabeb, Omatendeka, Ehi-Rovipuka, and ǂKhoadi-ǁHôas conservancies, from 2.5.2022-13.10.2022. © Lion Rangers data, CC BY-NC-ND 4.0.

Fig. 17.12 Cow killed by NPL-27 in ǂKhoadi-ǁHôas farming area, 13.6.2022. © Lion Rangers data, CC BY-NC-ND 4.0.
Normally this translocation would have emphasised returning NPL-27 to his previous home-range. However, his sudden departure raised questions as to whether the Otjiapa-Okavariona-Otjejekupe area remained a suitable destination. Neither rainfall data nor movements of other collared females in the area provided insight as to the cause of NPL-27’s seemingly sudden decision to leave the area. If NPL-27 would not re-settle here, it was considered likely he would continue to be a source of HLC.
A first clue as to NPL-27’s departure came from the collar data of two other males, who had recently moved their ranges further west, into the Otjiapa-Okavariona-Otjejekupe area. OPL-8 was first collared by MEFT and the Lion Rangers in the Hobatere Tourism Concession on 6 October 2021 along with his likely brother OPL-7: both were estimated between four and four-and-a-half years old at the time. Collar data from OPL-8 and OPL-7 indicated the two were closely bonded, rarely spending more than a day or two apart (see Figure 17.13). From October 2021 until late April 2022, these two lions primarily resided within the Hobatere Tourism Concession—some 60 kms from the Otjiapa-Okavariona-Otjejekupe area and separated by a rugged mountainous area. Perhaps in search of mating opportunities, in May 2022 OPL-8, and likely OPL-7 (whose collar had ceased to function), departed Hobatere, making their way south and west. These two males, moving into their prime years, would have been imposing adversaries for other male lions. Further data came from the Lion Rangers’ SMART patrols. These showed that, simultaneously, another male, OPL-3, spotted by Lion Rangers on numerous occasions, was moving into the area just south of Otjiapa-Okavariona-Otjejekupe. Although he was without a functioning collar at the time, OPL-3 was monitored by Rangers in the area, leading to him being collared in partnership with the Desert Lion Conservation Trust on 2 June 2022.

Fig. 17.13 Male lions OPL-7 and OPL-8 shown resting south of Otjiapa-Okavariona-Otjejekupe area, December 2022. © Lion Rangers data, CC BY-NC-ND 4.0.
On the night of 28 May 2022, NPL-27 came into close contact with OPL-8 (and likely OPL-7) north of Otjiapa-Okavariona-Otjejekupe (Figure 17.13). Whether a direct altercation took place is unknown, although NPL-27 and OPL-8’s collars recorded locations less than 200 m from each other at both 0600 and 0800. The result of this close encounter appears to be that NPL-27 moved further north, more than 40 kms in the next three days, to an area he had not previously been recorded in. By comparison, in the following two weeks OPL-8 resided in the area where the possible conflict took place, eventually settling into a home-range centred around Otjiapa-Okavariona-Otjejekupe, which he and OPL-7 have maintained as of this writing. These two males have also taken over the pride privileges of the females OPL-4, OPL-5, and OPL-15, who previously moved primarily with NPL-27.
The conflict leading to NPL-27’s departure, visible through available collar data, in combination with Lion Rangers’ ongoing work and SMART reports tracking available prey and lion movements, made the Otjiapa-Okavariona-Otjejekupe area a poor prospect for successfully translocating NPL-27. It was considered highly likely that NPL-27 would either be quickly chased out of the area, or killed in conflict with the other males.
Camera trap data suggested a viable alternative. From December 2021 to March 2022, the Lion Rangers research team deployed 81 camera traps to the Otjomombonde-Omirembue waterholes area in Omatendeka Conservancy. This mountainous and hard-to-reach area east of Otjiapa-Okavariona-Otjejekupe is considered something of a refuge for wildlife away from farming areas (see Chapter 3). Most crucially, trail camera images indicated minimal presence of lions. During the recent camera deployment covering 4,203 ‘trap nights’, in over 23,000 images containing carnivore or prey species, only 12 images contained lions. By comparison 72 images contained spotted hyena (Crocuta crocuta) and 162 contained brown hyena (Parahyaena brunnea). Five of these captures showed a male lion, known as NPL-33. Although considered to be resident within the Otjomombonde-Omirembue area, when the translocation of NPL-27 was being considered, NPL-33 was approximately 15 km to the north. Trail camera images also showed ample numbers of mountain zebra, giraffe, springbok, and even black-faced impala (Aepyceros melampus petersi) in the area. Ongoing Lion Ranger work in the area indicated prey species were still inhabiting the area.
Relying on the combination of collar data, trail camera images, and Lion Ranger reports around NPL-27’s former range of Otjiapa-Okavariona-Otjejekupe as well as surrounding Otjomombonde-Omirembue, the decision was taken by MEFT to translocate NPL-27 to Otjomombonde-Omirembue. During the early morning hours of 17 June 2022, NPL-27 was successfully immobilised and translocated from the ǂKhoadi-ǁHôas farming area (Figures 17.14 and 17.15). An approximately 30 hour operation was completed when NPL-27 was revived near the Omirembue waterhole. Follow-up monitoring by the Lion Rangers and via collar data indicated that he resided in the area through to the end of the month, making no attempt to return to either Otjiapa-Okavariona-Otjejekupe, or the farming areas where he previously caused conflict.
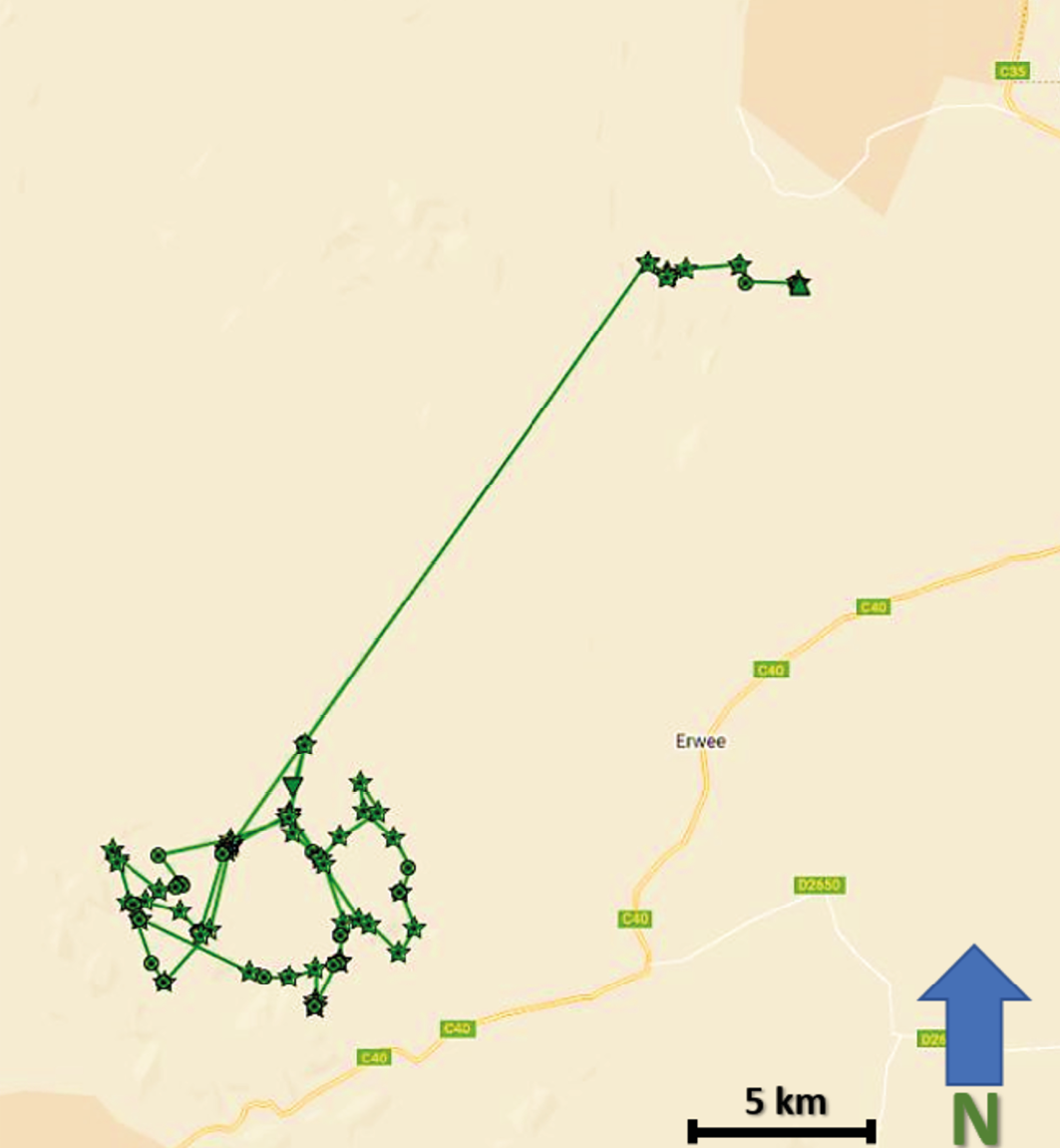
Fig. 17.14 Map showing visualised GPS/satellite collar data of NPL-27 translocation from ǂKhoadi-ǁHôas farming
(top right) to Otjomombonde-Omirembue waterholes area (bottom left), 16-21.6.2022. © Lion Rangers data,
CC BY-NC-ND 4.0.

Fig. 17.15 Lion Rangers, MEFT staff, and NPL-27 during translocation operation, 17.6.2022. © Lion Rangers data, CC BY-NC-ND 4.0.
17.5.1 Translocation postscript
Leading lion researchers are divided on the effectiveness of translocation.66 Translocated lions tend to return to their points of departure, sometimes with surprising speed, as our team has seen on numerous occasions. When it is used, translocation may simply be the best available option. Although NPL-27 would later be removed for encroaching on a separate farming area further south, the work of the Lion Rangers and researchers, supervised by MEFT, minimised remaining conflict and contributed to NPL-27 not being destroyed.
Because NPL-27 did not return to his previous range, risking near-certain conflict with OPL-8 and OPL-7, nor did he return to the ǂKhoadi-ǁHôas farming area, his translocation is considered a qualified success. In such a massive, unfenced landscape, there is no guarantee that lions will not encroach on human settlements. Rather the combination of remote sensing data and on-the-ground work of the Lion Rangers contributed to the conclusion that NPL-27 was failing to maintain a territory sufficiently distant from farming areas. He was now considered a “problem-causing” lion in need of removal. As it became apparent that other alternatives had been exhausted, NPL-27’s movements were monitored closely, leaving sufficient time to plan and execute a follow-up operation whereby NPL-27 was safely removed from the area. As of this writing he survives in his new location.
17.6 Conclusion
The combination of the Lion Rangers’ work and remote sensing data is pushing forward the prospect of lion monitoring and conservation based upon CBNRM principles in Kunene. The monitoring and translocation of NPL-27 provide a series of important insights for integrating technological and community-based approaches.
First, Kunene communal conservancies are farming areas. While conservancies have been gazetted to provide limited rights to wildlife for conservancy residents, potentially dangerous animals such as lions cannot too greatly negatively affect human lives and livelihoods (see Chapters 11 and 19). When such damage occurs, action must be taken to secure human well-being, but also to ensure continued support among conservancy residents for wildlife conservation. In the case of NPL-27, once he became a problem-causing lion it was necessary for him to be removed away from causing possible HLC, to ensure human well-being and continued support of lion conservation in the area. Through the work of the Lion Rangers, the conservancies took an active leadership role in monitoring the movements of NPL-27, limiting further HLC, and undertaking his translocation. These operations were performed in partnership with the local communities.
Second, the emphasis on remote sensing should not minimise the foundation of CBNRM upon which these specialised techniques become meaningfully operational in limiting HLC. The translocation of NPL-27 was able to take place because of the work of the Lion Rangers in monitoring wildlife, responding to and recording HLC, working with conservancy members to gauge their reactions, and providing on-the-ground information to researchers and government managers to make informed decisions. Remote sensing technologies can help researchers and Lion Rangers understand which lions are moving in which areas. But they cannot anticipate the likely effects of these movements, collect data on the human and more-than-human effects of these movements, nor forecast the response of local people. The interpretive element rests with researchers and the Lion Rangers to not only monitor and understand lion spatial ecology, but also to be able to react to HLC and potentially prevent conflict before it occurs. By providing more information to communities through remote sensing technologies, the Lion Rangers research team is helping grow the capacity of locals managing HLC.
Accordingly, by working with the Lion Rangers, researchers are better able to contextualise how certain types of data collection and analysis can support CBNRM. Much has been and will continue to be written about the challenges and successes of CBNRM in Kunene.67 The usefulness of lion collars, camera traps, and the SMART programme (see Chapter 18), rely on community tolerance of living with lions as well as local conservationists’ monitoring of the landscape for potential drivers of HLC, such as changing rainfall patterns, encroachment on farming areas by uncollared lion and other large carnivores, and prey and livestock movements. In Chapter 19 of this volume Muzuma explores another side of HLC: livestock movements across the landscape and how these can also drive HLC. Gaining as comprehensive a picture as practicable of the variables contributing to HLC refines the ability of the MEFT, the Lion Rangers, and researchers, to mitigate, manage, and even minimise HLC. This reinforces livelihoods as well as the survival of desert-adapted lions.
As noted by the late Garth Owen-Smith and Margaret Jacobsohn, who helped found Namibia’s communal conservancy movement (see Chapter 2), CBNRM must be a bottom-up approach in which process is also product.68 Caution should be the operative word when incorporating new technologies into community conservation. Remote sensing technologies such as lion collars and camera traps risk distancing the viewer from the real-life consequences of living with lions. As technology theorist and human-animal studies scholar Donna Haraway has noted, ‘situated knowledges’—those that are specifically relevant and forged by their time and place—are particularly powerful because they recognise the connectivity between actors, factors, and forces in each time and place.69 Remote sensing techniques should not replace, but augment, existing expertise of those living alongside lions. We have experienced numerous instances where lion collars have failed or been damaged, or trail cameras have malfunctioned, or even disappeared. People, and their knowledge of the landscapes they inhabit, are far more durable (as explored in Chapters 12, 13, 14 and 15). Desert-adapted lion conservation will continue to rely primarily on the willingness of conservancy residents to live alongside lions. The techniques outlined here merely support CBNRM of lions.
Bibliography
Balme, G.A., Hunter, L.T.B. and Slotow, R. 2009. Evaluating methods for counting cryptic carnivores. Journal of Wildlife Management 73: 433–41, https://doi.org/10.2193/2007-368
Belant, J.L., Bled, F., Mkasanga, I.J., Wilton, C.M., Mwampeta, S.B., Beyer, D.E., Mwakilema, W., and Fyumagwa, R. 2019. Track surveys do not provide accurate or precise lion density estimates in serengeti. Global Ecology and Conservation Journal 19: e00651, https://doi.org/10.1016/j.gecco.2019.e00651
Benson, E. 2010. Wired Wilderness: Technologies of Tracking and the Making of Modern Wildlife. Baltimore: Johns Hopkins University Press.
Bollig, M. 2016. Towards an arid Eden? Boundary-making, governance and benefit-sharing and the political ecology of the new commons of Kunene Region, northern Namibia. International Journal of the Commons 10(2): 771–99, https://thecommonsjournal.org/articles/10.18352/ijc.702
Bollig, M. 2020. Shaping the African Savannah: From Capitalist Frontier to Arid Eden in Namibia. Cambridge: Cambridge University Press, https://doi.org/10.1017/9781108764025
Donaldson, A.C., Carl, L., Meyer, R., Fuller, A. and Buss, P.E. 2023. Comparison of the cardiovascular effects of immobilization with three different drug combinations in free-ranging African lions. Conservation Physiology 11: 1–11, https://doi.org/10.1093/conphys/coac077
Dressler, W., Büscher, B., Schoon, M, Brockington, D., Hayes, T., Kull, C.A., McCarthy, J. and Shrestha, K. 2010. From hope to crisis and back again? A critical history of the global CBNRM narrative. Environmental Conservation 37(1): 5–15, https://doi.org/10.1017/S0376892910000044
Dröge, E., Creel, S., Becker, M.S., Loveridge, A.J., Sousa, L.L. & Macdonald, D.W. 2020. Assessing the performance of index calibration survey methods to monitor populations of wide-ranging low-density carnivores. Ecology and Evolution 1–17, https://doi.org/10.1002/ece3.6065
Haraway, D.J. 1990. Simians, Cyborgs, and Women: The Reinvention of Nature. New York and London: Routledge.
Hearn, M. 2003 Assessment of Biological and Human Factors Limiting the West Kunene Rhino Population. Report for the SADC Regional Programme for Rhino Conservation, http://www.rhinoresourcecenter.com/pdf_files/119/1196933961.pdf
Heydinger, J. 2021. Human-lion conflict and the reproduction of white supremacy in northwest Namibia. African Studies Review 64: 909–37, https://doi.org/10.1017/asr.2021.72
Heydinger, J., Diggle, R., Stuart-Hill, G., Dierkes, K. and Packer, C. 2022. Differentiated payments for ecosystem services based on estimated prey consumption by lions within communal conservancies. Ecosystem Services 53: 101403, https://doi.org/10.1016/j.ecoser.2021.101403
Heydinger, J.M., Packer, C. and Tsaneb, J. 2019. Desert-adapted lions on communal land: Surveying the costs incurred by, and perspectives of, communal-area livestock owners in northwest Namibia. Biological Conservation 236: 496–504, https://doi.org/10.1016/j.biocon.2019.06.003
Heydinger, J., Muzuma, U. and Packer, C. 2024. First systematic population survey of the desert-adapted lions, Northwest Namibia. African Journal of Ecology 62(2): e13226, https://doi.org/10.1111/aje.13266
Heydinger, J., Muzuma, U., and Tsaneb, J. in press. CBNRM and the desert-adapted lions: Centering local perspectives to limit human-lion conflict. In Anderson, D. and Bollig M. (eds.) Conservation in East and Southern Africa: People, Policy, Practice. Cambridge: Cambridge University Press.
Hoole, A.F. 2008. Community-Based Conservation and Protected Areas in Namibia: Social-Ecological Linkages for Biodiversity. Unpublished PhD Thesis, University of Manitoba.
Jacobsohn, M. 2019. Life is Like a Kudu Horn: A Conservation Memoir. Cape Town: Jacana.
Jones, B.T.B. 2001. The evolution of a community-based approach to wildlife management at Kunene, Namibia. In Hulme, D. and Murphree, M.W. (eds.) African Wildlife and Livelihoods: The Promise and Performance of Community Conservation. Oxford: James Currey, 160–76.
Jones, B.T.B. 2010. Ostrom and Namibian conservancies. Current Conservation 4: 21–23.
Jones, B.T.B. and Murphree, M.W. 2001. The evolution of policy on community conservation in Namibia and Zimbabwe. In Hulme, D. and Murphree, M.W. (eds.) African Wildlife and Livelihoods: The Promise and Performance of Community Conservation. Cape Town and Oxford: David Philip and James Currey, 38–58.
Karanth, K.U. and Nichols, J.D. 1998. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79: 2852–62, https://doi.org/10.1890/0012-9658(1998)079[2852:EOTDII]2.0.CO;2
Kock, M.D. and Burroughs, R. 2012. Chemical and Physical Restraint of Wild Animals: A Training and Field Manual for African Species. Greyton: International Wildlife Veterinary Services.
Lendelvo, S., Mechtilde, P. and Sullivan, S. 2020. A perfect storm? COVID-19 and community-based conservation in Namibia. Namibian Journal of Environment 4(B): 1–15, https://nje.org.na/index.php/nje/article/view/volume4-lendelvo/43
Mendelsohn, J., Jarvis, A., Roberts, C. and Robertson, T. 2002. Atlas of Namibia: A Portrait of the Land and its People. Cape Town: David Philip.
MET 2017. Human-Lion Conflict Management Plan for North West Namibia. Windhoek: Ministry of Environment and Tourism.
Muntifering, J.R., Linklater, W.L., Clark, S.G., !Uri-ǂKhob, S., Kasaona, J.K., |Uiseb, K., Du Preez, P., Kasaona, K., Beytell, P., Ketji, J., Hambo, B., Brown, M.A., Thouless, C., Jacobs, S. and Knight, A.T. 2017. Harnessing values to save the rhinoceros: Insights from Namibia. Oryx 51(1): 98–105, https://doi.org/10.1017/S0030605315000769
Muzuma, U. and Heydinger, J. 2024. Report on the Population Survey of the Free-ranging Lions of Northwest Namibia, with Results and Recommendations, 2022. Windhoek: Ministry of Environment, Forestry and Tourism.
NACSO 2016. The State of Community Conservation in Namibia: A Review of Communal Conservancies, Community Forests and other CBNRM Initiatives 2016. Windhoek: Namibian Association of CBNRM Support Organisations, https://www.nacso.org.na/sites/default/files/State%20of%20Community%20Conservation%20book%202016%20web.pdf
NACSO 2022. Game counts in north-west Namibia: May 2022, https://www.nacso.org.na/sites/default/files/North%20West%20Game%20Count-Regional%202022%20final.pdf
NNPC 2015. Macroeconomic Planning Department. Windhoek: Namibia National Planning Commission.
Ostrom, E. 1990. Governing the Commons: The Evolution of Institutions for Collective Action. Cambridge: Cambridge University Press.
Owen-Smith, G. 2010. An Arid Eden: One Man’s Mission in the Kaokoveld. Johannesburg: Jonathan Ball Publishers.
Packer, C., Loveridge, A., Canney, S. et al. 2013. Conserving large carnivores: Dollars and fence. Ecology Letters 16: 635–41, https://doi.org/10.1111/ele.12091
Pennycuick, C.J. and Rudnai, J. 1970. A method of identifying individual lion with an analysis of the reliability of identification. Journal of Zoology 160: 497–508.
Portas, R., Wachter, B., Beytell, P., Uiseb, K.H., Melzheimer, J. and Edwards, S. 2022. Leopard Panthera pardus camera trap surveys in the arid environments of northern Namibia. Mammalian Biology 102: 1185–98, https://doi.org/10.1007/s42991-022-00237-3
Rhino Ranger Incentive Programme 2014. Rhino Ranger Incentive Programme – 2014 Progress Report, http://www.savetherhinotrust.org/uploads/4/5/0/5/45057859/rhino_ranger_2014_update.pdf
Schaller, G.B. 1972. The Serengeti Lion: A Study in Predator-prey Relations. Chicago and London: University of Chicago Press.
Scott, J.C. 1998. Seeing Like a State: How Certain Schemes to Improve the Human Condition Have Failed. New Haven and London: Yale University Press.
SMART 2023. Spatial Monitoring and Reporting Tool, https://smartconservationtools.org/
Stander, P.E. 1999. Conservation of Lions and Other Large Carnivores in Etosha National Park and Khorixas District, Namibia. Windhoek.
Stander, P.E. 2000. Conservation of Lions and Other Large Carnivores in the Kunene Region, Namibia: Population Ecology and Long Term Monitoring of Free-ranging Populations in a Marginal and Arid Environment. Windhoek.
Stander, P.E. 2004. Population Ecology and Distribution of Lions in the Kunene and Erongo Regions, Namibia. Windhoek.
Stander, P.E. 2006. Population Ecology and Demography of Kunene Lions, January 2006. Windhoek.
Stander, P.E. 2007. Behaviour-Ecology and Conservation of Desert-Adapted Lions; 2007 Progress Report of the Kunene Lion Project, Namibia. Windhoek.
Stander, P.E. 2010. The Impact of Male-biased Mortality on the Population Structure of Desert-adapted Lions in Namibia. Unpublished Report, http://lionrangers.org/wp-content/uploads/2020/08/Stander2010Male-basedMortality.pdf
Stander, P.E. 2018. Vanishing Kings: Lions of the Namib Desert. Johannesburg: HPH Publishing.
Stander, P.E. and Hanssen, L. 2003. Population Ecology of Desert-adapted Lions in the Kunene Region, Namibia. Windhoek, Namibia. Windhoek, https://lionrangers.org/wp-content/uploads/2020/08/Stander2003LionStudyKunene.pdf
Stander, P.E. and Morkel, P. vdB. 1991. Field immobilization of lions using disassociative anaesthetics in combination with sedatives. African Journal of Ecology 29: 137–48.
Stuart-Hill, G., Diggle, R., Munali, B., Tagg, J. and Ward, D. 2005. The Event Book System: A community-based natural resource monitoring system from Namibia. Biodiversity and Conservation 14: 2611–31, https://link.springer.com/article/10.1007/s10531-005-8391-0
Sullivan, S. 2003. Protest, conflict and litigation: Dissent or libel in resistance to a conservancy in north-west Namibia. In Berglund, E. and Anderson, D. (eds.) Ethnographies of Conservation: Environmentalism and the Distribution of Privilege. Oxford: Berghahn Press, 69–86, https://doi.org/10.1515/9780857456748-008
Sullivan, S. 2016. Three of Namibia’s most famous lion family have been poisoned – why? The Conversation
23.8.2016, https://theconversation.com/three-of-namibias-most-famous-lion-family-have-been-poisoned-why-64322
Tavolaro, F.M., Woodgate, Z., Brown, C., Redpath, S.M. and O’Riain, M.J. 2022. Multispecies study of patterns and drivers of wildlife impacts on human livelihoods in communal conservancies. Conservation Science and Practice 4(9): e12773, https://doi.org/10.1111/csp2.12773
UNICEF 2013. Regional Education Analysis for Namibia. Windhoek: United Nations Children’s Fund.
Williams, K.S., Pitman, R.T., Mann, G.K.H., Whittington-Jones, G., Comley, J., Williams, S.T., Hill, R.A., Balme, G.A. and Parker, D.M. 2021. Utilizing bycatch camera-trap data for broad-scale occupancy and conservation: A case study of the brown hyaena Parahyaena brunnea. Oryx 55: 216–26, https://doi.org/10.1017/S0030605319000747
1 Acknowledgements: Thanks to Namibia’s Ministry of Environment, Forestry and Tourism (MEFT) and Namibia’s National Commission on Research, Science and Technology (NCRST) for overseeing this work. Thanks to the Community Conservation Fund of Namibia, International Union for Conservation of Nature (IUCN), Ultimate Safaris, and WWF-Namibia for supporting the Lion Rangers work and research. Thanks to Namibian Lion Trust for supplying lion collar data. Thanks to MEFT-Directorate of Scientific Services and Game Capture for assisting with lion collaring. Mathilde Brassine, Esau Matundu, Uakendisa Muzuma and Jendery Tsaneb assisted with camera trap deployment. Wide Horizons Aerial Technologies and Desert Lion Conservation Trust assisted with collar website management and visualisation. Thanks to all partnering conservancies.
2 Stander (2018)
3 MET (2017)
4 Ibid.
5 Packer et al. (2013)
6 Muzuma & Heydinger (2024); also see Heydinger et al. (2024)
7 Stander (2010)
8 Muzuma & Heydinger (2024)
9 Sullivan (2016)
10 MET (2017), Tavolaro et al. (2022)
11 Jones (2001)
12 Heydinger et al. (2019)
13 Heydinger et al. (in press)
15 Stander (2007)
16 Mendelsohn et al. (2002), Stander (2018)
17 Bollig (2020)
18 Heydinger et al. (2019)
19 NACSO (2022)
20 Heydinger et al. (2019)
21 Mendelsohn et al. (2002)
22 NNPC (2015)
23 Lendelvo et al. (2020)
24 UNICEF (2013)
25 Bollig (2016)
26 Heydinger (2021)
27 Sesfontein Pastoralist #4, Personal Communication, 23.11.2017.
28 Stander (1999, 2000), Stander & Hanssen (2003)
29 Stander (2004)
30 Stander (2006)
31 Dressler et al. (2010), Owen-Smith (2010)
32 Ostrom (1990), Jones (2010)
33 Jacobsohn (2019)
34 Heydinger et al. (in press)
35 MET (2017)
36 Hearn (2003), Owen-Smith (2010), Rhino Ranger Incentive Programme (2014), Muntifering et al. (2017)
38 Jones (2001)
39 Heydinger (2021)
40 Heydinger et al. (2019), Heydinger et al. (in press)
41 Jones & Murphree (2001)
43 Heydinger et al. (2022)
44 SMART (2023)
45 Stuart-Hill et al. (2005)
46 Stander (2018). Khorixas is named for xoris, the important food plant Salvadora persica.
47 Scott (1998)
48 Benson (2010)
49 Heydinger (2021)
52 Vanishing Kings (2015), https://intonatureproductions.com/films/vanishing-kings-i-lions-of-the-namib/
53 Benson (2010)
54 Schaller (1972)
55 Packer (pers. comm.)
56 Stander & Morkel (1991), Kock & Burroughs (2012), Donaldson et al. (2023)
57 Elsewhere collars have additional technologies for collecting other data such as respiration and heart rate—these have not been used to date in Kunene.
58 (1998)
59 Williams et al. (2021)
60 Balme et al. (2009), Williams et al. (2021), Portas et al. (2022)
61 Balme et al. (2009)
62 Belant et al. (2019), Dröge et al. (2020)
63 Balme et al. (2009)
64 Pennycuick & Rudnai (1970)
65 Muzuma & Heydinger (2024)
66 ALWG (African Lion Working Group) pers. comms., 2022.
67 e.g. Sullivan (2003), Hoole (2008), Bollig (2020)
68 Owen-Smith (2010), Jacobsohn (2019)
69 Haraway (1990)
